Influence of Eco-Friendly Processing Aids on Silica-Based Rubber Composites
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of the Rubber Composites
2.3. Characterization
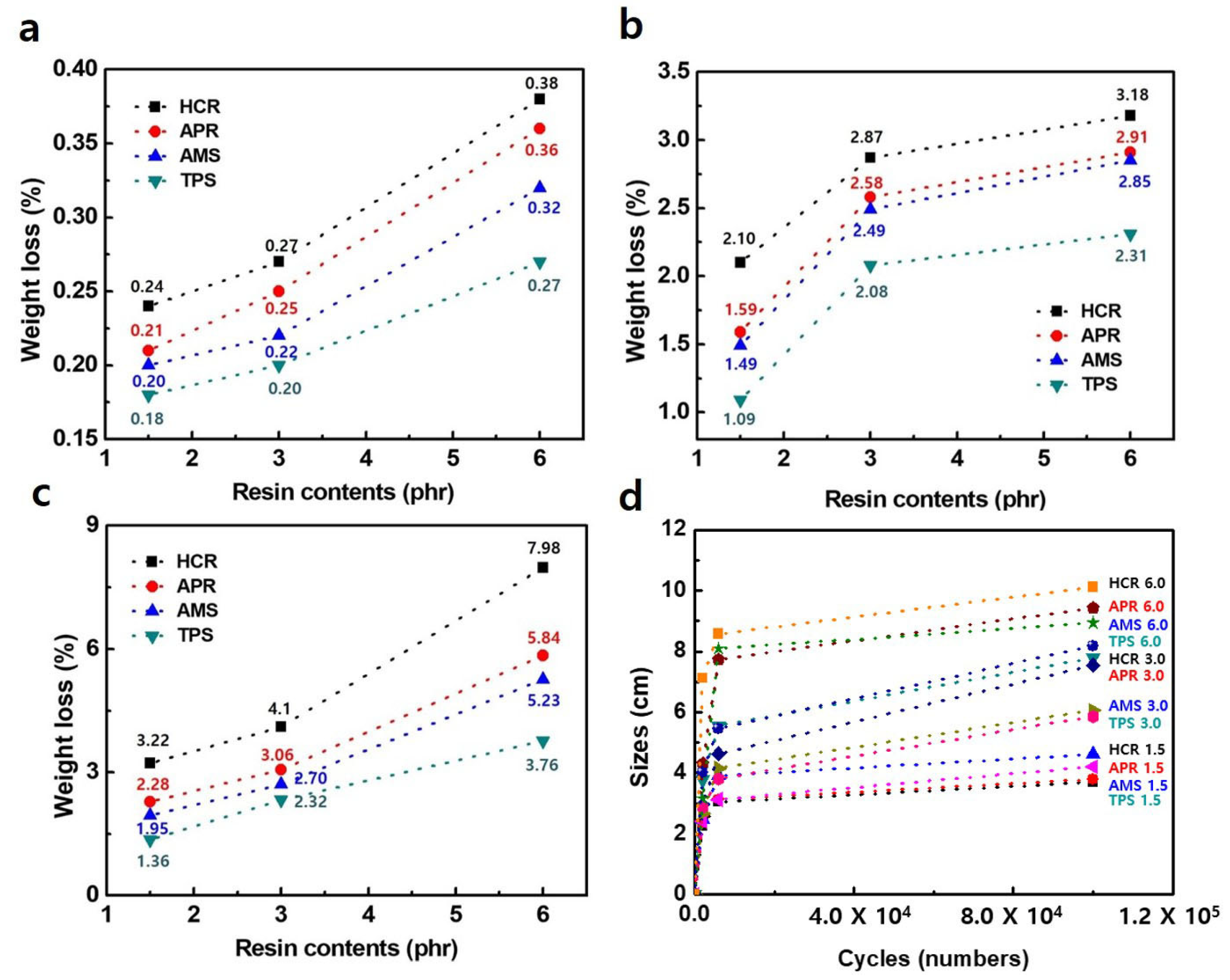
2.4. Measurement of Swelling
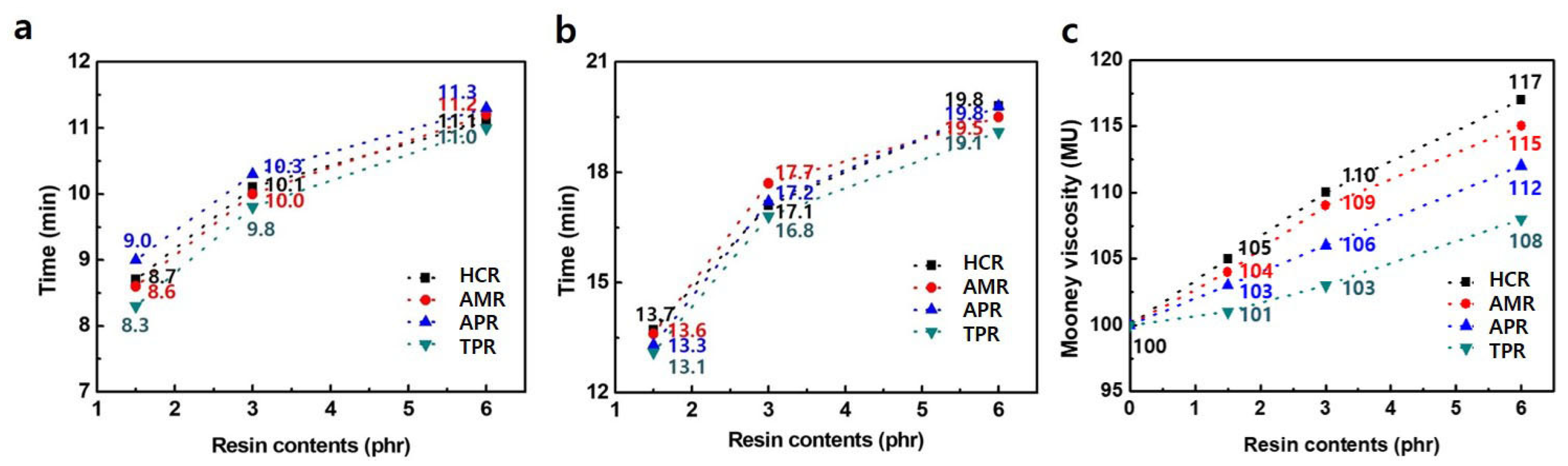
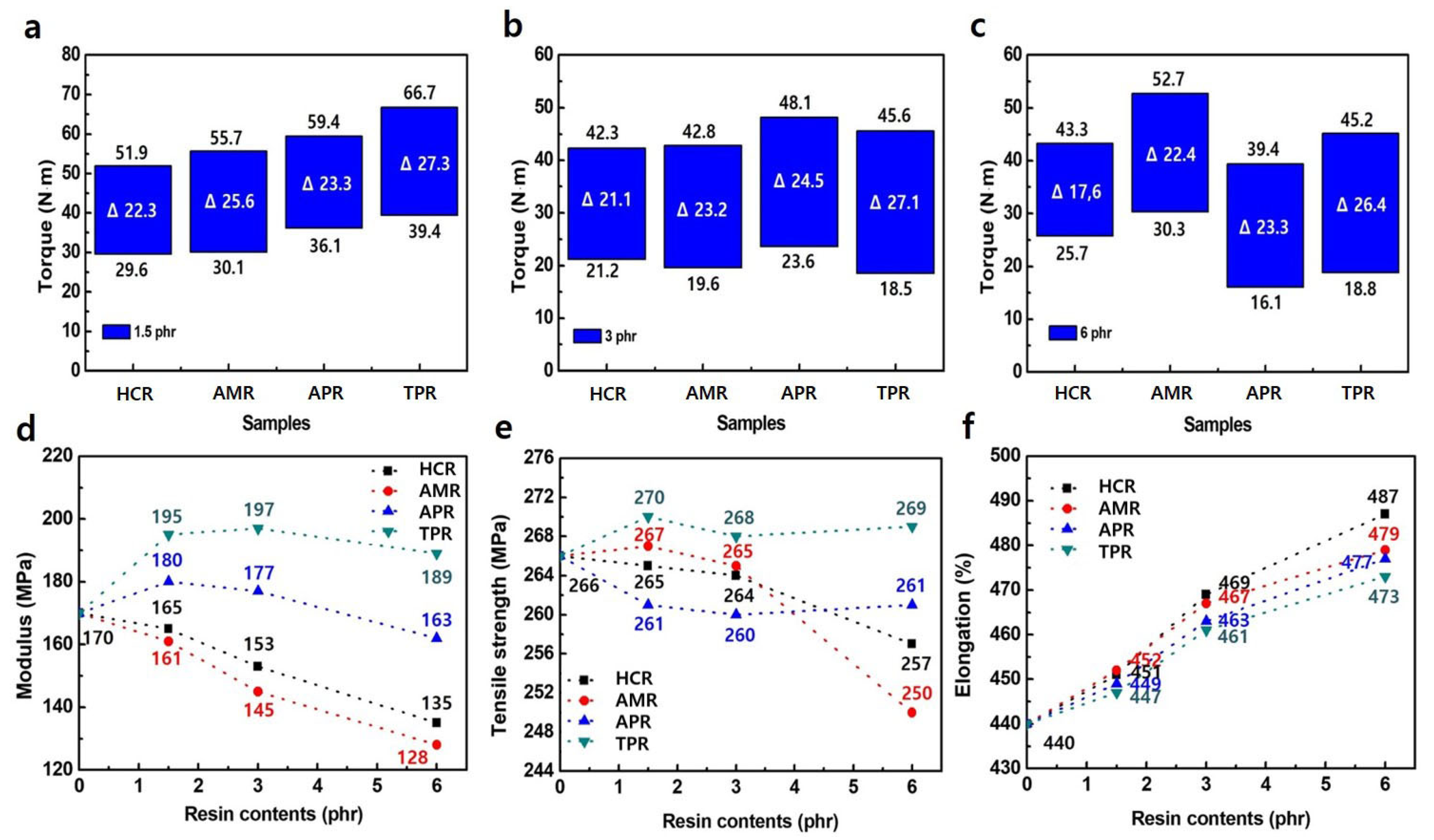
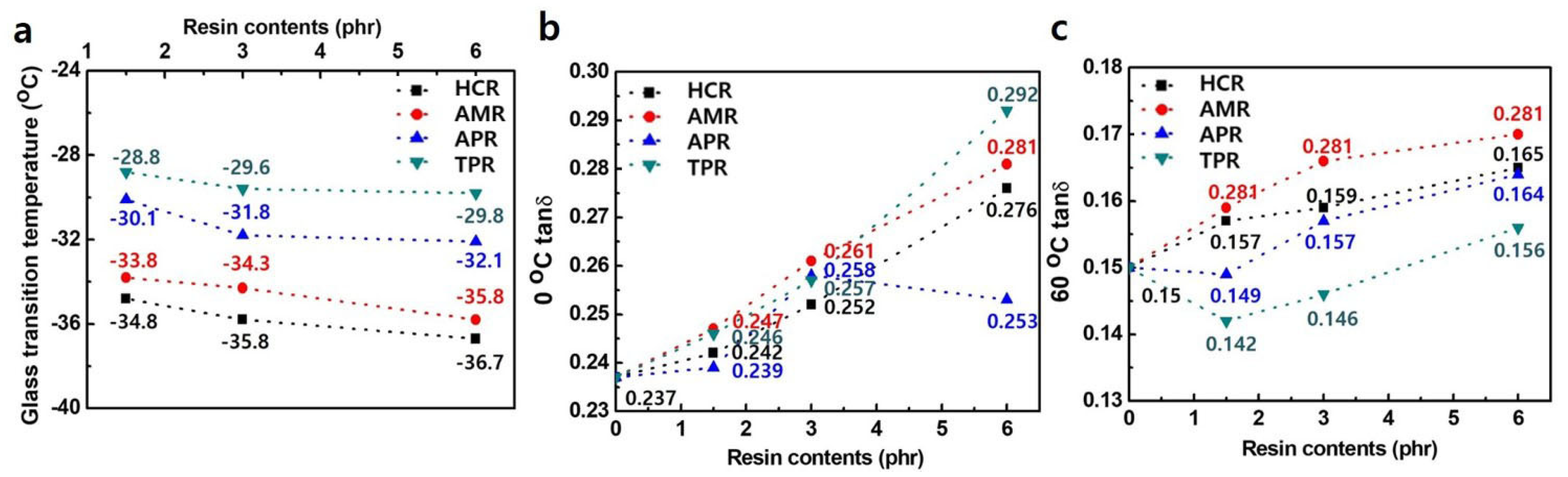
3. Results and Discussion
4. Conclusions
Supplementary Materials
Funding
Acknowledgments
Conflicts of Interest
References
- Leblanc, J.L. Rubber-filler interactions and rheological properties in filled compounds. Prog. Polym. Sci. 2002, 27, 627–687. [Google Scholar] [CrossRef]
- Essabir, H.; Raji, M.; Essassi, E.M.; Rodrigue, D.; Bouhfid, R. Morphological, thermal, mechanical, electrical and magnetic properties of ABS/PA6/SBR blends with Fe3O4 nanoparticles. J. Mater. Sci. 2017, 28, 17120–17130. [Google Scholar]
- Yang, S.; Liang, P.; Peng, X.; Zhou, Y.; Hua, K.; Wu, W.; Cai, Z. Improvement in mechanical properties of SBR/Fly ash composites by in-situ grafting-neutralization reaction. Macromol. Chem. Eng. J. 2018, 354, 849–855. [Google Scholar] [CrossRef]
- Liu, Z.; Zhang, Y. Enhanced mechanical and thermal properties of SBR composites by introducing graphene oxide nanosheets decorated with silica particles. Compos. Part A Appl. Sci. Manuf. 2017, 102, 236–242. [Google Scholar] [CrossRef]
- Rezende, C.A.; Bragança, F.C.; Doi, T.R.; Lee, L.-T.; Galembeck, F.; Boué, F. Natural rubber-clay nanocomposites: Mechanical and structural properties. Polymer 2010, 51, 3644–3652. [Google Scholar] [CrossRef]
- D’Apuzzo, M.; Evangelisti, A.; Nicolosi, V. An exploratory step for a general unified approach to labelling of road surface and tyre wet friction. Accid. Anal. Prev. 2020, 138, 105462. [Google Scholar] [CrossRef]
- Li, Y.; Han, B.; Liu, L.; Zhang, F.; Zhang, L.; Wen, S.; Lu, Y.; Yang, H.; Shen, J. Surface modification of silica by two-step method and properties of solution styrene butadiene rubber (SSBR) nanocomposites filled with modified silica. Compos. Sci. Technol. 2013, 88, 69–75. [Google Scholar] [CrossRef]
- Manna, A.K.; De, P.; Tripathy, D.K.; De, S.K.; Peiffer, D.G. Bonding between precipitated silica and epoxidied natural rubber in the presence of silane coupling agent. J. Appl. Polym. Sci. 1999, 74, 389–398. [Google Scholar] [CrossRef]
- Kim, K.; Lee, J.-Y.; Choi, B.-J.; Seo, B.; Kwag, G.-H.; Paik, H.-J.; Kim, W. Styrene-butadiene-glycidyl methacrylate terpolymer/silica composites: Dispersion of silica particles and dynamic mechanical properties. Compos. Interfaces 2014, 21, 685–702. [Google Scholar] [CrossRef]
- Sattayanurak, S.; Noordermeer, J.W.M.; Sahakaro, K.; Kaewsakul, W.; Dierkes, K.; Blume, A. Silica-Reinforced Natural Rubber: Synergistic Effects by Addition of Small Amounts of Secondary Fillers to Silica-Reinforced Natural Rubber Tire Tread Compounds. Adv. Mater. Sci. Eng. 2019, 2019, 1–8. [Google Scholar] [CrossRef]
- Ansarifar, M.A.; Nanapoolsin, T.; Jain, A. Silane-silica reinforcement of some natural rubber vulcanisates. J. Rubb. Res. 2002, 5, 11–27. [Google Scholar]
- Lin, Y.; Liu, S.; Peng, J.; Liu, L. The filler–rubber interface and reinforcement in styrene butadiene rubber composites with graphene/silica hybrids: A quantitative correlation with the constrained region. Compos. Part A Appl. Sci. Manuf. 2016, 86, 19–30. [Google Scholar] [CrossRef]
- Ansarifar, A.; Wang, L.; Ellis, R.J.; Kirtley, S.P. The reinforcement and crosslinking of styrene butadiene rubber with silanized precipitated silica nanofiller. Rubber Chem. Technol. 2006, 79, 39–54. [Google Scholar] [CrossRef]
- Ansarifar, A.; Wang, L.; Ellis, R.J.; Kirtley, S.P.; Riyazuddin, N. Enhancing the mechanical properties of styrene–butadiene rubber by optimizing the chemical bonding between silanized silica nanofiller and the rubber. J. Appl. Polym. Sci. 2007, 105, 322–332. [Google Scholar] [CrossRef]
- Kim, K.J.; VanderKooi, J. Effects of zinc ion containing surfactant on bifunctional silane treated silica compounds in natural rubber. J. Indust. Eng. Chem. 2002, 8, 334–347. [Google Scholar]
- Kim, K.J.; White, J.L. Silica agglomerate breakdown in three-stage mix including a continuous ultrasonic extruder. J. Indust. Eng. Chem. 2000, 6, 372–379. [Google Scholar]
- Eskandar, N.G.; Simovic, S.; Prestidge, C.A. Interactions of hydrophilic silica nanoparticles and classical surfactants at non-polar oil–water interface. J. Colloid Interf. Sci. 2011, 358, 217–225. [Google Scholar] [CrossRef] [PubMed]
- Simovic, S.; Prestidge, C.A. Hydrophilic Silica nanoparticles at the PDMS droplet−water interface. Langmuir 2003, 19, 3785–3792. [Google Scholar] [CrossRef]
- Vilmin, F.; Bottero, I.; Travert, A.; Malicki, N.; Gaboriaud, F.; Trivella, A. Thibault-Starzyk, F. Reactivity of bis [3-(triethoxysilyl)propyl] Tetrasulfide (TESPT) silane coupling agent over hydrated silica: Operando IR spectroscopy and chemometrics study. J. Phys. Chem. C 2014, 118, 4056–4071. [Google Scholar] [CrossRef]
- Dileep, P.; Varghese, G.A.; Sivakumar, S.; Narayanankutty, S.K. An innovative approach to utilize waste silica fume from zirconia industry to prepare high performance natural rubber composites for multi-functional applications. Polym. Test. 2020, 81, 106172. [Google Scholar] [CrossRef]
- Yatsuyanagi, F.; Suzuki, N.; Ito, M.; Kaidou, H. Effects of surface chemistry of silica particles on the mechanical properties of silica filled styrene–butadiene rubber systems. Polym. J. 2002, 34, 332. [Google Scholar] [CrossRef]
- Kuriakose, M.A.; Varghese, M. Use of rice bran oil and epoxidized rice bran oil in carbon black-filled natural rubber-polychloroprene blends. J. Appl. Polym. Sci. 2003, 90, 4084–4092. [Google Scholar] [CrossRef]
- Hassan, M.M.; Takahashi, T.; Koyama, K. Thermal and mechanical properties of novel composites of metyl silicone polymer and partially ceramized rice bran. Ind. Eng. Chem. Res. 2013, 52, 1275–1283. [Google Scholar] [CrossRef]
- Song, S.H. The effect of palm oil-based hybrid oils as green multifunctional oils on the properties of elastomer composites. Polymers 2018, 10, 1045. [Google Scholar] [CrossRef] [PubMed]
- Nandanan, V.; Joseph, R.; George, K.E. Rubber seed oil: A multipurpose additive in NR and SBR compounds. J. Appl. Polym. Sci. 1999, 72, 487–492. [Google Scholar] [CrossRef]
- Kukreja, T.R.; Chauhan, R.C.; Choe, S.; Kundu, P.P. Effect of the doses and natural of vegetable oil on carbon black/rubber interactions: Studies on caster oil and other vegetable oils. J. Appl. Polym. Sci. 2002, 87, 1574–1578. [Google Scholar] [CrossRef]
- Ashraf, S.M.; Sharif, A.; Ufana, R.; Manawwer, A.; Sharma, H.O. Compatibility studies on dehydrated caster oil epoxy blend with poly (methacrylic acid). J. Macromol. Sci. Part A 2005, 42, 1409–1421. [Google Scholar] [CrossRef]
- Brinker, C.J. Hydrolysis and condensation of silicates: Effects on structure. J. Non-Cryst Solids 1988, 100, 31–50. [Google Scholar] [CrossRef]
- Coltrain, B.K.; Kelts, L.W. The chemistry of hydrolysis and condensation of silica sol–gel precursors. Adv. Chem. 1994, 234, 403–418. [Google Scholar]
- Nassor, E.C.D.O.; Ávila, L.R.; Pereira, P.F.D.S.; Ciuffi, K.J.; Calefi, P.S.; Nassar, E.J. Influence of the hydrolysis and condensation time on the preparation of hybrid materials. Mater. Res. 2011, 14, 1–6. [Google Scholar] [CrossRef]
- Huang, R.; Pan, Q.; Feng, K. Maleic anhydride modified dicyclopentadiene resin for improving wet skid resistance of silica filled SSBR/BR composites. Appl. Sci. 2020, 10, 4478. [Google Scholar] [CrossRef]
- Lee, D.; Song, S.H. A study of silica reinforced rubber composites with eco-friendly processing aids for pneumatic tires. Macromol. Res. 2019, 27, 580–586. [Google Scholar] [CrossRef]
- Tian, C.; Feng, Y.; Chu, G.; Lu, Y.; Miao, C.; Ning, N.; Zhang, L.; Tian, M. Interfacial nanomechanical properties and chain segment dynamics of fibrillary silicate/elastomer nanocomposites. Compos.Part B Eng. 2020, 193, 108048. [Google Scholar] [CrossRef]
- Flory, P.J.; Rehner, J. Statistical mechanics of cross-linked polymer networks Ⅰ. Rubberlike elasticity. J. Chem. Phys. 1943, 11, 521–526. [Google Scholar] [CrossRef]
- Khalaf, A.I.; Hagazy, M.A. Synthesis and characterization of cationic surfactants in the preparation of organobentonite and study their effectiveness on the properties of styrenebutadiene rubber/bentonite composites. High Perform. Polym. 2013, 25, 115–125. [Google Scholar] [CrossRef]
- Qu, L.; Yu, G.; Xie, X.; Wang, L.; Li, J.; Zhao, Q. Effect of silane coupling agent on filler and rubber interaction of silica reinforced solution styrene butadiene rubber. Polym. Compos. 2013, 34, 1575–1582. [Google Scholar] [CrossRef]
- Ruggerone, R.; Geiser, V.; Vacche, S.D.; Leterrier, Y.; Manson, J.A.E. Immobilized polymer fraction in hyperbranched polymer/silica nanocomposite suspensions. Macromolecules 2010, 43, 10490–10497. [Google Scholar] [CrossRef]
- Sargsyan, A.; Tonoyan, A.; Davtyan, S.; Schick, C. The amount of immobilized polymer in PMMA SiO2 nanocomposites determined from calorimetric data. Eur. Polym. J. 2007, 43, 3113–3127. [Google Scholar] [CrossRef]
- Sengloyluan, K.; Sahakaro, K.; Dierkes, W.K.; Noordermeer, J.W.M. Silica-reinforced tire tread compounds compatibilized by using epoxidized natural rubber. Eur. Polym. J. 2014, 51, 69–79. [Google Scholar] [CrossRef]
- Li, Y.; Han, B.; Wen, S.; Lu, Y.; Yang, H.; Zhang, L.; Liu, L. Effect of the temperature on surface modification of silica and properties of modified silica filled rubber composites. Compos. Part A. Appl. Sci. Manuf. 2014, 62, 52–59. [Google Scholar] [CrossRef]
- Guo, H.; Jerrams, S.; Xu, Z.; Zhou, Y.; Jiang, L.; Zhang, L.; Liu, L.; Wen, S. Enhanced fatigue and durability of carbon black/natural rubber composites reinforced with graphene oxide and carbon nanotubes. Eng. Fract. Mech. 2020, 223, 106764. [Google Scholar] [CrossRef]
- Han, Q.; Zhang, L.; Wu, Y. Relationship between dynamic fatigue crack propagation properties and viscoelasticity of natural rubber/silicon rubber composites. RSC Adv. 2019, 9, 29813–29820. [Google Scholar] [CrossRef]
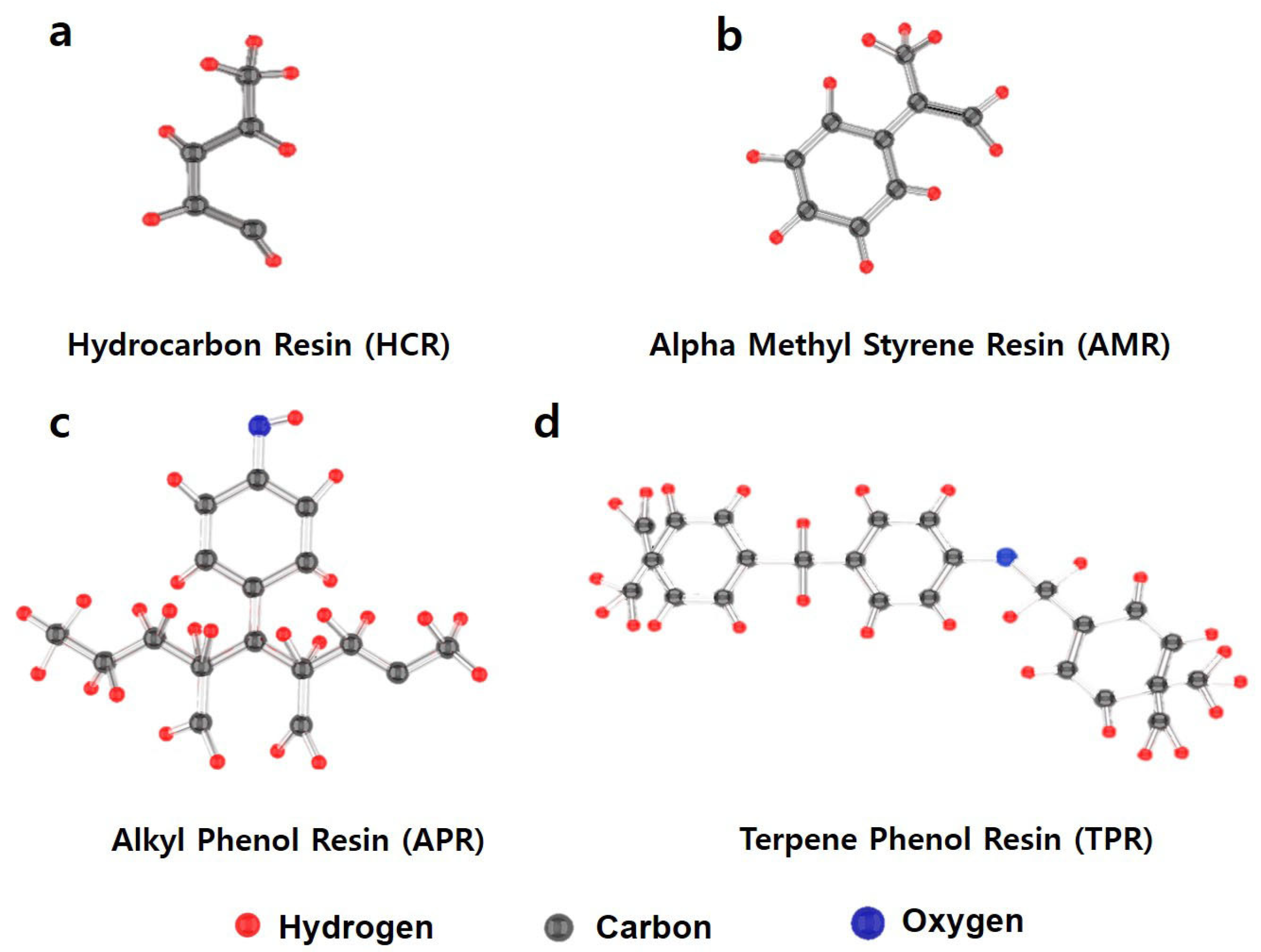
| STEP | Materials | HCR 1.5/3.0/6.0 | AMR 1.5/3.0/6.0 | APR 1.5/3.0/6.0 | TPR 1.5/3.0/6.0 | Injection Materials | Injection Time |
|---|---|---|---|---|---|---|---|
| Step 1 | SBR Latex | 50 | 50 | 50 | 50 |
SBR/SBR Latex Carbon Black Master Batch Chemical Drop |
0 30 100 180 300 |
| Silica | 40 | 40 | 40 | 40 | |||
| HCR | 1.5/3.0/6.0 | ||||||
| AMR | 1.5/3.0/6.0 | ||||||
| APR | 1.5/3.0/6.0 | ||||||
| TPR | 1.5/3.0/6.0 | ||||||
| Step 2 | SBR | 50 | 50 | 50 | 50 | ||
| Carbon Black | 20 | 20 | 20 | 20 | |||
| S/A | 1 | 1 | 1 | 1 | |||
| Sulfur | 1.75 | 1.75 | 1.75 | 1.75 | |||
| ZnO | 2 | 2 | 2 | 2 | |||
| TBBS | 1 | 1 | 1 | 1 |
| Materials | HCR 3.0 phr | AMR 3.0 phr | APR 3.0 phr | TPR 3.0 phr |
|---|---|---|---|---|
| Equilibrium swelling (%) | 145.13 ± 0.3 | 152.12 ± 0.2 | 165.72 ± 0.4 | 173.14 ± 0.3 |
| Crosslinking density (104 mol/g) | 2.35 ± 0.4 | 2.75 ± 0.5 | 2.87 ± 0.3 | 2.98 ± 0.5 |
| Materials | ω | ΔCp | ΔCpn | χim |
|---|---|---|---|---|
| HCR 3.0 phr | 3.0 | 0.485 | 0.5 | 20.0 |
| AMR 3.0 phr | 3.0 | 0.451 | 0.465 | 25.6 |
| APR 3.0 phr | 3.0 | 0.467 | 0.481 | 23.0 |
| TPR 3.0 phr | 3.0 | 0.423 | 0.436 | 30.2 |
| HCR 1.5 | HCR 3.0 | HCR 6.0 | APR 1.5 | APR 3.0 | APR 6.0 | AMS 1.5 | AMS 3.0 | AMS 6.0 | TPR 1.5 | TPR 3.0 | TPR 6.0 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 2000 | 2.47 | 3.78 | 7.12 | 2.42 | 2.95 | 4.31 | 2.36 | 2.67 | 3.16 | 2.26 | 2.87 | 4.01 |
| 6000 | 3.92 | 5.56 | 8.57 | 3.13 | 4.63 | 7.73 | 3.13 | 4.16 | 8.09 | 3.05 | 3.81 | 5.46 |
| 10,000 | 4.61 | 7.79 | 10.11 | 4.21 | 7.54 | 9.43 | 3.79 | 6.07 | 8.94 | 3.7 | 5.86 | 8.19 |
| dc/dn | 3.24 | 5.11 | 9.07 | 3.21 | 5.07 | 8.12 | 3.17 | 4.11 | 7.35 | 3.12 | 4.02 | 6.25 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Song, S.H. Influence of Eco-Friendly Processing Aids on Silica-Based Rubber Composites. Appl. Sci. 2020, 10, 7244. https://doi.org/10.3390/app10207244
Song SH. Influence of Eco-Friendly Processing Aids on Silica-Based Rubber Composites. Applied Sciences. 2020; 10(20):7244. https://doi.org/10.3390/app10207244
Chicago/Turabian StyleSong, Sung Ho. 2020. "Influence of Eco-Friendly Processing Aids on Silica-Based Rubber Composites" Applied Sciences 10, no. 20: 7244. https://doi.org/10.3390/app10207244
APA StyleSong, S. H. (2020). Influence of Eco-Friendly Processing Aids on Silica-Based Rubber Composites. Applied Sciences, 10(20), 7244. https://doi.org/10.3390/app10207244




