Synthesis and Characterization of Spherical Calcium Carbonate Nanoparticles Derived from Cockle Shells
Abstract
Featured Application
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
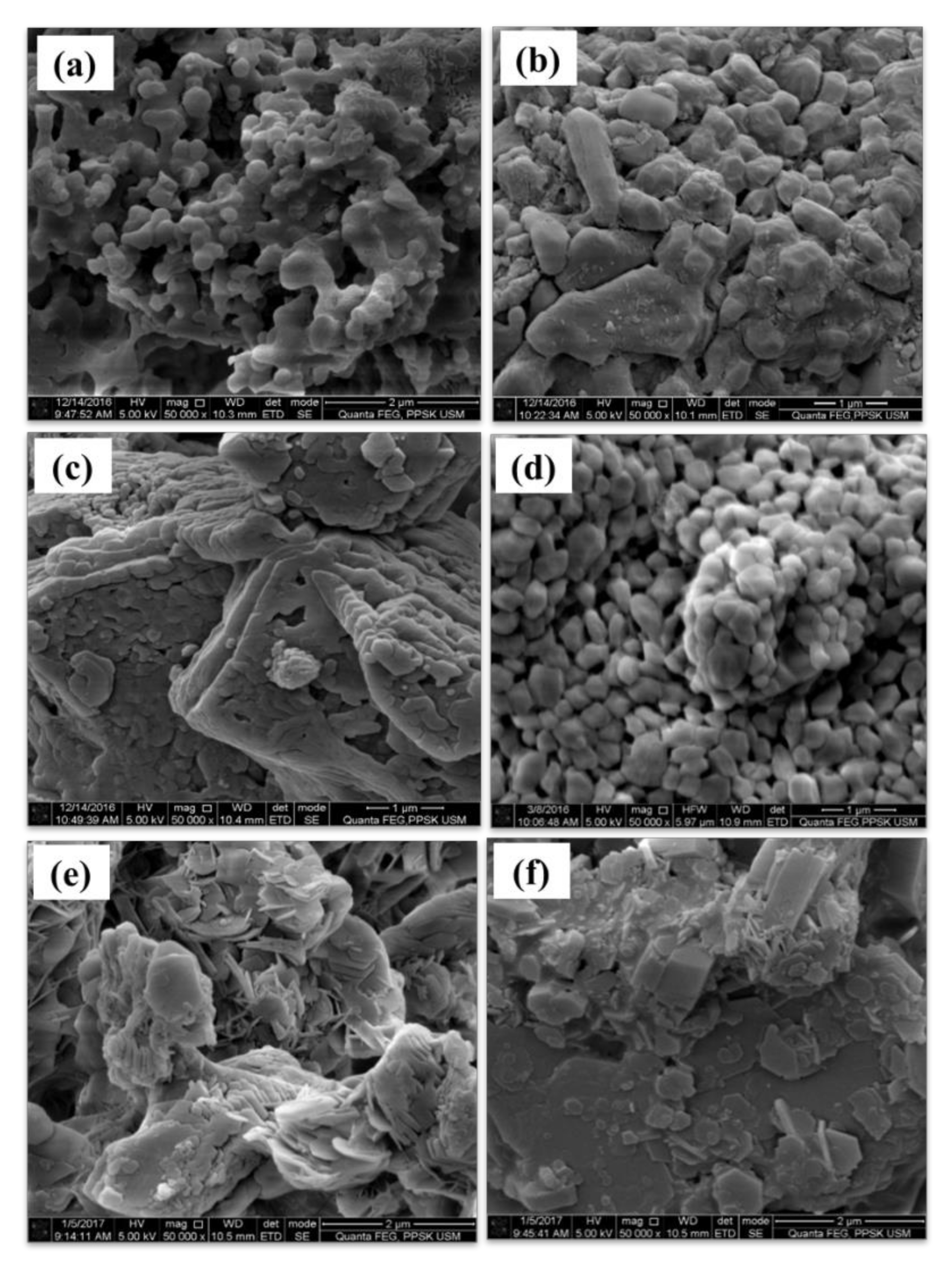
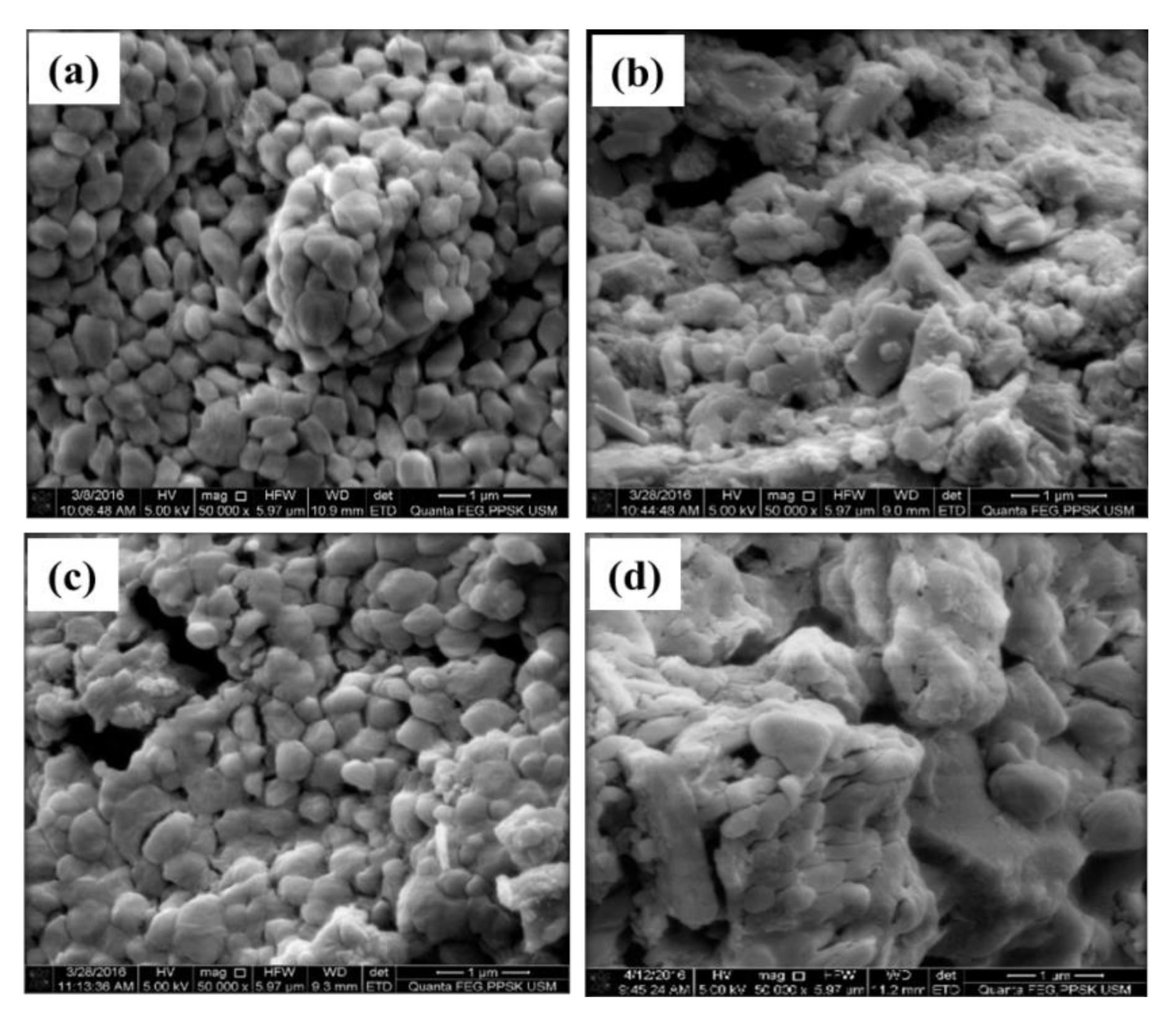
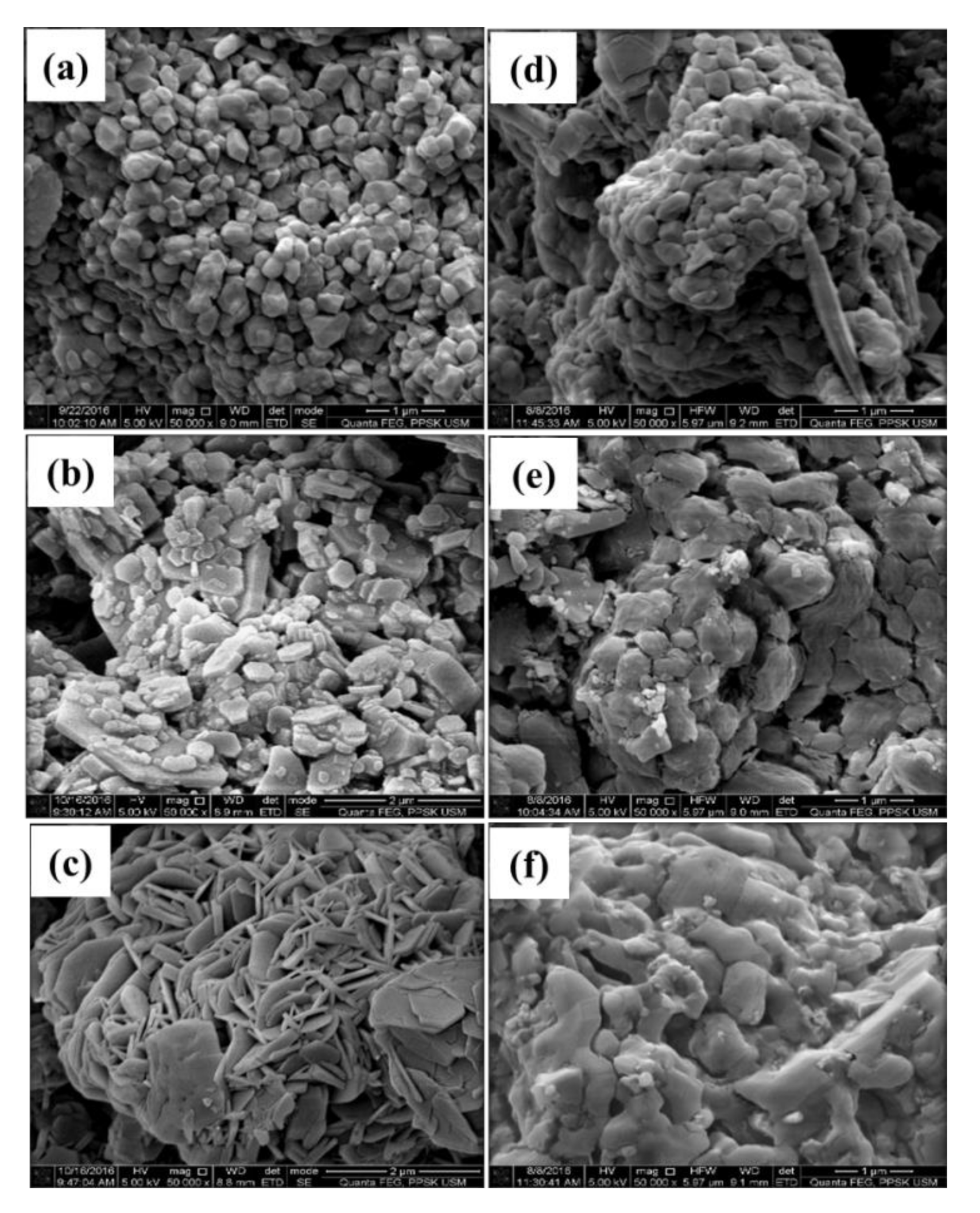
3.1. FESEM/EDX and BET Analyses of CaCO3 Particles Using Different Parameters
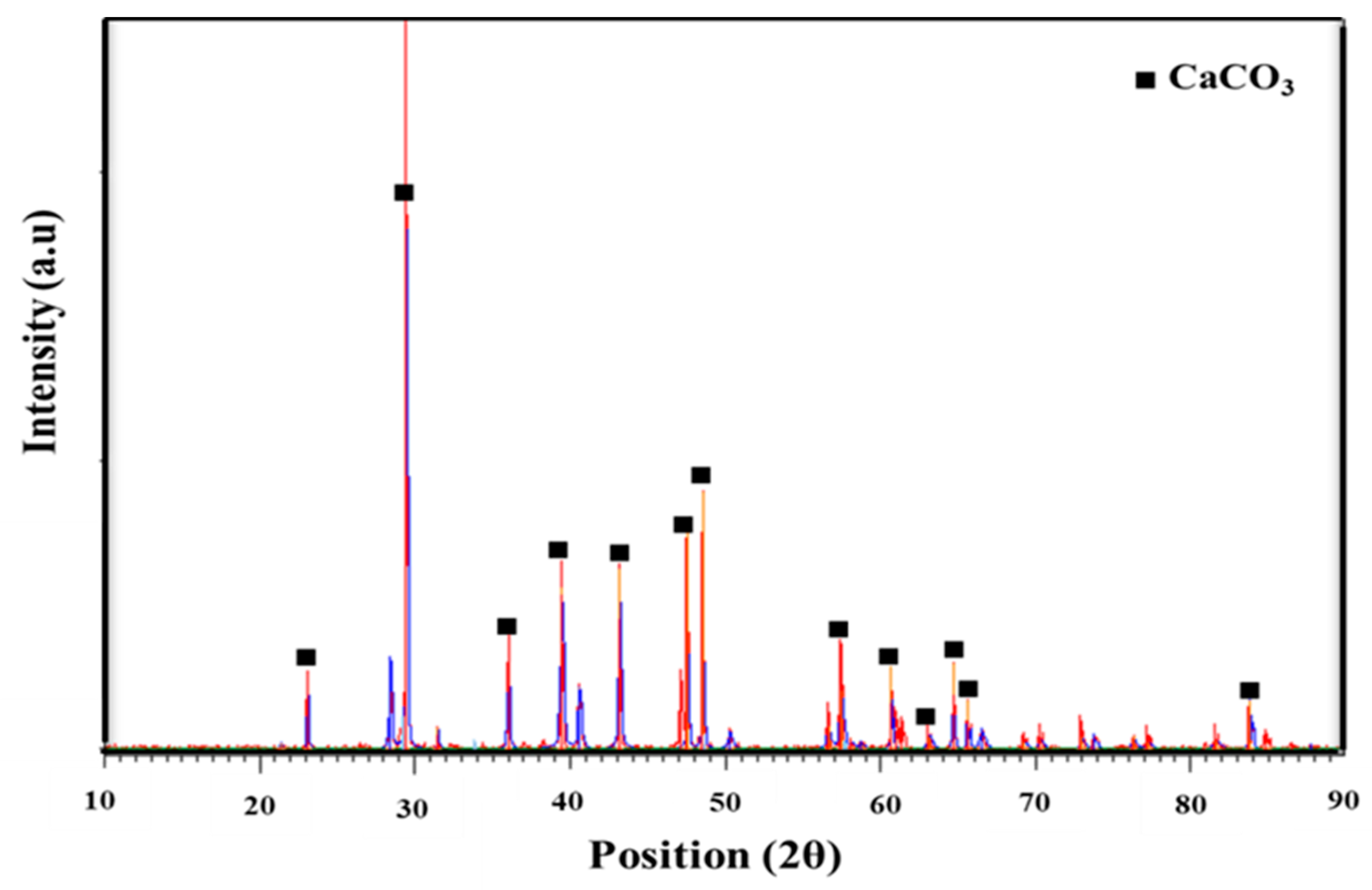
3.2. XRD Analysis of Optimized CaCO3 Nanoparticles
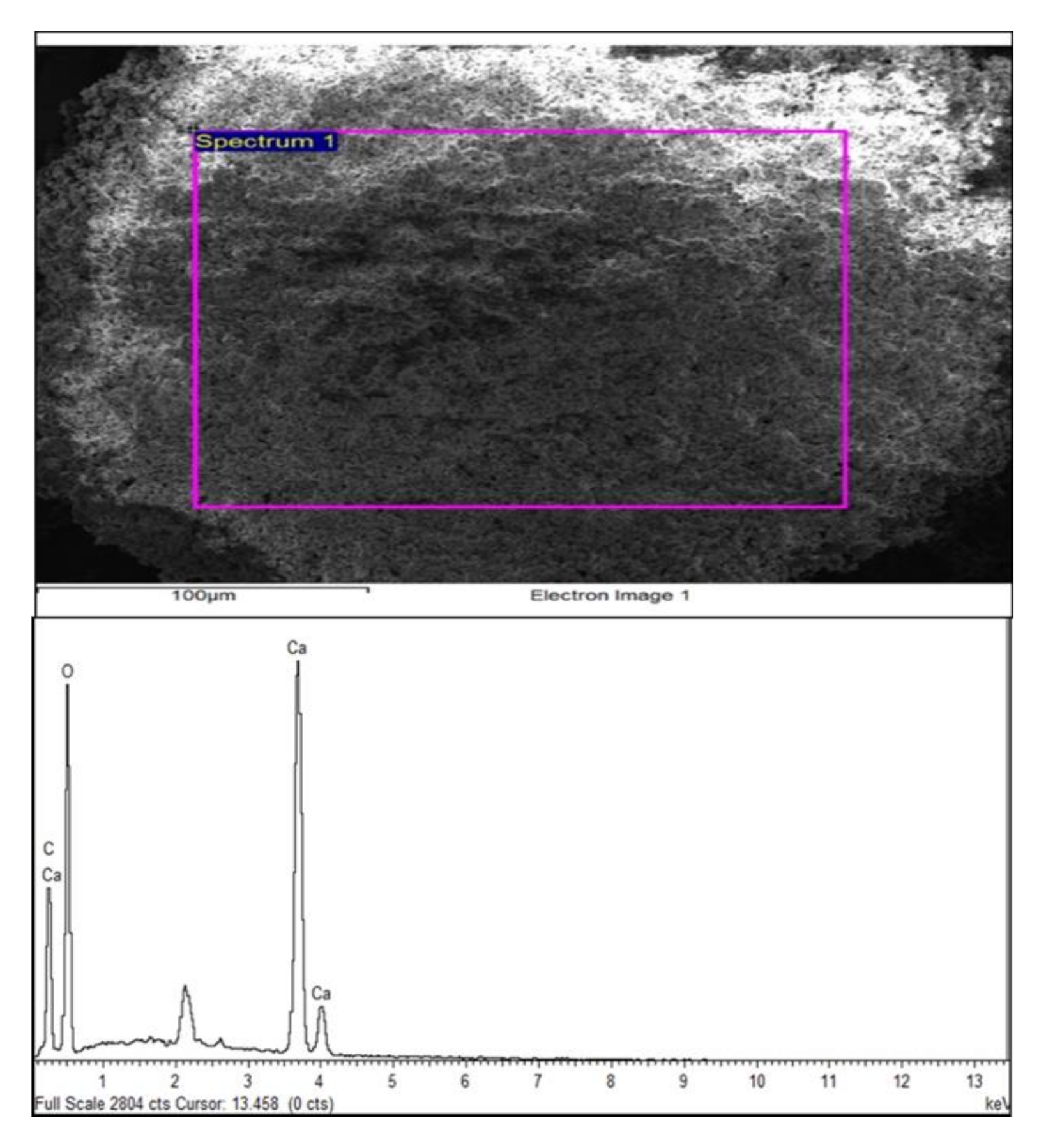
3.3. EDX Analysis of Optimized CaCO3 Nanoparticles
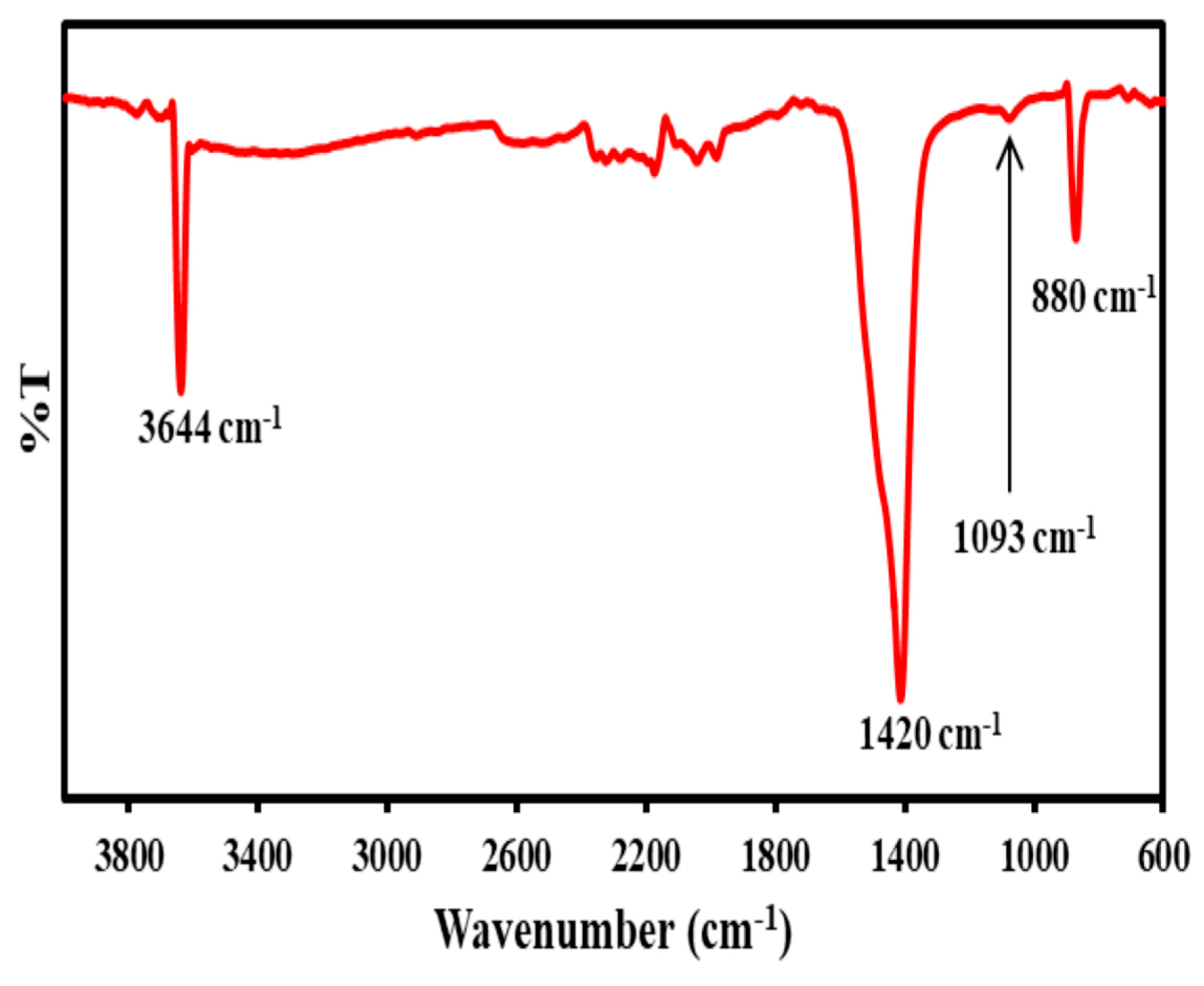
3.4. FTIR Analysis of Optimized CaCO3 Nanoparticles
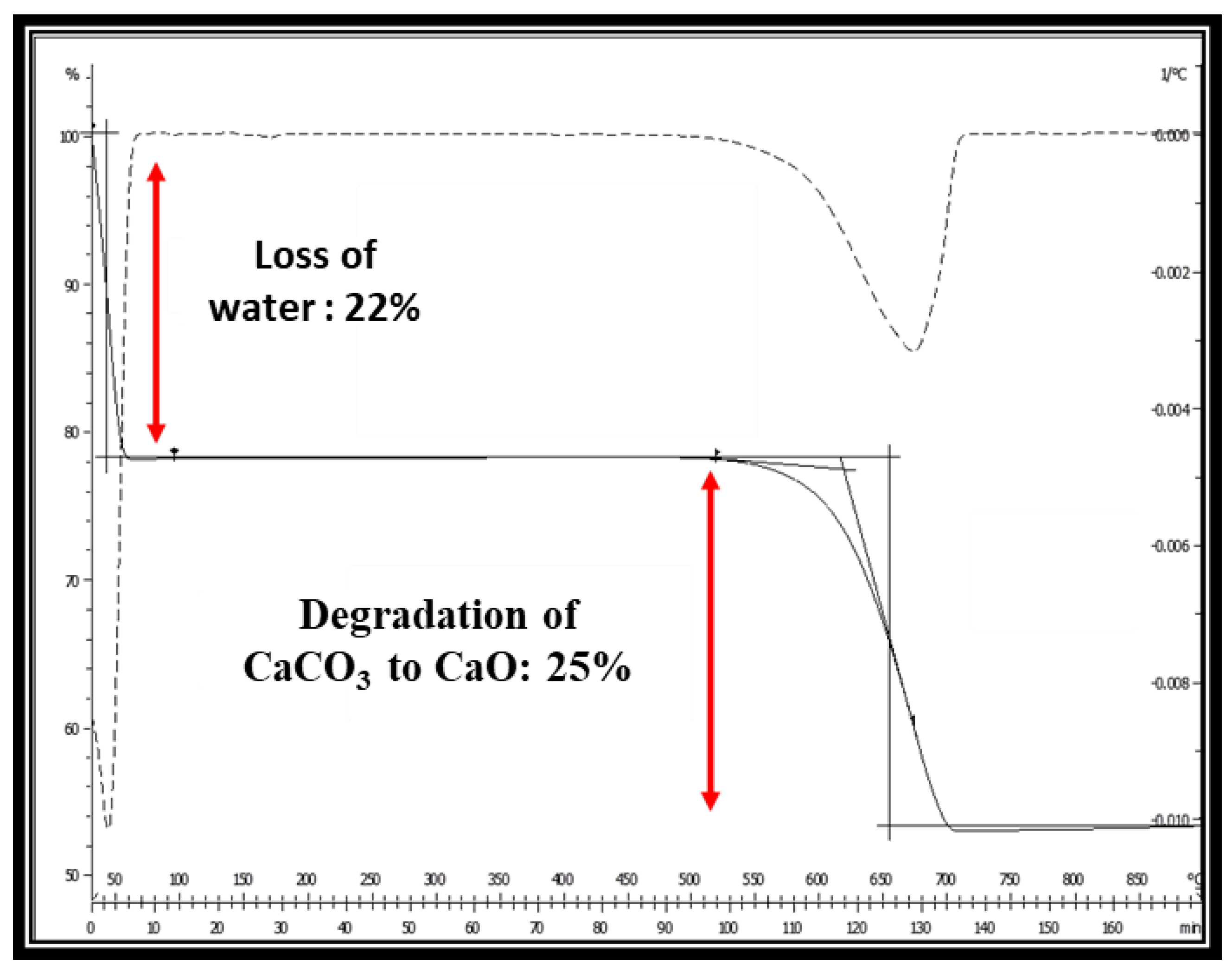
3.5. TGA of Optimized CaCO3 Nanoparticles
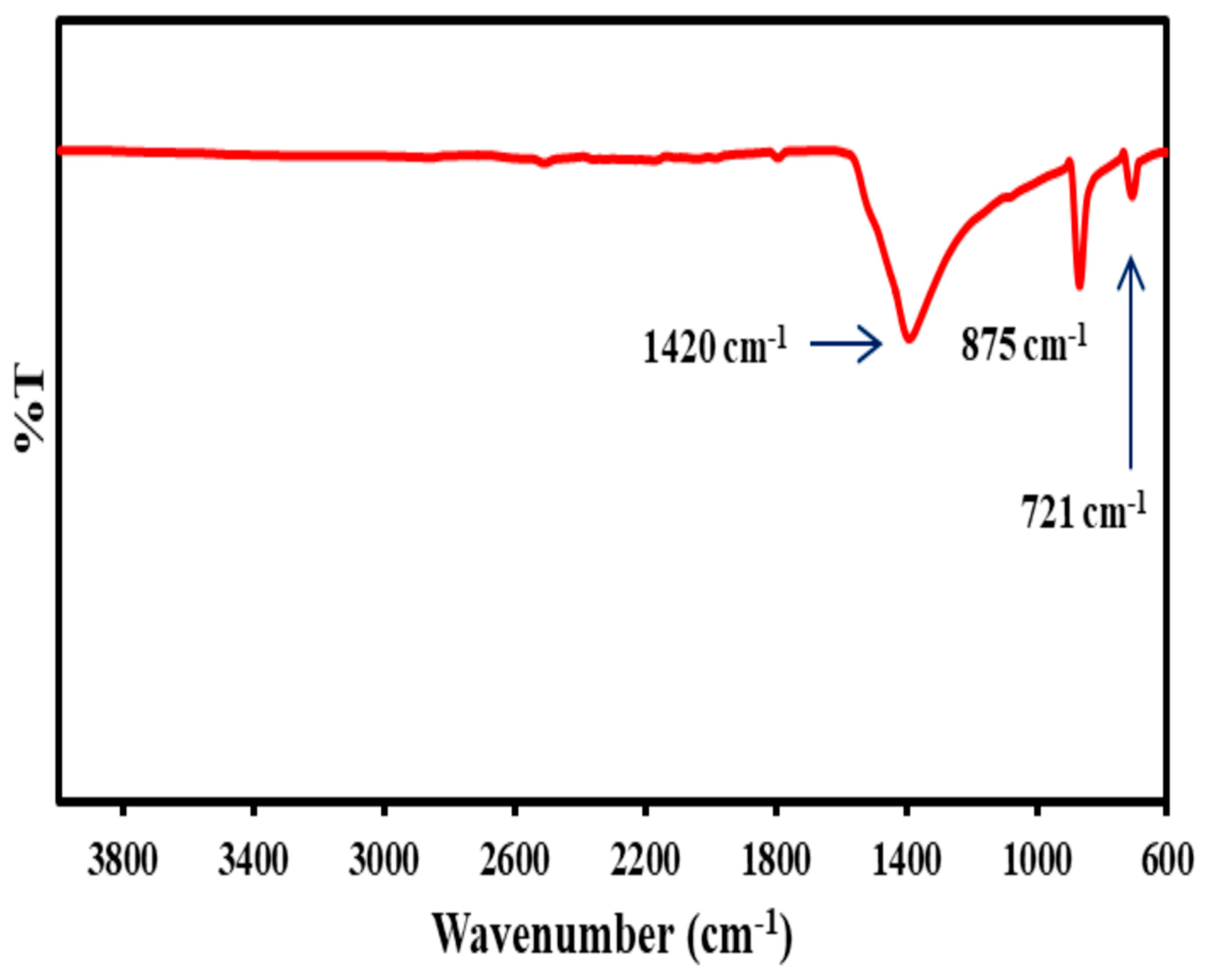
3.6. FTIR Analysis of CaO Nanoparticles
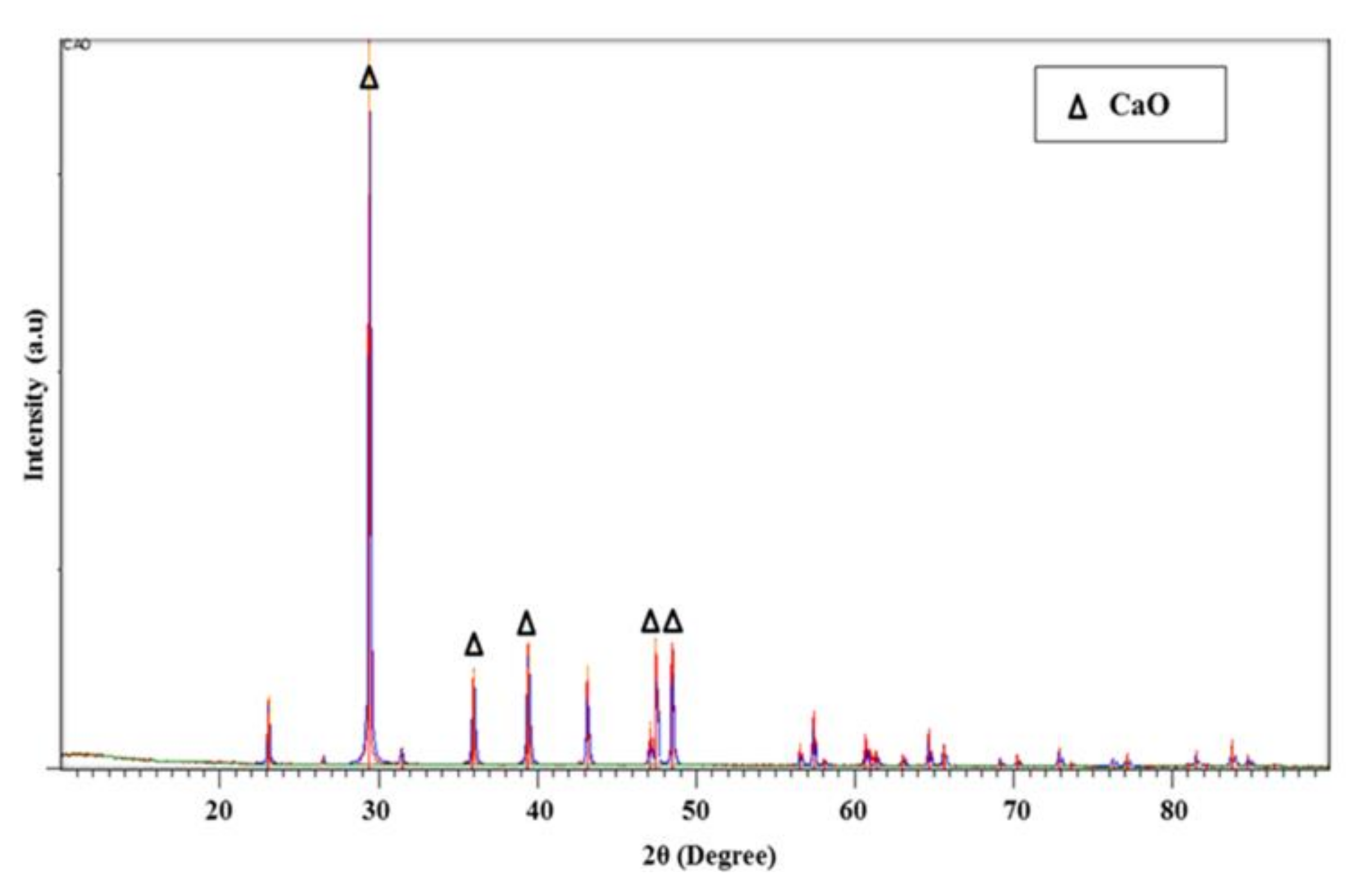
3.7. XRD Analysis of CaO Nanoparticles
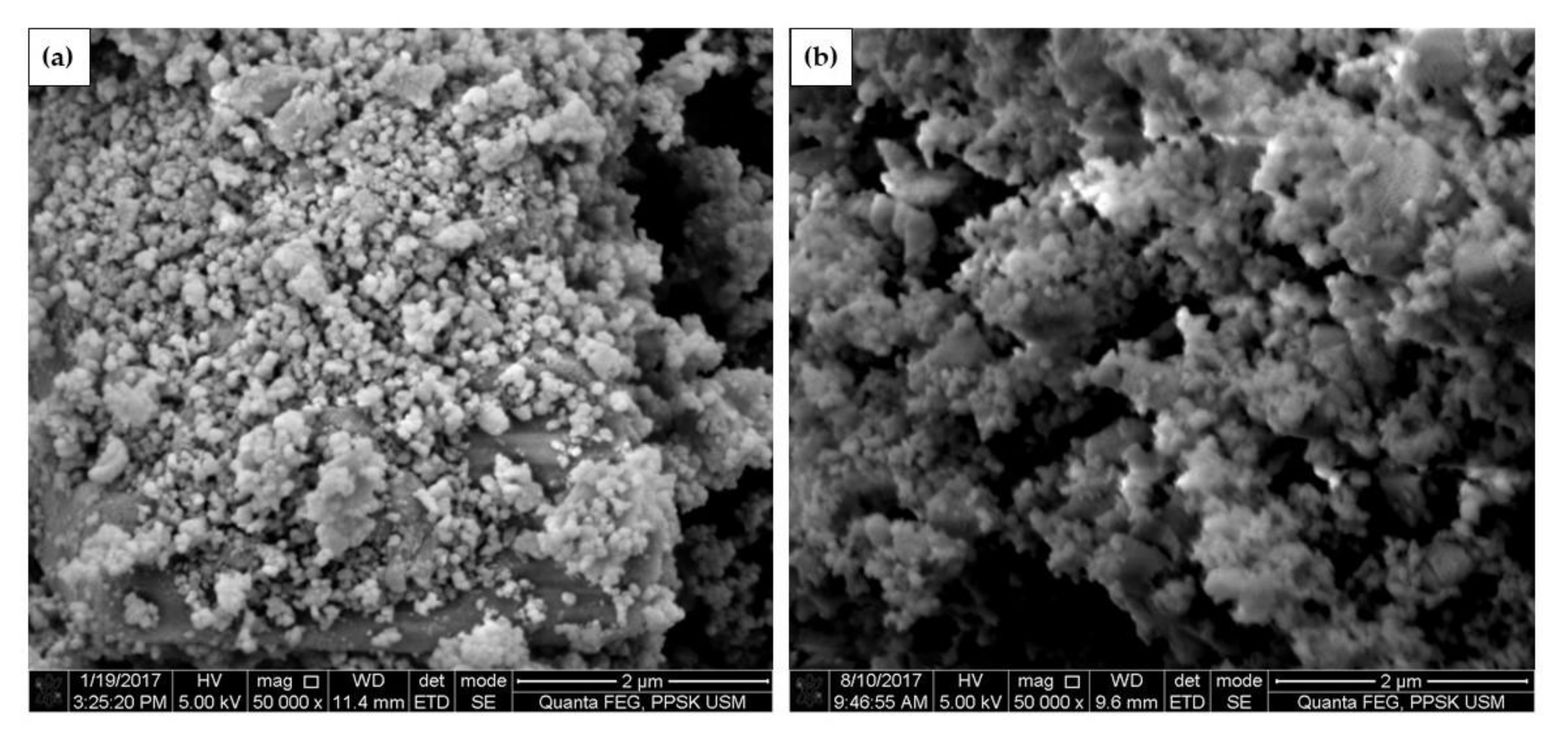
3.8. FESEM Analysis of CaO Nanoparticles
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Jaji, A.Z.; Zuki, A.; Mahmud, R.; Loqman, M.Y.; Hezmee, M.N.M.; Isa, T.; Wenliang, F.; Hammadi, N.I. Synthesis, characterization, and cytocompatibility of potential cockle shell aragonite nanocrystals for osteoporosis therapy and hormonal delivery. Nanotechnol. Sci. Appl. 2017, 10, 23–33. [Google Scholar] [CrossRef]
- Maleki, S.; Barzegar-Jalali, M.; Zarrintan, M.H.; Adibkia, K.; Lotfipour, F. Calcium carbonate nanoparticles; potential applications in bone and tooth disorders. Pharm. Sci. 2015, 20, 175–182. [Google Scholar]
- Kharissova, O.V.; Dias, H.R.; Kharisov, B.I.; Pérez, B.O.; Pérez, V.M.J. The greener synthesis of nanoparticles. Trends Biotechnol. 2013, 31, 240–248. [Google Scholar] [CrossRef]
- Awang-Hazmi, A.; Zuki, A.; Noordin, M.; Jalila, A.; Norimah, Y. Mineral Composition of the Cockle (Anadara granosa) Shells of West Coast of Peninsular Malaysia and It? s Potential as Biomaterial for Use in Bone Repair. J. Anim. Vet. Adv. 2007, 6, 591–594. [Google Scholar]
- Ghafar, S.L.M.A.; Hussein, M.Z.; Rukayadi, Y.; Zakaria, Z.A.B. Surface-functionalized cockle shell–based calcium carbonate aragonite polymorph as a drug nanocarrier. Nanotechnol. Sci. Appl. 2017, 10, 79–94. [Google Scholar] [CrossRef]
- Kamba, A.S.; Ismail, M.; Ibrahim, T.A.T.; Zuki, A. Synthesis and Characterisation of Calcium Carbonate Aragonite Nanocrystals from Cockle Shell Powder (Anadara granosa). J. Nanomater. 2013, 2013, 1–9. [Google Scholar] [CrossRef]
- Mahmood, S.; Zuki, A.; Razak, I.S.A.; Yusof, L.M.; Jaji, A.Z.; Tijani, I.; Hammadi, N.I. Preparation and characterization of cockle shell aragonite nanocomposite porous 3D scaffolds for bone repair. Biochem. Biophys. Rep. 2017, 10, 237–251. [Google Scholar] [CrossRef]
- Mailafiya, M.M.; Abubakar, K.; Danmaigoro, A.; Chiroma, S.M.; Rahim, E.B.A.; Moklas, M.A.M.; Zuki, A. Cockle Shell-Derived Calcium Carbonate (Aragonite) Nanoparticles: A Dynamite to Nanomedicine. Appl. Sci. 2019, 9, 2897. [Google Scholar] [CrossRef]
- Hussein, A.I.; Mat, A.N.C.; Wahab, N.A.A.A.; Ab Rahman, I.; Husein, A.; Ab-Ghani, Z. Synthesis and Properties of Novel Calcia-Stabilized Zirconia (Ca-SZ) with Nano Calcium Oxide Derived from Cockle Shells and Commercial Source for Dental Application. Appl. Sci. 2020, 10, 5751. [Google Scholar] [CrossRef]
- Vinas, M.T.; Zhang, F.; Vleugels, J.; Anglada, M. Effect of calcia co-doping on ceria-stabilized zirconia. J. Eur. Ceram. Soc. 2018, 38, 2621–2631. [Google Scholar] [CrossRef]
- Papanagiotou, H.P.; Morgano, S.M.; Giordano, R.A.; Pober, R. In vitro evaluation of low-temperature aging effects and finishing procedures on the flexural strength and structural stability of Y-TZP dental ceramics. J. Prosthet. Dent. 2006, 96, 154–164. [Google Scholar] [CrossRef]
- Beyth, N.; Houri-Haddad, Y.; Domb, A.J.; Khan, W.; Hazan, R. Alternative Antimicrobial Approach: Nano-Antimicrobial Materials. Evid. Based Complement. Altern. Med. 2015, 2015, 1–16. [Google Scholar] [CrossRef]
- Grochowska, K.; Siuzdak, K.; Karczewski, J.; Śliwiński, G. Functionalization of indium-tin-oxide electrodes by laser-nanostructured gold thin films for biosensing applications. Appl. Surf. Sci. 2015, 357, 1684–1691. [Google Scholar] [CrossRef]
- Espinosa, K.R.; Castillo, L.A.; Barbosa, S.E. Blown nanocomposite films from polypropylene and talc. Influence of talc nanoparticles on biaxial properties. Mater. Des. 2016, 111, 25–35. [Google Scholar] [CrossRef]
- Abt, T.; Sánchez-Soto, M. A Review of the Recent Advances in Cyclic Butylene Terephthalate Technology and its Composites. Crit. Rev. Solid State Mater. Sci. 2016, 42, 173–217. [Google Scholar] [CrossRef]
- Lu, J.; Lu, Z.; Li, X.; Xu, H.; Li, X. Recycling of shell wastes into nanosized calcium carbonate powders with different phase compositions. J. Clean. Prod. 2015, 92, 223–229. [Google Scholar] [CrossRef]
- Kurokawa, H. Temporal change of polished surface of composite resin. Jpn. J. Conserv. Dent. 2007, 50, 17. [Google Scholar]
- Afrashtehfar, K.I.; Del Fabbro, M. Clinical performance of zirconia implants: A meta-review. J. Prosthet. Dent. 2020, 123, 419–426. [Google Scholar] [CrossRef]
- Raigrodski, A.J. Contemporary materials and technologies for all-ceramic fixed partial dentures: A review of the literature. J. Prosthet. Dent. 2004, 92, 557–562. [Google Scholar] [CrossRef]
- Dion, I.; Rouais, F.; Baquey, C.; Lahaye, M.; Salmon, R.; Trut, L.; Cazorla, J.P.; Huong, P.V.; Monties, J.R.; Havlik, P. Physico-chemistry and cytotoxicity of ceramics: Part I—Characterization of ceramic powders. J. Mater. Sci. Mater. Electron. 1997, 8, 325–332. [Google Scholar] [CrossRef]
- Nakonieczny, D.; Paszenda, Z.; Drewniak, S.; Radko, T.; Lis, M. sZrO2-CeO2 ceramic powders obtained from a sol-gel process using acetylacetone as a chelating agent for potential application in prosthetic dentistry. Acta Bioeng. Biomech. 2016, 18, 53–60. [Google Scholar]
- Nakonieczny, D.; Walke, W.; Majewska, J.; Paszenda, Z. Characterization of magnesia-doped yttria-stabilized zirconia powders for dental technology applications. Acta Bioeng. Biomech. 2014, 16, 16. [Google Scholar]
- Piconi, C.; Maccauro, G. Zirconia as a ceramic biomaterial. Biomaterials 1999, 20, 1–25. [Google Scholar] [CrossRef]
- Tanaka, K.; Tamura, J.; Kawanabe, K.; Nawa, M.; Oka, M.; Uchida, M.; Kokubo, T.; Nakamura, T. Ce-TZP/Al2O3 nanocomposite as a bearing material in total joint replacement. J. Biomed. Mater. Res. 2002, 63, 262–270. [Google Scholar] [CrossRef]
- Christel, P.; Meunier, A.; Heller, M.; Torre, J.P.; Peille, C.N. Mechanical properties and short-termin vivo evaluation of yttrium-oxide-partially-stabilized zirconia. J. Biomed. Mater. Res. 1989, 23, 45–61. [Google Scholar] [CrossRef]
- Hannink, R.H.J.; Kelly, P.M.; Muddle, B.C. Transformation Toughening in Zirconia-Containing Ceramics. J. Am. Ceram. Soc. 2004, 83, 461–487. [Google Scholar] [CrossRef]
- Nath, S.; Sinha, N.; Basu, B. Microstructure, mechanical and tribological properties of microwave sintered calcia-doped zirconia for biomedical applications. Ceram. Int. 2008, 34, 1509–1520. [Google Scholar] [CrossRef]
- Gempita, G.; Hasratiningsih, Z.; Subrata, G.; Purwasasmita, B.S. Synthesis of mesh-shaped calcia partially stabilized zirconia using eggshell membrane template as filler composite. Padjadjaran J. Dent. 2017, 29, 29. [Google Scholar] [CrossRef]
- Papahadjopoulos-Sternberg, B.; Ackrell, J. Seeing Multifunctional Nano- and Micro-particles Suitable for Imaging & Therapy Using Freeze-fracture Electron Microscopy. Microsc. Microanal. 2008, 14, 1604–1605. [Google Scholar] [CrossRef]
- 3Kumar, A.; Yadav, N.; Bhatt, M.; Mishra, N.K.; Chaudhary, P.; Singh, R. Sol-gel derived nanomaterials and it’s applications: A review. Res. J. Chem. Sci. 2015, 5, 98–105. [Google Scholar]
- De Choudens-Sanchez, V.; Gonzalez, L.A. Calcite and Aragonite Precipitation Under Controlled Instantaneous Supersaturation: Elucidating the Role of CaCO3 Saturation State and Mg/Ca Ratio on Calcium Carbonate Polymorphism. J. Sediment. Res. 2009, 79, 363–376. [Google Scholar] [CrossRef]
- Górna, K.; Hund, M.; Vučak, M.; Gröhn, F.; Wegner, G. Amorphous calcium carbonate in form of spherical nanosized particles and its application as fillers for polymers. Mater. Sci. Eng. A 2008, 477, 217–225. [Google Scholar] [CrossRef]
- Buasri, A.; Chaiyut, N.; Loryuenyong, V.; Worawanitchaphong, P.; Trongyong, S. Calcium Oxide Derived from Waste Shells of Mussel, Cockle, and Scallop as the Heterogeneous Catalyst for Biodiesel Production. Sci. World J. 2013, 2013, 1–7. [Google Scholar] [CrossRef]
- Ghazali, S.S.; Kem, K.L.; Jusoh, R.; Abdullah, S.; Shariffuddin, J.H. Evaluation of La-Doped CaO Derived from Cockle Shells for Photodegradation of POME. Bull. Chem. React. Eng. Catal. 2019, 14, 205–218. [Google Scholar] [CrossRef]
- Laonapakul, T.; Sutthi, R.; Chaikool, P.; Mutoh, Y.; Chindaprasirtd, P. Optimum conditions for preparation of bio-calcium from blood cockle and golden apple snail shells and characterization. Sci. 2019, 45, 10–20. [Google Scholar] [CrossRef]
- Wang, C.; Zhao, J.; Zhao, X.; Bala, H.; Wang, Z. Synthesis of nanosized calcium carbonate (aragonite) via a polyacrylamide inducing process. Powder Technol. 2006, 163, 134–138. [Google Scholar] [CrossRef]
- Zdravkov, B.D.; Čermák, J.J.; Šefara, M.; Janků, J. Pore classification in the characterization of porous materials: A perspective. Open Chem. 2007, 5, 385–395. [Google Scholar] [CrossRef]
- Khodaee, P.; Najmoddin, N.; Shahrad, S. The effect of ethanol and temperature on the structural properties of mesoporous silica synthesized by the sol-gel method. In Proceedings of the 2018 25th National and 3rd International Iranian Conference on Biomedical Engineering (ICBME), Qom, Iran, 29–30 November 2018; pp. 1–5. [Google Scholar]
- Bahar, M.; Mozaffari, M.; Esmaeili, S. Effect of different alcohols, gelatinizing times, calcination and microwave on characteristics of TiO2 nanoparticles synthesized by sol–gel method. J. Theor. Appl. Phys. 2017, 11, 79–86. [Google Scholar] [CrossRef]
- Zhu, Y.; Zhang, L.; Gao, C.; Cao, L. The synthesis of nanosized TiO2 powder using a sol-gel method with TiCl4 as a precursor. J. Mater. Sci. 2000, 35, 4049–4054. [Google Scholar] [CrossRef]
- Stein, S.W.; Myrdal, P.B. The Relative Influence of Atomization and Evaporation on Metered Dose Inhaler Drug Delivery Efficiency. Aerosol Sci. Technol. 2006, 40, 335–347. [Google Scholar] [CrossRef]
- Smyth, H.D. Multimodal particle size distributions emitted from HFA-134a solution pressurized metered-dose inhalers. AAPS PharmSciTech 2003, 4, 76–86. [Google Scholar] [CrossRef]
- Chen, P.; Ye, N.; He, C.; Tang, L.; Li, S.; Sun, L.; Lia, Y. Preparation of Polyacrylate Hollow Microspheres via Facile Spray Drying. Appl. Sci. 2019, 9, 228. [Google Scholar] [CrossRef]
- Kumar, V.; Singh, K.; Kumar, A.; Kumara, M.; Singh, K.; Vij, A.; Thakur, A. Effect of solvent on crystallographic, morphological and optical properties of SnO 2 nanoparticles. Mater. Res. Bull. 2017, 85, 202–208. [Google Scholar] [CrossRef]
- Krokida, M.K.; Maroulis, Z.B. Effect of drying method on shrinkage and porosity. Dry. Technol. 1997, 15, 2441–2458. [Google Scholar] [CrossRef]
- Kontoyannis, C.G.; Vagenas, N.V. Calcium carbonate phase analysis using XRD and FT-Raman spectroscopy. Analyst 2000, 125, 251–255. [Google Scholar] [CrossRef]
- Wang, Y.; Moo, Y.X.; Chen, C.; Gunawan, P.; Xu, R. Fast precipitation of uniform CaCO3 nanospheres and their transformation to hollow hydroxyapatite nanospheres. J. Colloid Interface Sci. 2010, 352, 393–400. [Google Scholar] [CrossRef]
- Alba-Rubio, A.C.; Santamaría-González, J.; Mérida-Robles, J.M.; Moreno-Tost, R.; Martín-Alonso, D.; Jiménez-López, A.; Maireles-Torres, P. Heterogeneous transesterification processes by using CaO supported on zinc oxide as basic catalysts. Catal. Today 2010, 149, 281–287. [Google Scholar] [CrossRef]
- Karunadasa, K.S.; Manoratne, C.; Pitawala, H.; Rajapakse, R. Thermal decomposition of calcium carbonate (calcite polymorph) as examined by in-situ high-temperature X-ray powder diffraction. J. Phys. Chem. Solids 2019, 134, 21–28. [Google Scholar] [CrossRef]
- Popescu, M.-A.; Isopescu, R.; Matei, C.; Fagarasan, G.; Plesu, V. Thermal decomposition of calcium carbonate polymorphs precipitated in the presence of ammonia and alkylamines. Adv. Powder Technol. 2014, 25, 500–507. [Google Scholar] [CrossRef]
- Putra, R.S.; Liyanita, A.; Arifah, N.; Puspitasari, E.; Sawaludin; Hizam, M.N. Enhanced Electro-Catalytic Process on the Synthesis of FAME Using CaO from Eggshell. Energy Procedia 2017, 105, 289–296. [Google Scholar] [CrossRef]
- Guan, W.; Ji, F.; Cheng, Y.; Fang, Z.; Fang, D.; Yan, P.; Chen, Q. A Novel Synthesis Method of Porous Calcium Silicate Hydrate Based on the Calcium Oxide/Polyethylene Glycol Composites. J. Nanomater. 2013, 2013, 1–7. [Google Scholar] [CrossRef]
- Bai, H.-X.; Shen, X.-Z.; Liu, X.-H.; Liu, S.-Y. Synthesis of porous CaO microsphere and its application in catalyzing transesterification reaction for biodiesel. Trans. Nonferrous Met. Soc. China 2009, 19, s674–s677. [Google Scholar] [CrossRef]


| Volume of DDW (mL) | Surface Area (m2/g) | Average Pore Diameter (Å) |
|---|---|---|
| 0 | 8.53 | 386 |
| 10 | 14.9 | 235 |
| 20 | 16.8 | 281 |
| 50 | 26.0 | 180 |
| 60 | 25.0 | 183 |
| 70 | 25.8 | 192 |
| Solvent | Surface Area (m2/g) | Average Pore Diameter (Å) |
|---|---|---|
| Propanol | 0.96 | 523 |
| Ethanol | 7.1 | 409 |
| Methanol | 6.3 | 703 |
| Feeding Rate of K2CO3 (h) | Surface Area (m2/g) | Average Pore Diameter (Å) |
|---|---|---|
| 1.0 | 26 | 580 |
| 1.5 | 8.4 | 606 |
| 2.0 | 5.9 | 758 |
| 2.5 | 4.1 | 325 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hussein, A.I.; Ab-Ghani, Z.; Che Mat, A.N.; Ab Ghani, N.A.; Husein, A.; Ab. Rahman, I. Synthesis and Characterization of Spherical Calcium Carbonate Nanoparticles Derived from Cockle Shells. Appl. Sci. 2020, 10, 7170. https://doi.org/10.3390/app10207170
Hussein AI, Ab-Ghani Z, Che Mat AN, Ab Ghani NA, Husein A, Ab. Rahman I. Synthesis and Characterization of Spherical Calcium Carbonate Nanoparticles Derived from Cockle Shells. Applied Sciences. 2020; 10(20):7170. https://doi.org/10.3390/app10207170
Chicago/Turabian StyleHussein, Abbas Ibrahim, Zuryati Ab-Ghani, Ahmad Nazeer Che Mat, Nur Atikah Ab Ghani, Adam Husein, and Ismail Ab. Rahman. 2020. "Synthesis and Characterization of Spherical Calcium Carbonate Nanoparticles Derived from Cockle Shells" Applied Sciences 10, no. 20: 7170. https://doi.org/10.3390/app10207170
APA StyleHussein, A. I., Ab-Ghani, Z., Che Mat, A. N., Ab Ghani, N. A., Husein, A., & Ab. Rahman, I. (2020). Synthesis and Characterization of Spherical Calcium Carbonate Nanoparticles Derived from Cockle Shells. Applied Sciences, 10(20), 7170. https://doi.org/10.3390/app10207170





