Abstract
High-intensity ultrasonic waves have great potential for the green synthesis of various nanomaterials under mild conditions and offer an excellent alternative for hazardous chemical methods. Herein a facile approach for the eco-friendly synthesis of Au-ZrO2 nanocatalyst with a high catalytic activity using a facile ultrasonic method is presented. Gold (Au) in the nanosize regime was successfully deposited on the surface of solvothermally synthesized monodispersed ZrO2 nanoparticles (ZrO2 NPs) in a very short period of time (5 min) at room temperature. Spherical shape small size Au nanoparticles that are uniformly dispersed on the surface of ZrO2 nanoparticles were obtained. Notably, in the absence of ZrO2 nanoparticles, HAuCl4 could not be reduced, indicating that nano-sized ZrO2 not only acted as support but also helped to reduce the gold precursor at the surface. The as-prepared Au-ZrO2 nanocatalyst was characterized by various techniques. The Au-ZrO2 nanocatalyst served as a highly efficient reducing catalyst for the reduction of 4-nitrophenol. The reaction time decreased with increasing the amount of catalyst.
1. Introduction
Recently, heterogeneous catalysis based on gold nanoparticles (Au NPs) supported on various metal oxides has become popular due to their high catalytic activities [1]. Bulk gold is chemically inert and often regarded as an ineffective catalyst due to its poor affinity towards most molecules [2]. However, when gold in the nano-size regime is dispersed on suitable supports such as metal oxides, it has displayed excellent catalytic activities towards various important reactions including hydrogenation, oxidation, and reduction etc. [3]. Since the discovery of exceptionally high catalytic activity of small size Au NPs towards CO oxidation, the interest in oxide-supported Au nanocatalysts has increased significantly [4,5]. Particularly, the type of support plays an important role in increasing the catalytic activity of gold-based catalysts [6].
Therefore, the selection of proper support is decisive in enhancing the interaction between Au NPs and support, which eventually improves the catalytic properties of the resulting material [7]. Au nanoparticles deposited on metal oxide domains resulted in enhanced applications [8,9,10,11]. Metal oxides based supports with large surface areas and porosities are known to enhance the catalytic activity and selectivity of gold-based catalysts [12,13]. In this regard, zirconia (ZrO2) is of particular interest due to its tunable porosity and surface area, high thermal stability, unique amphoteric character and redox properties [14,15]. Traditionally, the metal oxide supported Au based nanocatalysts were often prepared by impregnation, deposition-precipitation, physical blending or chemical precipitation followed by surface adsorption methods [16,17,18]. However, these methods usually yield insoluble materials with little control over the size, morphology and dispersion of the metal component. Furthermore, most of these methods also require high temperatures, rigorous reaction conditions, post-reaction treatment, long durations, and toxic reducing and stabilizing chemicals [19]. Therefore, due to environmental concerns, these methods have limited applications and the development of the non-toxic, facile, and green method is highly required to prepare supported gold-based nanocatalyst [20].
Recently, sonochemical methods are considered as effective techniques for the preparation of different types of nanomaterials [21]. The dissociation of water molecules under ultrasonic conditions produces respective H and OH radicals from water vapors, that act as active species, which facilitates the oxidation and/or reduction of precursors [22]. Herein, we successfully prepared the Au-ZrO2 nanocatalyst under ultrasonic irradiation using ZrO2 support (cf. Scheme 1). The ZrO2 nanoparticles were prepared to employ a solvothermal approach [23]. The reaction was performed under facile conditions at room temperature within a very short time period (i.e., 5 min). The preparation of Au-ZrO2 was confirmed by various characterization techniques. The as-prepared Au-ZrO2 sample was tested as a reduction catalyst for the conversion of 4-nitrophenol to 4-aminophenol under stirring at room temperature, and the progress of catalytic conversion was monitored using UV-Vis analysis.
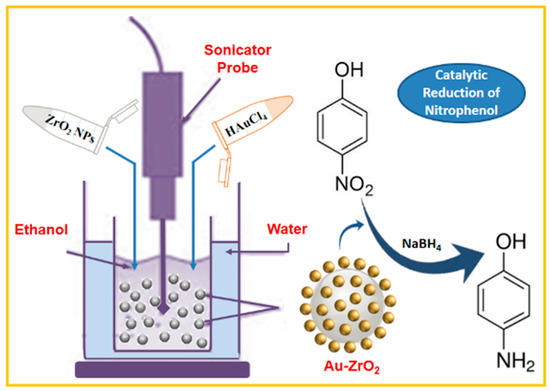
Scheme 1.
Schematic representation of the preparation of Au-ZrO2 nanocatalyst via the sonochemical method and its application towards the reduction of 4-nitrophenol.
2. Materials and Methods
2.1. Materials
All chemicals, such as benzyl alcohol (99.0%), tetrachloroauric (III) acid trihydrate (HAuCl4.3H2O, 99%), Zirconium (IV) isopropoxide (99.9%), and 4-nitrophenol were of reagent grade and were purchased from Sigma Aldrich.
2.2. Preparation of Au-ZrO2
For the synthesis of Au-ZrO2, initially, ZrO2 was prepared via solvothermal method according to our previously reported method [23]. Briefly, 387.67 mg (1 mmol) of zirconium isopropoxide was dissolved in 30 mL of benzyl alcohol in a beaker (25 mL) under constant stirring. The benzyl alcohol solution of zirconium isopropoxide was transferred into a Teflon cup which was placed in a 50 mL stainless steel autoclave and heated up to 210 °C. The vessel was cooled down after 72 hrs. A white powder was obtained, which was separated by centrifugation and washed repeatedly with tetrahydrofuran (THF) and dried in vacuo. The as-obtained ZrO2 powder was used to prepare Au-ZrO2 employing typical ultrasonic method as follows: initially, 196.91 mg (0.5 mmol) of tetrachloroauric (III) acid trihydrate (HAuCl4.3H2O, 99%) was dissolved in 30 mL of ethanol (bright yellow solution obtained) in a 50 mL beaker. To this solution, 246.56 mg (2 mmol) of ZrO2 NPs was added (1:4 ratio with respect to gold precursor), the mixture was briefly stirred. Subsequently, the solution was kept in a probe sonicator (Biologics Inc, Model 3000 MP, 115 volts, Cary, NC, USA) with a supply of 25 kHz ultrasonic waves with an input power of 460 W. After 5 min, the mixture turned to light purple, the sonication was stopped, and the product was separated through centrifuge (9000 rpm). The product formed was confirmed with UV-Vis analysis. Notably, in the absence of ZrO2 NPs, the gold precursor did not reduce to form Au NPs even under the same conditions. Therefore, for the comparison of UV results, pristine Au NPs are prepared under similar conditions using citric acid as a reducing agent.
2.3. Reduction of 4-Nitrophenol Using Au-ZrO2 Catalyst
The reduction was performed according to the following method: Initially, stock solutions of 4-nitrophenol (2 mM) and NaBH4 (0.03 M) were prepared. Subsequently, 1.4 mL of DI water, 0.3 mL of 4-nitrophenol, and 1 mL of NaBH4 solutions were mixed in a 3 mL standard quartz cuvette. Thereafter, 0.25 mL of Au-ZrO2 catalyst (from 0.25 mg/mL dispersion) was added to the mixture, which was slowly stirred at room temperature. The color of the solution changed after several minutes. The formation of the product was confirmed by UV analysis. To investigate the effect of the concentration of the catalyst on the rate of reduction of 4-nitrophenol, four different samples were prepared by varying the amount of catalyst (0.25, 0.5, 0.75, and 1.0 mL). In order to perform the reusability tests for the catalysts, reactions were performed under similar conditions using a large number of substrates. For example, 50 mL of DI water, 15 mL of 2 mM stock solution of 4-nitrophenol, 15 mL of 0.03 M freshly prepared NaBH4 solution, and 25 mg of Au-ZrO2 catalyst are mixed in a 100 mL round bottom flask.
2.4. Characterization
For UV-Analysis including the reduction of 4-nitrophenol studies, PerkinElmer lambda 35 spectrophotometer (Waltham, MA, USA) was used. FT-IR spectra were measured using the Perkin Elmer spectrometer (1000), Waltham, MA, USA. XRD diffractograms are obtained from the D2 Phaser X-ray diffractometer equipped with Cu Ka radiation (k = 1.5418 A°) (Bruker, Germany). SEM, TEM and EDX were measured on Jeol, JED-2200 series (Japan) and Jeol TEM model JEM-1011 (Japan) at 100 keV, respectively.
3. Results and Discussion
The ZrO2 NPs required in this study were prepared by a solvothermal method as reported [23]. In this case, solvent benzyl alcohol was also acted as a stabilizing agent by physically adsorbing on the surfaces of the NPs. XRD diffractogram confirmed the preparation of ZrO2 NPs. For the preparation of Au-ZrO2, freshly prepared ZrO2 NPs together with gold precursor was dispersed in ethanol under slow stirring. Then, the resulting mixture was subsequently treated in a probe sonicator for a short time period (i.e., 5 min), which led to the change in color of dispersion milky white to light purple, indicating the formation of Au NPs on the surface of ZrO2 NPs.
Initially, UV analysis confirmed the preparation of Au-ZrO2, as shown in Figure 1a. To compare the influence of Au in the absorption properties of Au-ZrO2 nanoparticles, we also measured the UV-Vis spectroscopy of pristine ZrO2 and Au nanoparticles respectively. Interestingly, it was observed that ZrO2 NPs also acted as a catalyst in the formation of Au NPs, since under similar conditions in the absence of ZrO2, Au precursor did not reduce to produce Au NPs. While, in the presence of ZrO2 nanoparticles, Au was deposited within 5 min, which was reflected by the color change of the solution (Figure 1b) and confirmed by UV-analysis. To compare the surface plasmon band, Au NPs are synthesized using the same concentration of Au precursor (HAuCl4) but using citric acid as a reducing agent. The UV spectrum of Au-ZrO2 exhibited a broad absorption peak centered at ~560 nm, which indicated the formation of smaller size Au NPs [24]. The citrate reduced Au nanoparticles showed surface plasmon band centered at ~523 nm. As expected, there was a redshift of about 27 nm in the plasmon bands of Au-ZrO2 nanoparticles as compared to pristine Au nanoparticles. The red-shift in the plasmon band can be attributed to the close contact of the Au and ZrO2 interface that results in the effective local dielectric function around the gold [10], which was found to be in line with the classical Mie theory [25]. Besides, the shift in surface plasmon band Au, the absorption band of ZrO2 also red-shifted from 280 nm to 320 nm. This could be due to surface defects created by the binding of Au NPs on the surface of the ZrO2 NPs [26].
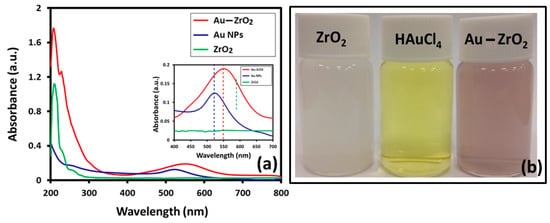
Figure 1.
(a) Comparison of the UV-Vis spectra of Au-ZrO2 with pristine ZrO2 nanoparticles (NPs) stabilized with benzyl alcohol (b) digital photos of the dispersion of ZrO2 nanoparticles, HAuCl4 and Au-ZrO2 in ethanol.
These results are consistent with the evidence obtained from FT-IR analysis. The FT-IR spectrum of Au-ZrO2 is almost similar to the IR spectrum of pristine ZrO2 NPs containing benzyl alcohol as stabilizing ligands. The IR spectrum of Au-ZrO2 contains various characteristic peaks of benzyl alcohol including the peaks between 3600 to 2900 cm−1 corresponding to hydroxyl groups, C-H stretching of the aromatic ring, and C−H stretching of CH2 (methylene) groups. The vibrations of phenyl rings appeared in the regions of 1900 to 1600 cm−1. Whereas, the characteristic peaks of O−H, and C−O stretching occurred in the range of 1420 to 1330 cm−1 and 1080–1022 cm−1 respectively. The existence of benzyl alcohol on ZrO2 can be confirmed by comparing the IR spectra of ZrO2 and Au-ZrO2 (cf. Figure 2) indicate the presence of benzyl alcohol attached ZrO2 and Au-ZrO2 NPs. However, the concentration of benzyl alcohol is much reduced in the case of Au-ZrO2 NPs.
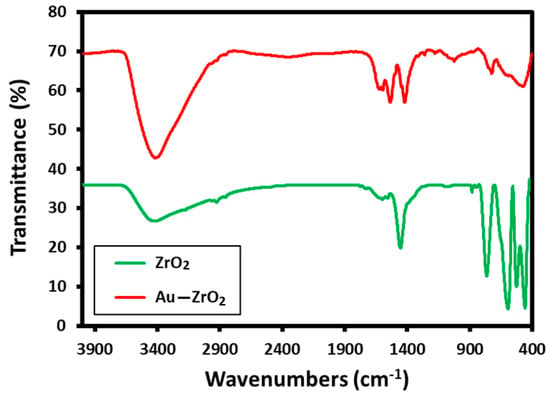
Figure 2.
Comparison of the FT-IR spectra of Au-ZrO2 with pristine ZrO2 NPs stabilized with benzyl alcohol.
X-ray diffraction measurements are performed to establish the nature of the crystallographic phase of ZrO2 support and to confirm the occurrence of Au NPs in the Au-ZrO2 composite. The comparison of the XRD spectra of pristine ZrO2 NPs and the Au-ZrO2 are reported in Figure 3. XRD indicates the cubic phase of ZrO2 in Au-ZrO2, as diffraction peaks perfectly matched with the reported data (JCPDS No. 27-0997). For instance, several prominent peaks observed at 2θ values of 30.46°, 34.54°, 50.45°, 60.37°, 74.56°, and 81.55° correspond to the (111), (200), (220), (311), (400), and (331) planes of crystalline zirconia, respectively. In addition, due to the high loading of gold nanoparticles (25% of ZrO2), sharp and prominent peaks belonging to the cubic fcc phase of Au are also clearly visible in the XRD pattern of Au-ZrO2. The peaks at 2θ values of 38.31° (111), 44.46° (200), 64.67° (220), 77.45° (311), and 82.30° (222) are matched with the standard gold metal (Au°) with JCPDS data card No. 04-0784 [27].
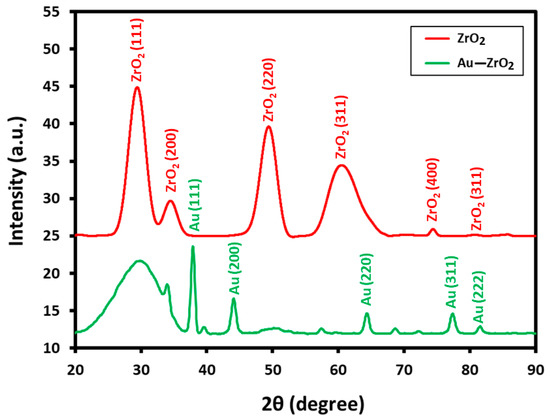
Figure 3.
XRD diffractograms of Au-ZrO2 and pristine ZrO2 NPs depicting the cubic phase of both Au and ZrO2 nanoparticles in Au-ZrO2.
Figure 4 shows the corresponding SEM and TEM images of Au-ZrO2 nanoparticles. Figure 4a shows a representative electron micrograph. Although it is difficult to conclude the exact size of gold domains, a dark contrast could be due to the presence of high-density metal (Au) present on the surface of ZrO2 nanoparticles. Figure 4b shows the SEM micrographs which demonstrated that the Au NPs are deposited on the ZrO2 NPs in the Au–ZrO2 hybrid and shows the distribution of elements present therein, while the EDX elemental mapping spectra confirm the presence of representative elements (Figure 4c).
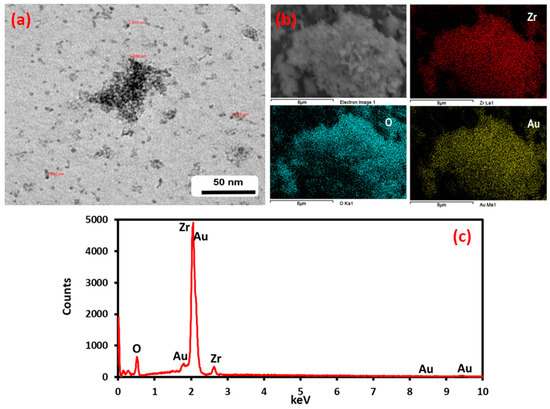
Figure 4.
TEM, SEM, and EDX analysis of Au-ZrO2. (a) The dark contrast in the TEM image indicates the presence of gold nanoparticles (b) the color mapping of the sample under SEM analysis clearly indicates the presence of Zr and gold in the sample. (c) EDX also points towards the presence of Au and Zr in The Au-ZrO2 Sample.
Catalytic Properties of Au-ZrO2
The catalytic efficiency of Au-ZrO2 was tested for the reduction of 4-nitrophenol, which is a highly toxic pollutant, typically derived from industrial wastes [28]. 4-nitrophenol is typically removed from the wastewater by various processes including adsorption, degradation, and electrochemical treatment, and so on [29]. On the other hand, the catalytic reduction of 4-nitrophenol and other nitro-aromatics to amino-phenols has gained significant interest in the scientific community [30]. The catalytic reduction of nitrophenols to amino-phenols is an important class of reaction due to the significance of amino-phenols as intermediates in various processes [31]. Particularly, 4-aminophenol has several applications such as a starting material and intermediate for various drugs, as a corrosion inhibitor, etc. [32,33]. Additionally, the 4-nitrophenol reduction is widely used as a model catalytic reaction, due to its well-controlled process which usually yields a single product at mild temperature and ideally requires a catalyst to proceed [34,35]. The reduction of 4-nitrophenol is usually carried out in the presence of NaBH4 as a reductant and various metal nanoparticles such as Pd, Pt, Ni, Ag, and Au as catalysts [36]. Among several metal NPs, Au NPs often exhibit superior catalytic properties for this type of reaction due to their unique properties. For instance, the catalytic properties of Au NPs are dependent on their particle size, where smaller size NPs show higher catalytic activities and also are active under mild conditions [37,38]. For example, gold aryl based core-shell nanoparticles demonstrated high catalytic activity under mild conditions towards the reduction of 4-nitrophenol in short reaction time (5 min) [39].
Usually, the catalytic property of smaller size, naked Au NPs is adversely affected by the aggregation of NPs [3]. To address this issue, different types of ligands are applied as stabilizers and/or the NPs are dispersed on various supports to prevent the aggregation and to enhance the surface area of the catalysts [40]. Recently, activated coke was used as a support for anchoring the Au NPs to prepare Au/AC catalysts, which exhibited high catalytic properties during 4-nitrophenol reduction [39]. Very recently, a trace amount of Pd was used to improve the catalytic properties of Au@ZrO2 nanocatalyst. The Pd1/Au20@ZrO2 composite consists of gold nuclei surrounded by zirconia shell which is decorated with Pd. The composite was prepared using a distinct ratio of Au:Pd. Although, the as-prepared catalyst demonstrated superior catalytic activity and strong stability towards the reduction of 4-nitrophenol, however, the catalyst was prepared by a complicated process which requires several steps [41]. However, in this study, smaller sizes Au NPs are uniformly distributed on the ZrO2 surface in a single step which enhanced the catalytic properties of the resulting composite.
The reaction can be easily tracked by the change in the characteristic absorption peak of 4-nitrophenol at ~400 nm. In the presence of NaBH4 and catalyst, the absorption band of 4-nitrophenol at ~400 nm, disappear and a new band emerges at a lower wavelength at ~300 nm, which indicates the successful transformation of 4-nitrophenol to 4-aminophenol [42]. Although, the transformation of nitro to amino phenol is thermodynamically favored in the presence of NaBH4, still, a catalyst is required to clear the kinetic barrier caused by the difference of potential between donor and acceptor [43]. The Au-ZrO2 nanocatalyst may facilitate the transfer of an electron from BH4− ions to the nitro group of 4-nitrophenol and reduce it to 4-aminophenol with improved reaction rate. Upon addition of Au-ZrO2, the intensity of the absorption band of 4-nitrophenol at ~400 nm gradually decreased with the emergence of a new band at ~300 nm indicating the formation of 4-aminophenol. The reaction was completed within a short period of time between 5 to 30 min depending upon the amount of catalyst used. With increasing the amount of Au-ZrO2 nanocatalyst from 0.25 mL to 1 mL the reaction time decreased from 30 min to 5 min, respectively, as shown in Figure 5. Notably, in the absence of catalyst, the peak at the 400 nm decreased slightly even after a long reaction time (several hours). This clearly depicts the inefficiency of NaBH4 towards the reduction of 4-nitrophenol and the importance of Au-ZrO2 during this process.
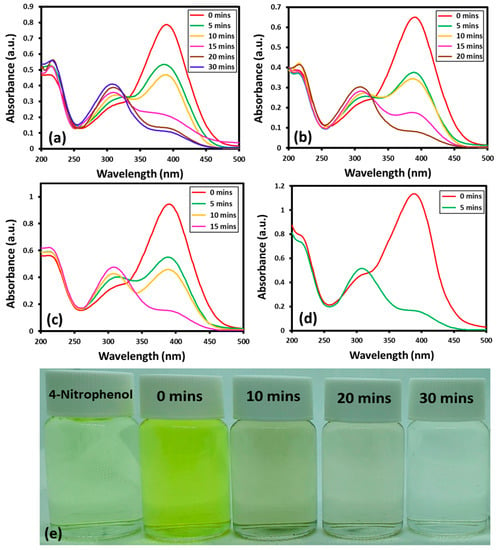
Figure 5.
UV spectra of 4-nitrophenol reduction by the addition of different volumes of Au-ZrO2 nanocatalyst: (a) 0.25 mL, (b) 0.5 mL, (c) 0.75 mL, (d) 1.00 mL, and (e) digital photos of the color transformation during the conversion of nitrophenol to aminophenol the amount of nanocatalyst was taken from freshly prepared dispersion of Au-ZrO2 with a concentration of 0.25 mg/mL.
Furthermore, the stability of the Au-ZrO2 nanocatalyst was tested by repeated use of the same catalyst for the reduction of 4-nitrophenol several times. The reusability of the catalyst has significant importance from both academic and commercial aspects. In this case, to evaluate the reusability of Au-ZrO2, the reduction of 4-nitrophenol was performed five times using the same catalyst. After every reaction, the catalyst is isolated by centrifuge and reused for another reaction. All the reusability tests are carried out using 0.25 mL concentration of catalyst; however, the substrates and catalysts are taken in large amounts to recover a sufficient amount of catalyst required for further reactions. The results of the recycling of Au-ZrO2 are shown in Figure 6. The results revealed that the catalytic activity of Au-ZrO2 was only slightly affected even after five cycles. During the reusability tests, the conversion of 4-nitrophenol was slightly decreased in each reaction which could be due to the weight loss of catalyst while recovery (during filtration). For instance, during the first reaction using fresh catalyst, 4-nitrophenol was completely converted to 4-aminophenol in 30 min, however in subsequent reactions conversion was slightly reduced after every reaction during the same reaction time, as reflected by the decrease in the intensity of the absorption peak of 4-aminophenol at ~300 nm (cf. Figure 6).
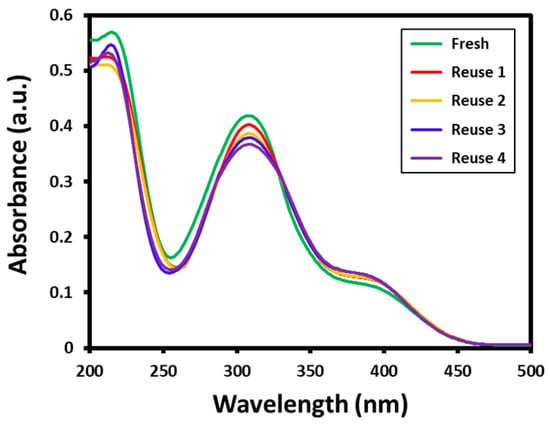
Figure 6.
Reusability of Au-ZrO2 nanocatalyst for the reduction of 4-nitrophenol. All the reactions are performed for 30 min using the same catalyst which was recovered by centrifugation.
4. Conclusions
Herein, we have successfully prepared Au-ZrO2 nanocatalyst using a facile ultrasonic method at room temperature. Using this method, the Au-ZrO2 nanocatalyst preparation was completed in a very short period of time (5 min) under mild conditions in the absence of any additives or surfactant. Au in the nano-size regime was found to be uniformly deposited on the surface of ZrO2 NPs. The formation of a nanocatalyst was confirmed using UV–Vis, XRD, FT-IR spectroscopy along with TEM, and SEM analysis. The as-prepared Au-ZrO2 nanocatalyst demonstrated excellent catalytic activity towards the reduction of 4-nitrophenol to 4-aminophenol in the presence of NaBH4. The reduction was completed in a very short period of time (between 5–30 min) under mild conditions, and with increasing the amount of catalyst, the conversion time decreased accordingly. Therefore, this approach i.e., ultrasonic method, can be potentially extended for the green synthesis of various other nanoparticles based composite materials. Additionally, the Au-ZrO2 nanocatalyst can be further tested for various other reactions as a potential reduction catalyst.
Author Contributions
M.K., S.F.A., and M.R.S. contributed to the design of the project. M.R.S., S.F.A., M.I.A., M.S., and M.K. contributed to the experiment and characterization. M.R.S., S.F.A., and M.K. contributed to the drafting of the manuscript. A.A., M.R.H.S., and M.N.T. provided scientific guidance. All authors have read and agreed to the published version of the manuscript.
Funding
Researchers Supporting Project Number (RSP-2019/60), King Saud University, Riyadh, Saudi Arabia.
Acknowledgments
The authors would like to extend their sincere appreciation to the Researchers Supporting Project Number (RSP-2019/60), King Saud University, Riyadh, Saudi Arabia.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Zhao, J.; Jin, R. Heterogeneous catalysis by gold and gold-based bimetal nanoclusters. Nano Today 2018, 18, 86–102. [Google Scholar] [CrossRef]
- Freakley, S.J.; He, Q.; Kiely, C.J.; Hutchings, G.J. Gold Catalysis: A Reflection on Where We are Now. Catal. Lett. 2015, 145, 71–79. [Google Scholar] [CrossRef]
- Corma, A.; Garcia, H. Supported gold nanoparticles as catalysts for organic reactions. Chem. Soc. Rev. 2008, 37, 2096–2126. [Google Scholar] [CrossRef] [PubMed]
- Haruta, M.; Yamada, N.; Kobayashi, T.; Iijima, S. Gold catalysts prepared by coprecipitation for low-temperature oxidation of hydrogen and of carbon monoxide. J. Catal. 1989, 115, 301–309. [Google Scholar] [CrossRef]
- Haruta, M. Catalysis of Gold Nanoparticles Deposited on Metal Oxides. Cattech 2002, 6, 102–115. [Google Scholar] [CrossRef]
- Stratakis, M.; Garcia, H. Catalysis by Supported Gold Nanoparticles: Beyond Aerobic Oxidative Processes. Chem. Rev. 2012, 112, 4469–4506. [Google Scholar] [CrossRef] [PubMed]
- Comotti, M.; Li, W.-C.; Spliethoff, B.; Schüth, F. Support Effect in High Activity Gold Catalysts for CO Oxidation. J. Am. Chem. Soc. 2006, 128, 917–924. [Google Scholar] [CrossRef] [PubMed]
- Oschmann, B.; Tahir, M.N.; Mueller, F.; Bresser, D.; Lieberwirth, I.; Tremel, W.; Passerini, S.; Zentel, R. Precursor Polymers for the Carbon Coating of Au@ZnO Multipods for Application as Active Material in Lithium-Ion Batteries. Macromol. Rapid Commun. 2015, 36, 1075–1082. [Google Scholar] [CrossRef]
- Tahir, M.N.; Natalio, F.; Cambaz, M.A.; Panthöfer, M.; Branscheid, R.; Kolb, U.; Tremel, W. Controlled synthesis of linear and branched Au@ZnO hybrid nanocrystals and their photocatalytic properties. Nanoscale 2013, 5, 9944–9949. [Google Scholar] [CrossRef]
- Schladt, T.D.; Shukoor, M.I.; Schneider, K.; Tahir, M.N.; Natalio, F.; Ament, I.; Becker, J.; Jochum, F.D.; Weber, S.; Köhler, O.; et al. Au@MnO Nanoflowers: Hybrid Nanocomposites for Selective Dual Functionalization and Imaging. Angew. Chem. Int. Ed. 2010, 49, 3976–3980. [Google Scholar] [CrossRef]
- Wooh, S.; Lee, Y.-G.; Tahir, M.N.; Song, D.; Meister, M.; Laquai, F.; Tremel, W.; Bisquert, J.; Kang, Y.S.; Char, K. Plasmon-enhanced photocurrent in quasi-solid-state dye-sensitized solar cells by the inclusion of gold/silica core–shell nanoparticles in a TiO2 photoanode. J. Mater. Chem. A 2013, 1, 12627–12634. [Google Scholar] [CrossRef]
- Wang, Y.; Arandiyan, H.; Scott, J.; Bagheri, A.; Dai, H.; Amal, R. Recent advances in ordered meso/macroporous metal oxides for heterogeneous catalysis: A review. J. Mater. Chem. A 2017, 5, 8825–8846. [Google Scholar] [CrossRef]
- Assal, M.E.; Kuniyil, M.; Shaik, M.R.; Khan, M.; Al-Warthan, A.; Siddiqui, M.R.H.; Adil, S.F. Synthesis, Characterization, and Relative Study on the Catalytic Activity of Zinc Oxide Nanoparticles Doped MnCO3,–MnO2, and –Mn2O3 Nanocomposites for Aerial Oxidation of Alcohols. J. Chem. 2017, 2017, 2937108. [Google Scholar] [CrossRef]
- Puigdollers, A.R.; Pacchioni, G. CO oxidation on Au nanoparticles supported on ZrO2: Role of metal/oxide interface and oxide reducibility. ChemCatChem 2017, 9, 1119–1127. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, H.; Xu, B.-Q. Remarkable nanosize effect of zirconia in Au/ZrO2 catalyst for CO oxidation. J. Phys. Chem. B 2005, 109, 9678–9683. [Google Scholar] [CrossRef] [PubMed]
- Menegazzo, F.; Pinna, F.; Signoretto, M.; Trevisan, V.; Boccuzzi, F.; Chiorino, A.; Manzoli, M. Highly dispersed gold on zirconia: Characterization and activity in low-temperature water gas shift tests. ChemSusChem 2008, 1, 320–326. [Google Scholar] [CrossRef]
- Menegazzo, F.; Pinna, F.; Signoretto, M.; Trevisan, V.; Boccuzzi, F.; Chiorino, A.; Manzoli, M. Quantitative determination of sites able to chemisorb CO on Au/ZrO2 catalysts. Appl. Catal. A 2009, 356, 31–35. [Google Scholar] [CrossRef]
- Hong, Y.-C.; Sun, K.-Q.; Han, K.-H.; Liu, G.; Xu, B.-Q. Comparison of catalytic combustion of carbon monoxide and formaldehyde over Au/ZrO2 catalysts. Catal. Today 2010, 158, 415–422. [Google Scholar] [CrossRef]
- Shaik, M.R.; Alam, M.; Adil, S.F.; Kuniyil, M.; Al-Warthan, A.; Siddiqui, M.R.H.; Tahir, M.N.; Labis, J.P.; Khan, M. Solvothermal Preparation and Electrochemical Characterization of Cubic ZrO2 Nanoparticles/Highly Reduced Graphene (HRG) based Nanocomposites. Materials 2019, 12, 711. [Google Scholar] [CrossRef]
- Adil, S.F.; Assal, M.E.; Khan, M.; Al-Warthan, A.; Siddiqui, M.R.H.; Liz-Marzán, L.M. Biogenic synthesis of metallic nanoparticles and prospects toward green chemistry. Dalton Trans. 2015, 44, 9709–9717. [Google Scholar] [CrossRef]
- Xu, H.; Zeiger, B.W.; Suslick, K.S. Sonochemical synthesis of nanomaterials. Chem. Soc. Rev. 2013, 42, 2555–2567. [Google Scholar] [CrossRef] [PubMed]
- Okitsu, K.; Cavalieri, F. Synthesis of Metal Nanomaterials with Chemical and Physical Effects of Ultrasound and Acoustic Cavitation. In Sonochemical Production of Nanomaterials; Springer: Berlin/Heidelberg, Germany, 2018; pp. 19–37. [Google Scholar]
- Shaik, M.R.; Al-Marri, A.H.; Adil, S.F.; Mohri, N.; Barton, B.; Siddiqui, M.R.; Al-Warthan, A.; Labis, J.P.; Tremel, W.; Khan, M. Benzyl alcohol assisted synthesis and characterization of highly reduced graphene oxide (HRG)@ ZrO2 nanocomposites. ChemistrySelect 2017, 2, 3078–3083. [Google Scholar] [CrossRef]
- Haiss, W.; Thanh, N.T.; Aveyard, J.; Fernig, D.G. Determination of size and concentration of gold nanoparticles from UV− Vis spectra. Anal. Chem. 2007, 79, 4215–4221. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Pinchuk, A.O.; McMahon, J.M.; Li, S.; Ausman, L.K.; Atkinson, A.L.; Schatz, G.C. Methods for describing the electromagnetic properties of silver and gold nanoparticles. Acc. Chem. Res. 2008, 41, 1710–1720. [Google Scholar] [CrossRef]
- Tahir, M.N.; Ragg, R.; Natalio, F.; Sahoo, J.K.; Daniel, P.; Koynov, K.; Strand, D.; Strand, S.; Tremel, W. Amine functionalized ZrO2 nanoparticles as biocompatible and luminescent probes for ligand specific cellular imaging. J. Mater. Chem. B 2015, 3, 2371–2377. [Google Scholar] [CrossRef]
- Rajakumar, G.; Gomathi, T.; Abdul Rahuman, A.; Thiruvengadam, M.; Mydhili, G.; Kim, S.-H.; Lee, T.-J.; Chung, I.-M. Biosynthesis and biomedical applications of gold nanoparticles using Eclipta prostrata leaf extract. Appl. Sci. 2016, 6, 222. [Google Scholar] [CrossRef]
- Thawarkar, S.R.; Thombare, B.; Munde, B.S.; Khupse, N.D. Kinetic investigation for the catalytic reduction of nitrophenol using ionic liquid stabilized gold nanoparticles. RSC Adv. 2018, 8, 38384–38390. [Google Scholar] [CrossRef]
- Xiong, Z.; Zhang, H.; Zhang, W.; Lai, B.; Yao, G. Removal of nitrophenols and their derivatives by chemical redox: A review. Chem. Eng. J. 2019, 359, 13–31. [Google Scholar] [CrossRef]
- Pasinszki, T.; Krebsz, M.; Lajgut, G.G.; Kocsis, T.; Kótai, L.; Kauthale, S.; Tekale, S.; Pawar, R. Copper nanoparticles grafted on carbon microspheres as novel heterogeneous catalysts and their application for the reduction of nitrophenol and one-pot multicomponent synthesis of hexahydroquinolines. New J. Chem. 2018, 42, 1092–1098. [Google Scholar] [CrossRef]
- Nasreen, S.; Rafique, U.; Ehrman, S.; Ashraf, M.A. Hybrid mesoporous silicates: A distinct aspect to synthesis and application for decontamination of phenols. Saudi J. Biol. Sci. 2019, 26, 1161–1170. [Google Scholar] [CrossRef]
- Mohamed, F.A.; AbdAllah, M.A.; Shammat, S.M. Selective spectrophotometric determination of p-aminophenol and acetaminophen. Talanta 1997, 44, 61–68. [Google Scholar] [CrossRef]
- Muller, B.; Shahid, M.; Kinet, G. Nitro-and aminophenols as corrosion inhibitors for aluminium and zinc pigments. Corros. Sci. 1999, 41, 1323–1331. [Google Scholar] [CrossRef]
- Kong, X.; Zhu, H.; Chen, C.; Huang, G.; Chen, Q. Insights into the reduction of 4-nitrophenol to 4-aminophenol on catalysts. Chem. Phys. Lett. 2017, 684, 148–152. [Google Scholar] [CrossRef]
- Xu, W.; Kong, J.S.; Yeh, Y.-T.E.; Chen, P. Single-molecule nanocatalysis reveals heterogeneous reaction pathways and catalytic dynamics. Nat. Mater. 2008, 7, 992–996. [Google Scholar] [CrossRef]
- Ma, Y.; Wu, X.; Zhang, G. Core-shell Ag@Pt nanoparticles supported on sepiolite nanofibers for the catalytic reduction of nitrophenols in water: Enhanced catalytic performance and DFT study. Appl. Catal. B Environ. 2017, 205, 262–270. [Google Scholar] [CrossRef]
- Zhao, S.; Das, A.; Zhang, H.; Jin, R.; Song, Y.; Jin, R. Mechanistic insights from atomically precise gold nanocluster-catalyzed reduction of 4-nitrophenol. Prog. Nat. Sci. Mater. Int. 2016, 26, 483–486. [Google Scholar] [CrossRef]
- Liu, B.; Yan, S.; Zhang, A.; Song, Z.; Sun, Q.; Huo, B.; Yang, W.; Barrow, C.J.; Liu, J. Insight into Catalytic Mechanisms for the Reduction of Nitrophenol via Heterojunctions of Gold Nanoclusters on 2D Boron Nitride Nanosheets. ChemNanoMat 2019, 5, 784–791. [Google Scholar] [CrossRef]
- Fu, Y.; Xu, P.; Huang, D.; Zeng, G.; Lai, C.; Qin, L.; Li, B.; He, J.; Yi, H.; Cheng, M.; et al. Au nanoparticles decorated on activated coke via a facile preparation for efficient catalytic reduction of nitrophenols and azo dyes. Appl. Surf. Sci. 2019, 473, 578–588. [Google Scholar] [CrossRef]
- Zhang, J.; Chen, G.; Chaker, M.; Rosei, F.; Ma, D. Gold nanoparticle decorated ceria nanotubes with significantly high catalytic activity for the reduction of nitrophenol and mechanism study. Appl. Catal. B Environ. 2013, 132, 107–115. [Google Scholar] [CrossRef]
- Acosta, B.; Evangelista, V.; Miridonov, S.; Fuentes, S.; Simakov, A. The Decoration of Gold Core in Au@ZrO2 Nanoreactors with Trace Amounts of Pd for the Effective Reduction of 4-Nitrophenol to 4-Aminophenol. Catal. Lett. 2019, 149, 1621–1632. [Google Scholar] [CrossRef]
- Shen, W.; Qu, Y.; Pei, X.; Li, S.; You, S.; Wang, J.; Zhang, Z.; Zhou, J. Catalytic reduction of 4-nitrophenol using gold nanoparticles biosynthesized by cell-free extracts of Aspergillus sp. WL-Au. J. Hazard. Mater. 2017, 321, 299–306. [Google Scholar] [CrossRef] [PubMed]
- Zhao, P.; Feng, X.; Huang, D.; Yang, G.; Astruc, D. Basic concepts and recent advances in nitrophenol reduction by gold-and other transition metal nanoparticles. Coord. Chem. Rev. 2015, 287, 114–136. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).