Exploration of Fall-Evaluation Scores Using Clinical Tools with the Short-Form Berg Balance Scale and Timed Up and Go and Motion Detection Sensors
Abstract
1. Introduction
2. Materials and Methods
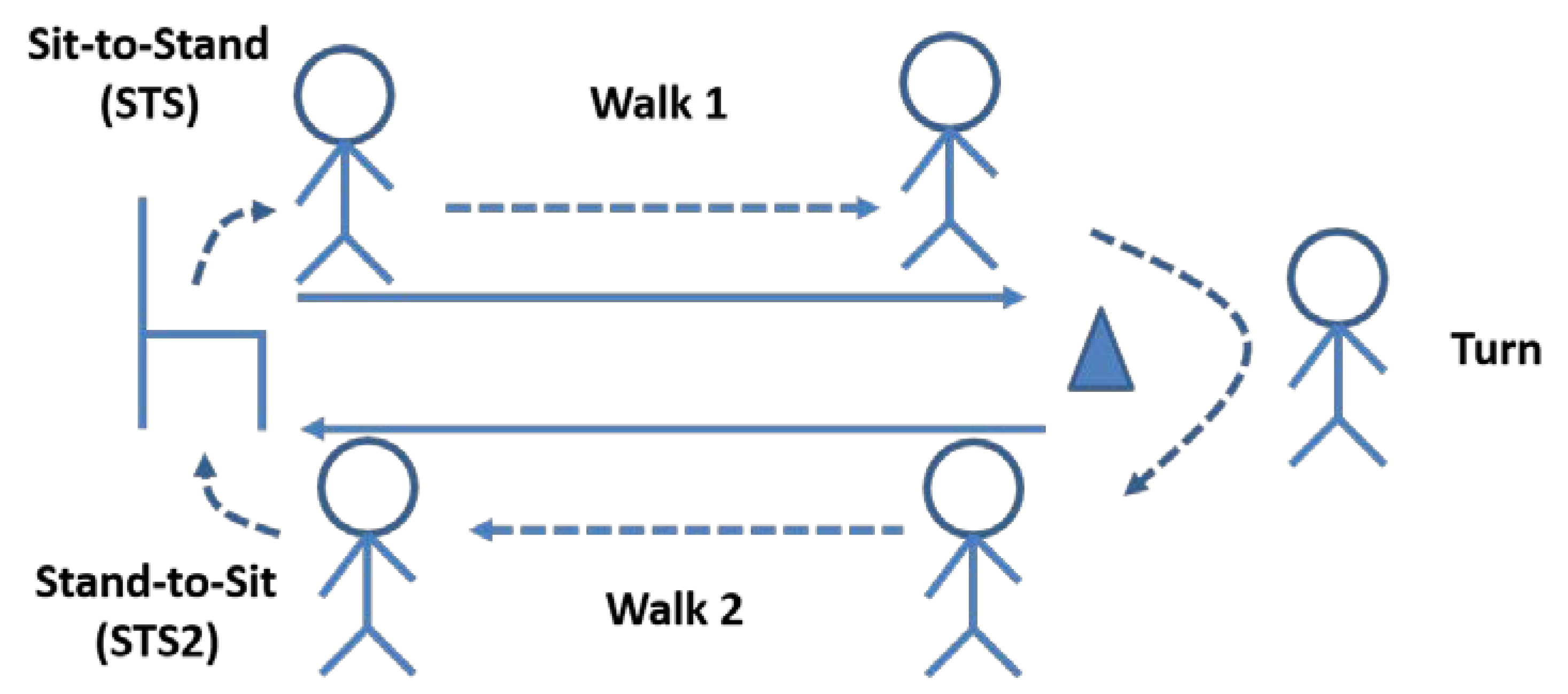
2.1. Protocol
2.2. Subjects
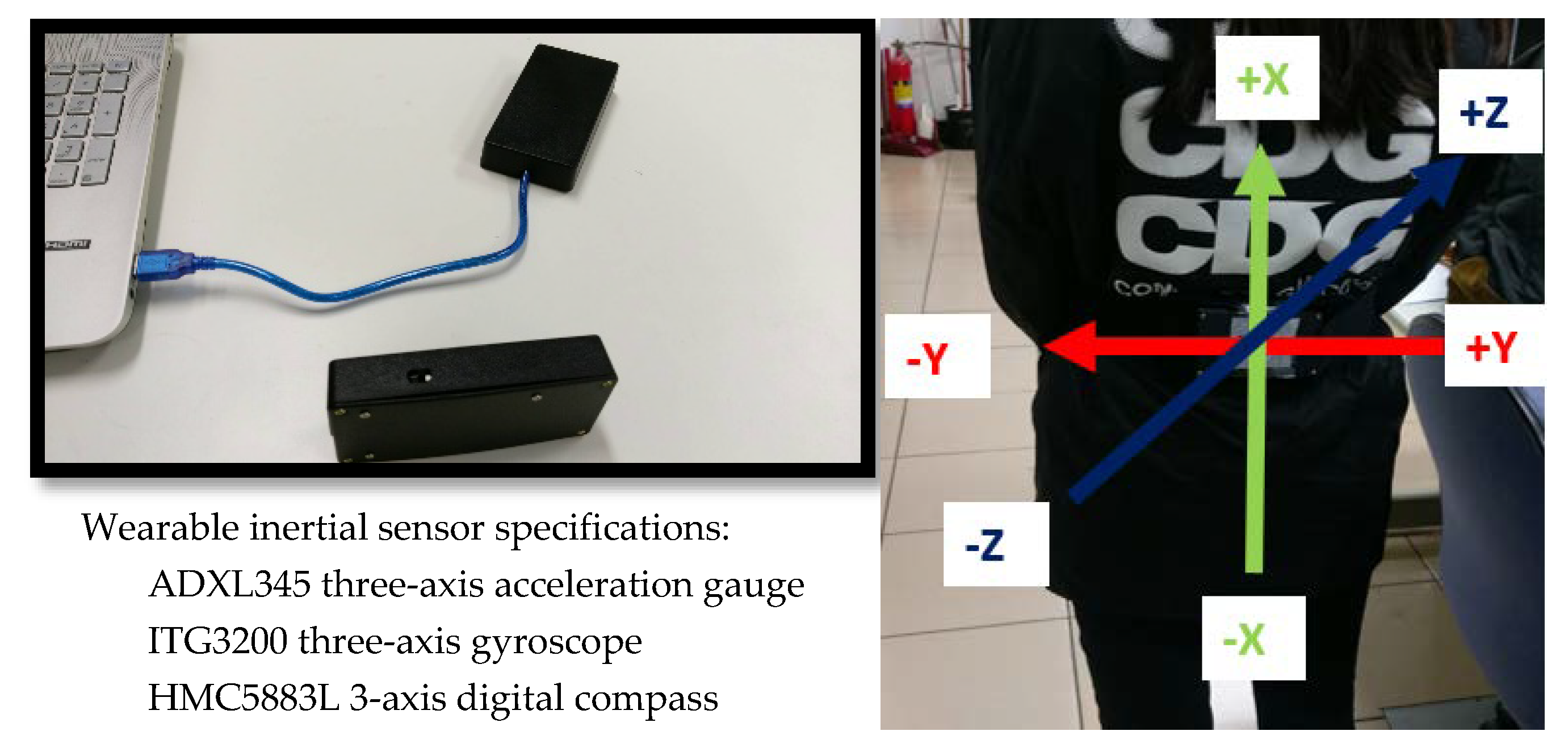
2.3. Inertial Sensor
2.4. Clinical Tests
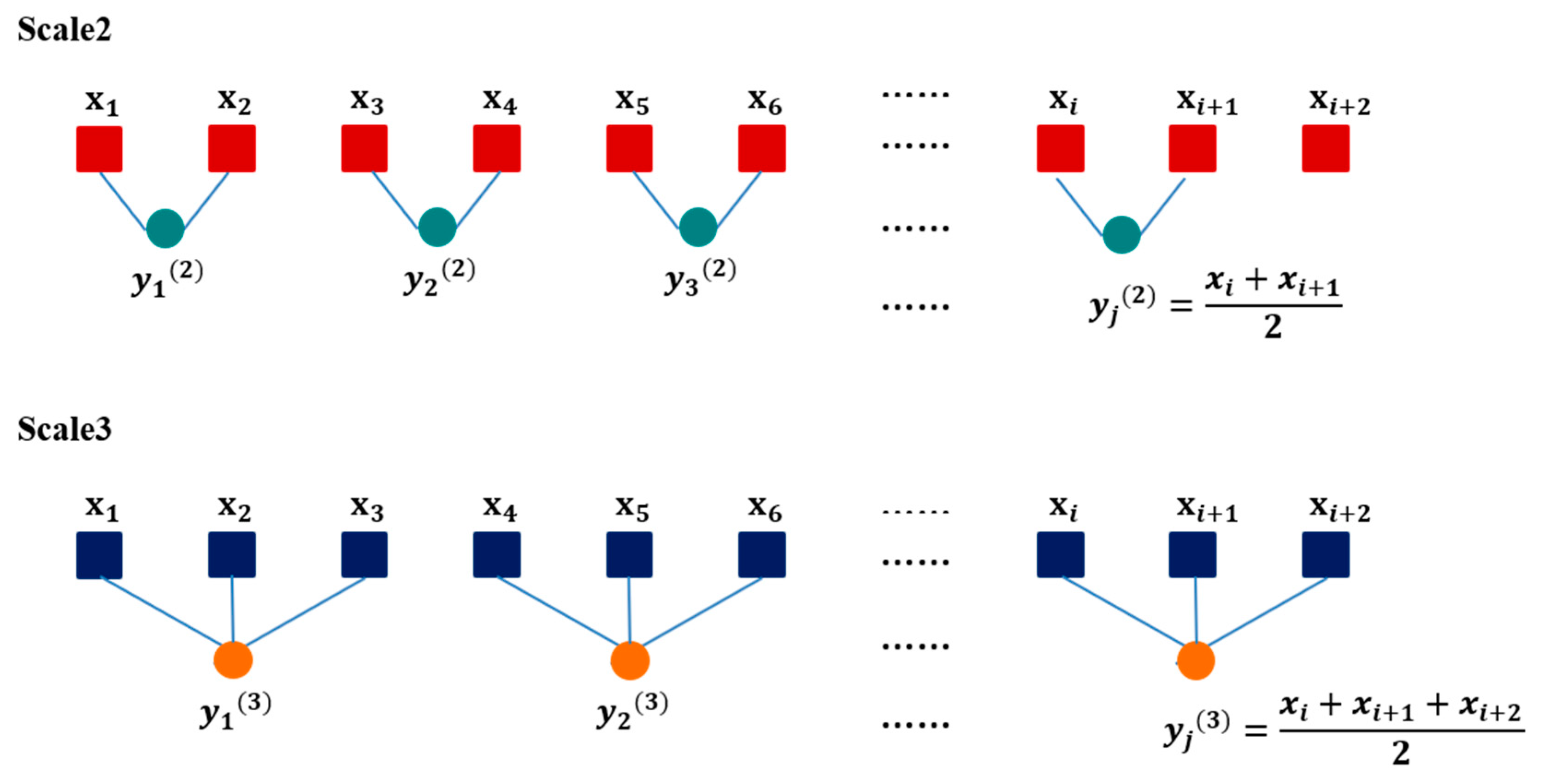
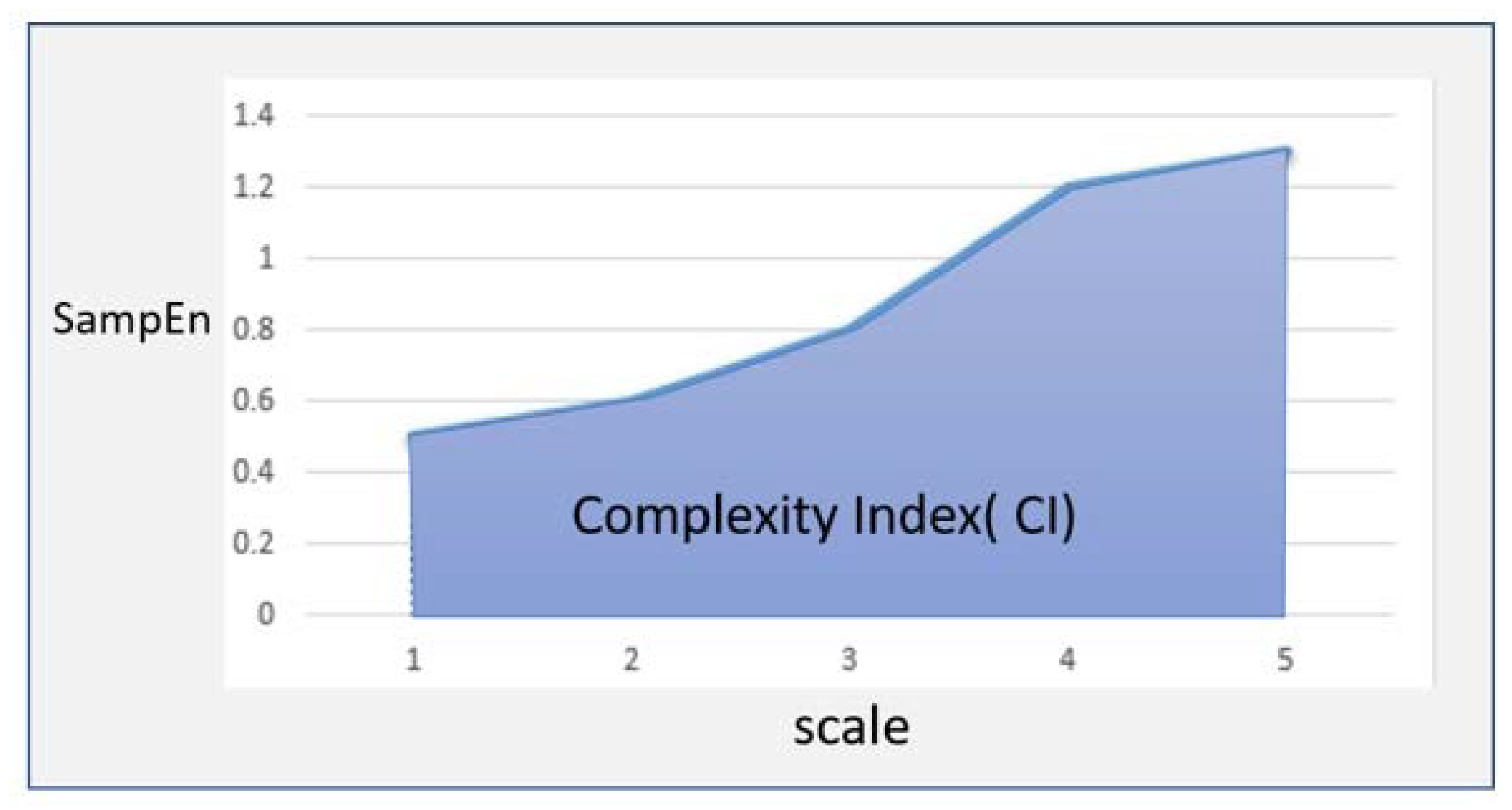
2.5. Data Analysis
3. Results
3.1. Clinical Test of Cut Point Based on Experienced Clinician’s Fall-Risk Evaluation
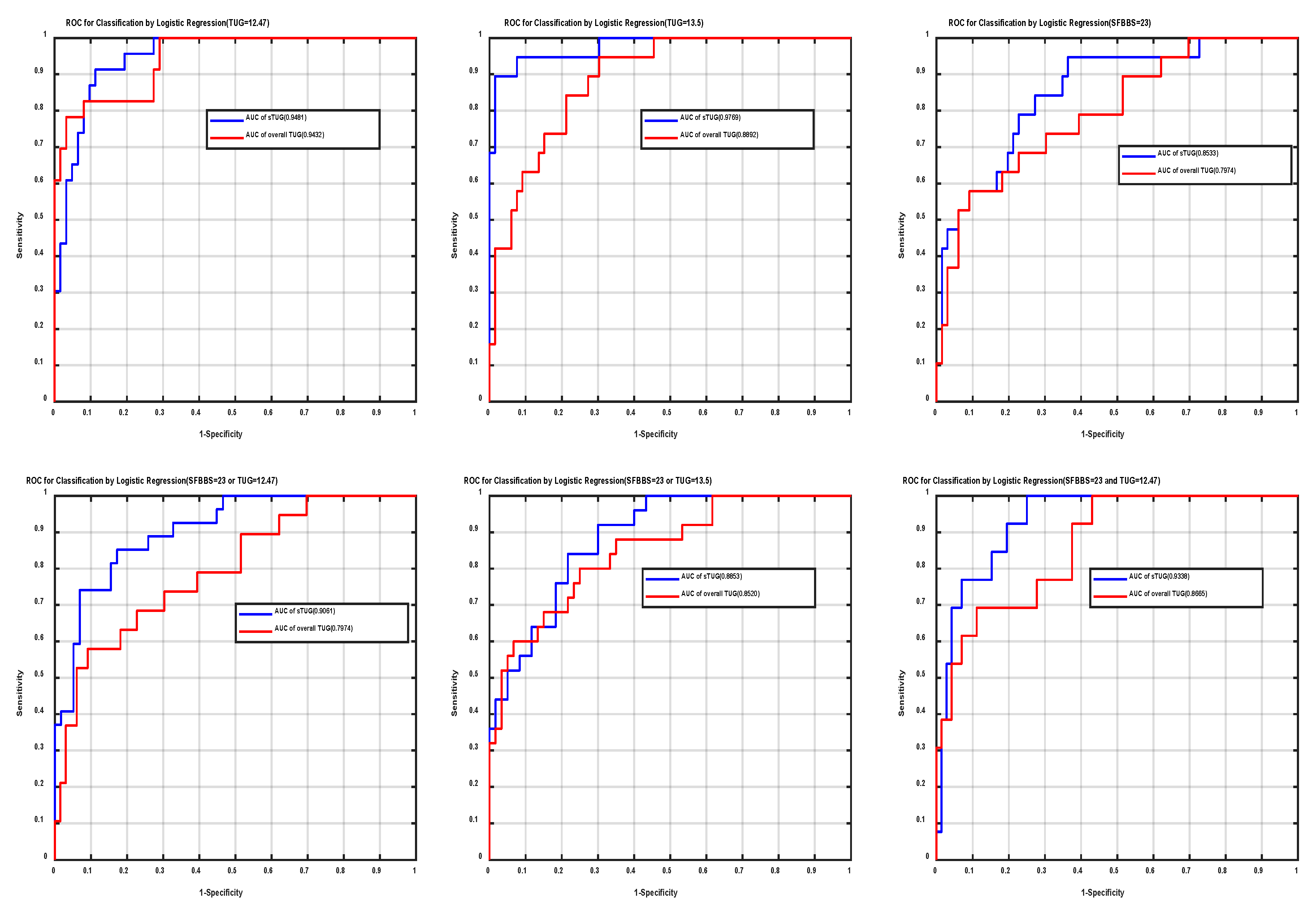
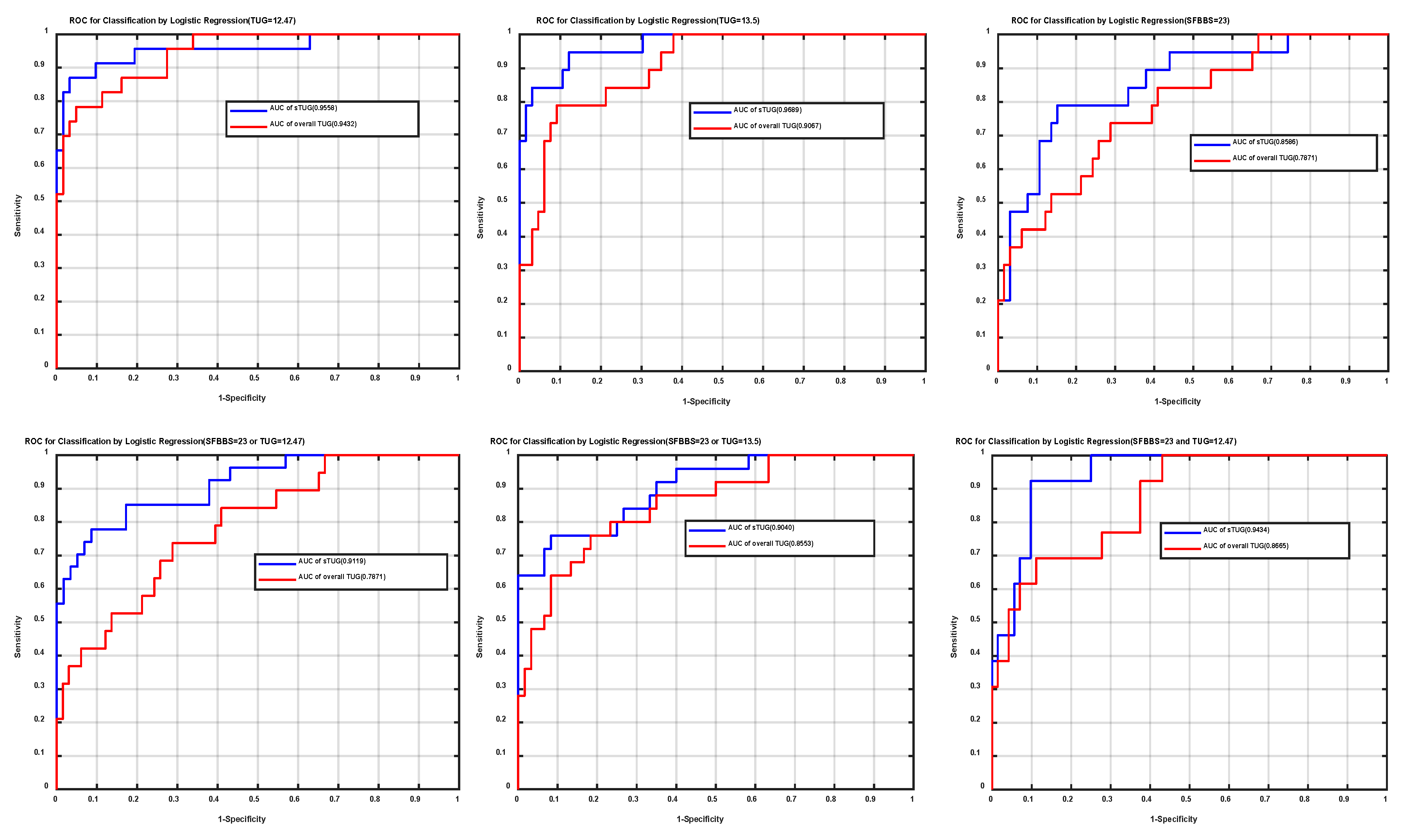
3.2. Clinical Test of Stepwise Logistic Regression as Fall-Risk Evaluation (Prediction Results)
3.3. Comparing (a) and (b)
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lin, S. Risk of falls in community-dwelling older adults: A review of evidence and Taiwan experiences. Taiwan Geriatr. Gerontol. 2013, 8, 239–251. [Google Scholar]
- WHO. Number of People Over 60 Years Set to Double by 2050; Major Societal Changes Required. 2015. Available online: https://www.who.int/mediacentre/news/releases/2015/older-persons-day/en/ (accessed on 11 September 2020).
- Mion, L.C.; Chandler, A.M.; Waters, T.M.; Dietrich, M.S.; Kessler, L.A.; Miller, S.T.; Shorr, R.I. Is it possible to identify risks for injurious falls in hospitalized patients? Jt. Comm. J. Qual. Patient Saf. 2012, 38, 408–413. [Google Scholar] [CrossRef]
- Najafi, B.; Aminian, K.; Loew, F.; Blanc, Y.; Robert, P.A. Measurement of stand-sit and sit-stand transitions using a miniature gyroscope and its application in fall risk evaluation in the elderly. IEEE Trans. Biomed. Eng. 2002, 49, 843–851. [Google Scholar] [CrossRef] [PubMed]
- Montesinos, L.; Castaldo, R.; Pecchia, L. Wearable inertial sensors for fall risk assessment and prediction in older adults: A systematic review and meta-analysis. IEEE Transactions on Neural Systems and Rehabilitation Engineering,.. 2018, 26, 573–582. [Google Scholar] [CrossRef]
- Kear, B.M.; Guck, T.P.; McGaha, A.L. Timed up and go (TUG) test: Normative reference values for ages 20 to 59 years and relationships with physical and mental health risk factors. J. Prim. Care Commun. Health 2016, 8, 9–13. [Google Scholar] [CrossRef]
- Stienen, M.N.; Maldaner, N.; Joswig, H.; Corniola, M.V.; Bellut, D.; Prömmel, P.; Regli, L.; Weyerbrock, A.; Schaller, K.; Gautschi, O.P. Objective functional assessment using the “Timed Up and Go” test in patients with lumbar spinal stenosis. Neurosurg. Focus 2019, 46, E4. [Google Scholar] [CrossRef]
- Howcroft, J.; Kofman, J.; Lemaire, E. Prospective fall-risk prediction models for older adults based on wearable sensors. IEEE Trans. Neural Syst. Rehabil. Eng. 2017, 25, 1812–1820. [Google Scholar] [CrossRef]
- Lee, C.H.; Sun, T.L.; Jiang, B.C.; Choi, V.H. Using wearable accelerometers in a community service context to categorize falling behavior. Entropy 2016, 18, 257. [Google Scholar] [CrossRef]
- Cesari, M.; Penninx, B.W.; Pahor, M.; Lauretani, F.; Corsi, A.M.; Williams, G.R.; Guralnik, J.M.; Ferrucci, L. Inflammatory markers and physical performance in older persons: The InCHIANTI study. J. Gerontol. Biol. Sci. Med. Sci. 2004, 59, 242–248. [Google Scholar] [CrossRef]
- Bourke, A.K.; Van de Ven, P.; Gamble, M.; O’Connor, R.; Murphy, K.; Bogan, E.; Mcquade, E.; Finucane, P.; Olaighin, G.; Nelson, J. Evaluation of waist-mounted tri-axial accelerometer-based fall-detection algorithms during scripted and continuous unscripted activities. J. Biomech. 2010, 43, 3051–3057. [Google Scholar] [CrossRef]
- Bian, Z.P.; Hou, J.; Chau, L.P.; Magnenat-Thalmann, N. Fall detection based on body part tracking using a depth camera. IEEE J. Biomed. Health Inf. 2015, 19, 430–439. [Google Scholar] [CrossRef] [PubMed]
- Bianchi, F.; Redmond, S.J.; Narayanan, M.R.; Cerutti, S.; Lovell, N.H. Barometric pressure and triaxial accelerometry-based falls event detection. IEEE Trans. Neural Syst. Rehabil. Eng. 2010, 18, 619–627. [Google Scholar] [CrossRef] [PubMed]
- Shumway-Cook, A.; Brauer, S.; Woollacott, M. Predicting the probability for falls in community-dwelling older adults using the Timed Up & Go Test. Phys. Ther. 2000, 80, 896–903. [Google Scholar] [PubMed]
- Steffen, T.M.; Hacker, T.A.; Mollinger, L. Age and gender-related test performance in community-dwelling elderly people: Six-Minute Walk test, Berg Balance Scale, timed Up & Go Test, and gait speeds. Phys. Ther. 2002, 82, 128–137. [Google Scholar]
- Phelan, E.A.; Mahoney, J.E.; Voit, J.C.; Stevens, J.A. Assessment and management of fall risk in primary care settings. Med. Clin. North. Am. 2015, 99, 281–293. [Google Scholar] [CrossRef]
- Caulfield, B.M.; Donnelly, S.C. What is connected health and why will it change your practice? QJM Int. J. Med. 2013, 106, 703–707. [Google Scholar] [CrossRef]
- Rajagopalan, R.; Litvan, I.; Jung, T.P. Fall prediction and prevention systems: Recent trends, challenges, and future research directions. Sensors 2017, 17, 2509. [Google Scholar] [CrossRef]
- Kim, E.A.; Mordiffi, S.Z.; Bee, W.H.; Devi, K.; Evans, D. Evaluation of three fall-risk assessment tools in an acute care setting. J. Adv. Nurs. 2007, 60, 427–435. [Google Scholar] [CrossRef]
- Data Visualization and Infographics Graphics. Available online: https://www.smashingmagazine.com/2008/01/monday-inspiration-data-visualization-and-infographics/ (accessed on 11 September 2020).
- Bellazzi, R.; Zupan, B. Predictive data mining in clinical medicine: Current issues and guidelines. Int. J. Med. Inf. 2008, 77, 81–97. [Google Scholar] [CrossRef]
- Migut, M.; Worring, M. Visual exploration of classification models for various data types in risk assessment. Inf. Vis. 2012, 11, 237–251. [Google Scholar] [CrossRef]
- Hurd, W.J.; Morrow, M.M.; Kaufman, K.R. Tri-axial accelerometer analysis techniques for evaluating functional use of the extremities. J. Electromyogr. Kinesiol. 2013, 23, 924–929. [Google Scholar] [CrossRef] [PubMed]
- Tochigi, Y.; Segal, N.A.; Vaseenon, T.; Brown, T.D. Entropy analysis of tri-axial leg acceleration signal waveforms for measurement of decrease of physiological variability in human gait. J. Orthop. Res. 2012, 30, 897–904. [Google Scholar] [CrossRef] [PubMed]
- Pincus, S.M.; Goldberger, A.L. Physiological time-series analysis: What does regularity quantify? Am. J. Physiol. Heart Circ. Physiol. 1994, 266, H1643–H1656. [Google Scholar] [CrossRef] [PubMed]
- Costa, M.; Goldberger, A.L.; Peng, C.K. Multiscale entropy analysis of complex physiologic time series. Phys. Rev. Lett. 2002, 89, 068102. [Google Scholar] [CrossRef] [PubMed]
- Costa, M.; Goldberger, A.L.; Peng, C.K. Multiscale entropy analysis of biological signals. Phys. Rev. E 2005, 71, 021906. [Google Scholar] [CrossRef]
- Wu, C.H.; Lee, C.H.; Jiang, B.C.; Sun, T.L. Multiscale entropy analysis of postural stability for estimating fall risk via domain knowledge of timed-up-and-go accelerometer data for elderly people living in a community. Entropy 2019, 21, 1076. [Google Scholar] [CrossRef]
- Hamm, J.; Money, A.; Atwal, A. Fall prevention self-assessments via mobile 3D visualization technologies: Community dwelling older adults’ perceptions of opportunities and challenges. JMIR Hum. Factors 2017, 4, e15. [Google Scholar] [CrossRef]
- Chau, P.Y.; Hu, P.J. Examining a model of information technology acceptance by individual professionals: An exploratory study. J. Manag. Inf. Syst. 2014, 18, 191–229. [Google Scholar] [CrossRef]
- Perell, K.L.; Nelson, A.; Goldman, R.L.; Luther, S.L.; Prieto-Lewis, N.; Rubenstein, L.Z. Fall risk assessment measures: An analytic review. J. Gerontol. Biol. Sci. Med. Sci. 2001, 56, M761–M766. [Google Scholar] [CrossRef]
- Lin, M.R.; Tsai, S.L.; Chen, S.Y.; Tzeng, S.J. Risk factors for elderly falls in a rural community of central Taiwan. Taiwan J. Public Health 2002, 21, 73–82. [Google Scholar]
- Okumiya, K.; Matsubayash, K.; Nakamura, T.; Fujisawa, M.; Osaki, Y.; Doi, Y.; Ozawa, T. The timed “up and go” test is a useful predictor of falls in community-dwelling older people. J. Am. Geriatr. Soc. 1998, 46, 928–930. [Google Scholar] [CrossRef] [PubMed]
- Issacs, B. Clinical and laboratory studies of falls in old people: Prospects on prevention. Clin. Geriatr. Med. 1985, 1, 513–520. [Google Scholar] [CrossRef]
- Gates, S.; Smith, L.A.; Fisher, J.D.; Lamb, S.E. Systematic review of accuracy of screening instruments for predicting fall risk among independently living older adults. J. Rehabil. Res. Dev. 2008, 45, 1105–1116. [Google Scholar] [CrossRef] [PubMed]
- Vassallo, M.; Poynter, L.; Sharma, J.C.; Kwan, J.; Allen, S. Fall risk-assessment tools compared with clinical judgment: An evaluation in a rehabilitation ward. Age Ageing 2008, 37, 277–281. [Google Scholar] [CrossRef][Green Version]
- Weiss, A.; Herman, T.; Plotnik, M.; Brozgol, M.; Maidan, I.; Giladi, N.; Gurevich, T.; Yamada, M.; Arai, H.; Nagai, K.; et al. Development of a new fall risk assessment index for older adults. Int. J. Gerontol. 2012, 6, 160–162. [Google Scholar]
- Pohl, P.; Nordin, E.; Lundquist, A.; Bergstrom, U.; Lundin-Olsson, L. Community-dwelling older people with an injurious fall are likely to sustain new injurious falls within 5 years-a prospective long-term follow-up study. BMC Geriatr. 2014, 14, 120. [Google Scholar] [CrossRef]
- Huang, C.H.; Sun, T.L. Interactive visualization to support fall risk assessment for community-dwelling elderly. Inf. Vis. 2017, 18, 33–44. [Google Scholar]
- Mischel, P.S.; Cloughesy, T. Using molecular information to guide brain tumor therapy. Nat. Clin. Pract. Neurol. 2006, 2, 232–233. [Google Scholar] [CrossRef]
- Azzam, T.; Evergreen, S.; Germuth, A.A.; Kistler, S.J. Data visualization and evaluation. New Directions Eval. 2013, 2013, 7–32. [Google Scholar] [CrossRef]
- Riva, F.; Toebes, M.J.P.; Pijnappels, M.A.G.M.; Stagni, R.; Van Dieën, J.H. Estimating fall risk with inertial sensors using gait stability measures that do not require step detection. Gait Posture 2013, 38, 170–174. [Google Scholar] [CrossRef]
- Karthikeyan, G.; Sheikh, S.G.; Chippala, P. Test-retest reliability of short form of berg balance scale in elderly people. Glob. Adv. Res. J. Med. Med. Sci. 2012, 1, 139–144. [Google Scholar]
- Chien, W.L. Prediction of Falling Risk of the Elderly by Data Mining Techniques. Master’s Thesis, Tatung University, Taipei City, Taiwan, February 2012. [Google Scholar]
- Alexandre, T.S.; Meira, D.M.; Rico, N.C.; Mizuta, S.K. Accuracy of timed up and go test for screening risk of falls among community-dwelling elderly. Rev. Bras. Fisioter. 2012, 16, 381–388. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Wei, Q.; Fan, S.; Lu, C.; Lin, T.; Abbod, M.F.; Shieh, J. Adaptive computation of multiscale entropy and its application in EEG signals for monitoring depth of anesthesia during surgery. Entropy 2012, 14, 978–992. [Google Scholar] [CrossRef]
- Costa, M.; Priplata, A.A.; Lipsitz, L.A.; Wu, Z.; Huang, N.E.; Goldberger, A.L.; Peng, C.K. Noise and poise: Enhancement of postural complexity in the elderly with a stochastic-resonance–based therapy. Europhys. Lett. 2007, 77, 68008. [Google Scholar] [CrossRef] [PubMed]
- O’Neill, T.W.; Varlow, J.; Silman, A.J.; Reeve, J.; Reid, D.M.; Todd, C.; Woolf, A.D. Age and sex influences on fall characteristics. Ann. Rheum. Dis. 1994, 53, 773–775. [Google Scholar]
- Meyns, P.; Bruijn, S.M.; Duysens, J. The how and why of arm swing during human walking. Gait Posture 2013, 38, 555–562. [Google Scholar] [CrossRef]
- Podsiadlo, D.; Richardson, S. The timed “Up & Go”: A test of basic functional mobility for frail elderly persons. J. Am. Geriatr. Soc. 1991, 39, 142–148. [Google Scholar]
- Freeland, K.N.; Thompson, A.N.; Zhao, Y.; Leal, J.E.; Mauldin, P.D.; Moran, W.P. Medication use and associated risk of falling in a geriatric outpatient population. Ann. Pharmacother. 2012, 46, 1188–1192. [Google Scholar] [CrossRef]
- Russell, M.A.; Hill, K.D.; Day, L.M.; Blackberry, I.; Gurrin, L.C.; Dharmage, S.C. Development of the falls risk for older people in the community (FROP-Com) screening tool. Age Ageing 2009, 38, 40–46. [Google Scholar] [CrossRef]
- Russell, M.A.; Hill, K.D.; Blackberry, I.; Day, L.M.; Dharmage, S.C. The reliability and predictive accuracy of the falls risk for older people in the community assessment (FROP-Com) tool. Age Ageing 2008, 37, 634–639. [Google Scholar] [CrossRef]
- Sun, R.; Sosnoff, J.J. Novel sensing technology in fall risk assessment in older adults: A systematic review. BMC Geriatr. 2018, 18, 14. [Google Scholar] [CrossRef] [PubMed]
- Howcroft, J.; Kofman, J.; Lemaire, E.D. Review of fall risk assessment in geriatric populations using inertial sensors. J. Neuroeng. Rehabil. 2013, 10, 91. [Google Scholar] [CrossRef] [PubMed]
- Shi, G.; Zhang, J.; Dong, C.; Han, P.; Jin, Y.; Wang, J. Fall detection system based on inertial mems sensors: Analysis design and realization. In Proceedings of the IEEE International Conference on Cyber Technology in Automation, Control, and Intelligent Systems, Shenyang, China, 8–12 June 2015; pp. 1834–1839. [Google Scholar]
| TUG | ||||||
| Code | T1 | T2 | T3 | |||
| Name | CI_X | CI_Y | CI_Z | |||
| Segment-based TUG (sTUG) | ||||||
| Code | F1 | F2 | F3 | F4 | F5 | F6 |
| Name | CI_X_STS | CI_X_Turn | CI_X_STS2 | CI_X_walk | CI_Y_STS | CI_Y_Turn |
| Code | F7 | F8 | F9 | F10 | F11 | F12 |
| Name | CI_Y_STS2 | CI_Y_walk | CI_Z_STS | CI_Z_Turn | CI_Z_STS2 | CI_Z_walk |
| Cutoff Point | TUG = 12.47 | TUG = 13.5 | SFBBS = 23 | SFBBS = 23 or TUG = 12.47 | SFBBS = 23 or TUG = 13.5 | SFBBS = 23 and TUG = 13.5/12.47 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| TUG | code | p-value | code | p-value | code | p-value | code | p-value | code | p-value | code | p-value |
| T1 | 0.015 | T1 | 0.130 | T1 | 0.027 | T1 | 0.015 | T1 | 0.045 | T1 | 0.085 | |
| T2 | 0.000 | T2 | 0.191 | T2 | 0.000 | T2 | 0.000 | T2 | 0.000 | T2 | 0.000 | |
| T3 | 0.000 | T3 | 0.023 | T3 | 0.000 | T3 | 0.000 | T3 | 0.000 | T3 | 0.000 | |
| sTUG | F1 | 0.233 | F1 | 0.513 | F1 | 0.213 | F1 | 0.252 | F1 | 0.305 | F1 | 0.390 |
| F2 | 0.292 | F2 | 0.038 | F2 | 0.022 | F2 | 0.268 | F2 | 0.039 | F2 | 0.020 | |
| F3 | 0.08 | F3 | 0.203 | F3 | 0.059 | F3 | 0.026 | F3 | 0.044 | F3 | 0.283 | |
| F4 | 0.489 | F4 | 0.000 | F4 | 0.192 | F4 | 0.316 | F4 | 0.199 | F4 | 0.182 | |
| F5 | 0.078 | F5 | 0.018 | F5 | 0.007 | F5 | 0.039 | F5 | 0.016 | F5 | 0.002 | |
| F6 | 0.009 | F6 | 0.066 | F6 | 0.639 | F6 | 0.146 | F6 | 0.309 | F6 | 0.204 | |
| F7 | 0.003 | F7 | 0.000 | F7 | 0.001 | F7 | 0.002 | F7 | 0.006 | F7 | 0.001 | |
| F8 | 0.000 | F8 | 0.000 | F8 | 0.000 | F8 | 0.000 | F8 | 0.000 | F8 | 0.000 | |
| F9 | 0.000 | F9 | 0.000 | F9 | 0.001 | F9 | 0.000 | F9 | 0.000 | F9 | 0.000 | |
| F10 | 0.007 | F10 | 0.025 | F10 | 0.025 | F10 | 0.017 | F10 | 0.035 | F10 | 0.007 | |
| F11 | 0.000 | F11 | 0.000 | F11 | 0.001 | F11 | 0.000 | F11 | 0.000 | F11 | 0.000 | |
| F12 | 0.000 | F12 | 0.000 | F12 | 0.018 | F12 | 0.000 | F12 | 0.001 | F12 | 0.016 | |
| Cutoff Point | TUG = 12.47 | TUG = 13.5 | SFBBS = 23 | SFBBS = 23 or TUG = 12.47 | SFBBS = 23 or TUG = 13.5 | SFBBS = 23 and TUG = 13.5/12.47 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| TUG | code | Frequent | code | Frequent | code | Frequent | code | Frequent | code | Frequent | code | Frequent |
| T1 | 14 | T1 | 35 | T1 | 10 | T1 | 1 | T1 | 4 | T1 | 7 | |
| T2 | 100 | T2 | 100 | T2 | 68 | T2 | 100 | T2 | 97 | T2 | 62 | |
| T3 | 100 | T3 | 100 | T3 | 91 | T3 | 100 | T3 | 100 | T3 | 100 | |
| sTUG | F1 | 9 | F1 | 19 | F1 | 7 | F1 | 2 | F1 | 6 | F1 | 10 |
| F2 | 5 | F2 | 19 | F2 | 79 | F2 | 1 | F2 | 48 | F2 | 88 | |
| F3 | 5 | F3 | 7 | F3 | 26 | F3 | 6 | F3 | 15 | F3 | 9 | |
| F4 | 76 | F4 | 86 | F4 | 93 | F4 | 84 | F4 | 93 | F4 | 95 | |
| F5 | 4 | F5 | 11 | F5 | 19 | F5 | 8 | F5 | 17 | F5 | 9 | |
| F6 | 46 | F6 | 30 | F6 | 1 | F6 | 2 | F6 | 3 | F6 | 21 | |
| F7 | 4 | F7 | 9 | F7 | 66 | F7 | 12 | F7 | 3 | F7 | 38 | |
| F8 | 100 | F8 | 99 | F8 | 74 | F8 | 100 | F8 | 100 | F8 | 61 | |
| F9 | 31 | F9 | 96 | F9 | 45 | F9 | 55 | F9 | 74 | F9 | 73 | |
| F10 | 10 | F10 | 9 | F10 | 15 | F10 | 18 | F10 | 7 | F10 | 20 | |
| F11 | 52 | F11 | 97 | F11 | 8 | F11 | 60 | F11 | 55 | F11 | 60 | |
| F12 | 75 | F12 | 0 | F12 | 23 | F12 | 46 | F12 | 26 | F12 | 24 | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, C.-H.; Wu, C.-H.; Jiang, B.C.; Sun, T.-L. Exploration of Fall-Evaluation Scores Using Clinical Tools with the Short-Form Berg Balance Scale and Timed Up and Go and Motion Detection Sensors. Appl. Sci. 2020, 10, 6931. https://doi.org/10.3390/app10196931
Lee C-H, Wu C-H, Jiang BC, Sun T-L. Exploration of Fall-Evaluation Scores Using Clinical Tools with the Short-Form Berg Balance Scale and Timed Up and Go and Motion Detection Sensors. Applied Sciences. 2020; 10(19):6931. https://doi.org/10.3390/app10196931
Chicago/Turabian StyleLee, Chia-Hsuan, Chi-Han Wu, Bernard C. Jiang, and Tien-Lung Sun. 2020. "Exploration of Fall-Evaluation Scores Using Clinical Tools with the Short-Form Berg Balance Scale and Timed Up and Go and Motion Detection Sensors" Applied Sciences 10, no. 19: 6931. https://doi.org/10.3390/app10196931
APA StyleLee, C.-H., Wu, C.-H., Jiang, B. C., & Sun, T.-L. (2020). Exploration of Fall-Evaluation Scores Using Clinical Tools with the Short-Form Berg Balance Scale and Timed Up and Go and Motion Detection Sensors. Applied Sciences, 10(19), 6931. https://doi.org/10.3390/app10196931





