Cold Atmospheric Pressure Plasma in Wound Healing and Cancer Treatment
Abstract
1. Introduction
2. CAP Treatment to Promote Healing of Acute and Chronic Wounds
3. CAP Application for Cancer Treatment
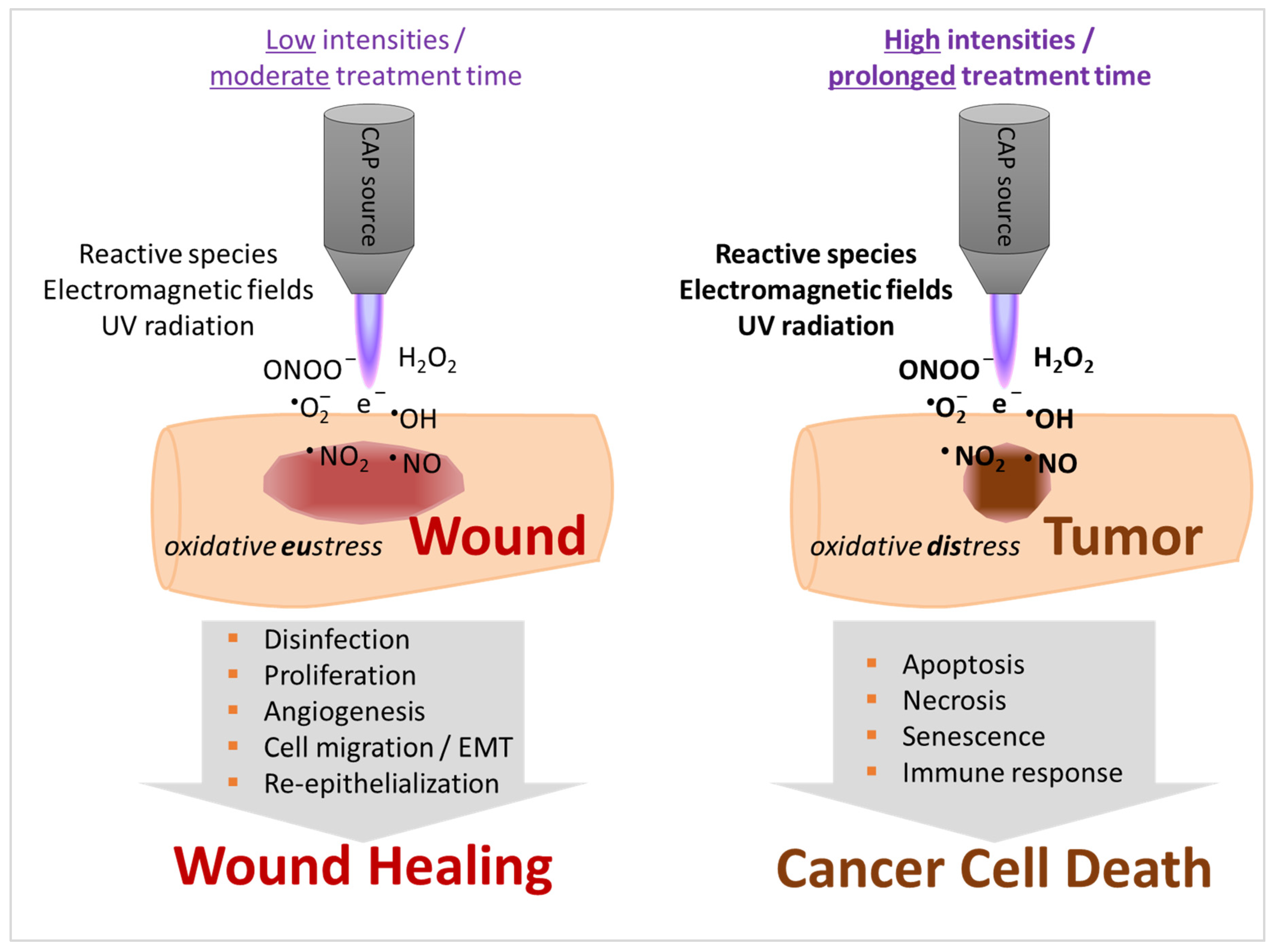
4. Properties of CAP Stimulate Tissue Regeneration but May also Induce Cell Death
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Von Woedtke, T.; Reuter, S.; Masur, K.; Weltmann, K.-D. Plasmas for medicine. Phys. Rep. 2013, 530, 291–320. [Google Scholar] [CrossRef]
- Fridman, G.; Friedman, G.; Gutsol, A.; Shekhter, A.B.; Vasilets, V.N.; Fridman, A. Applied Plasma Medicine. Plasma Process. Polym. 2008, 5, 503–533. [Google Scholar] [CrossRef]
- Metelmann, H.-R.; Woedtke, T.; Weltmann, K.-D. Comprehensive Clinical Plasma Medicine: Cold Physical Plasma for Medical Application; Springer International Publishing: Midtown Manhattan, NY, USA, 2018. [Google Scholar]
- Bernhardt, T.; Semmler, M.L.; Schäfer, M.; Bekeschus, S.; Emmert, S.; Boeckmann, L. Plasma Medicine: Applications of Cold Atmospheric Pressure Plasma in Dermatology. Oxid. Med. Cell. Longev. 2019, 2019, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Boeckmann, L.; Bernhardt, T.; Schäfer, M.; Semmler, M.L.; Kordt, M.; Waldner, A.; Wendt, F.; Sagwal, S.; Bekeschus, S.; Berner, J.; et al. Aktuelle Indikationen der Plasmatherapie in der Dermatologie. Der Hautarzt 2020, 71, 109–113. [Google Scholar] [CrossRef] [PubMed]
- Isbary, G.; Morfill, G.; Schmidt, H.; Georgi, M.; Ramrath, K.; Heinlin, J.; Karrer, S.; Landthaler, M.; Shimizu, T.; Steffes, B.; et al. A first prospective randomized controlled trial to decrease bacterial load using cold atmospheric argon plasma on chronic wounds in patients. Br. J. Dermatol. 2010, 163, 78–82. [Google Scholar] [CrossRef] [PubMed]
- Isbary, G.; Heinlin, J.; Shimizu, T.; Zimmermann, J.; Morfill, G.; Schmidt, H.-U.; Monetti, R.; Steffes, B.; Bunk, W.; Li, Y.; et al. Successful and safe use of 2 min cold atmospheric argon plasma in chronic wounds: Results of a randomized controlled trial. Br. J. Dermatol. 2012, 167, 404–410. [Google Scholar] [CrossRef]
- Klebes, M.; Ulrich, C.; Kluschke, F.; Patzelt, A.; Vandersee, S.; Richter, H.; Bob, A.; Von Hutten, J.; Krediet, J.T.; Kramer, A.; et al. Combined antibacterial effects of tissue-tolerable plasma and a modern conventional liquid antiseptic on chronic wound treatment. J. Biophotonics 2015, 8, 382–391. [Google Scholar] [CrossRef]
- Ulrich, C.; Kluschke, F.; Patzelt, A.; Vandersee, S.; Czaika, M.V.A.; Richter, H.; Bob, A.; Von Hutten, J.; Painsi, C.; Huge, R.; et al. Clinical use of cold atmospheric pressure argon plasma in chronic leg ulcers: A pilot study. J. Wound Care 2015, 24, 196–203. [Google Scholar] [CrossRef]
- Isbary, G.; Stolz, W.; Shimizu, T.; Monetti, R.; Bunk, W.; Schmidt, H.-U.; Morfill, G.E.; Klämpfl, T.G.; Steffes, B.; Thomas, H.M.; et al. Cold atmospheric argon plasma treatment may accelerate wound healing in chronic wounds: Results of an open retrospective randomized controlled study in vivo. Clin. Plasma Med. 2013, 1, 25–30. [Google Scholar] [CrossRef]
- Brehmer, F.; Haenssle, H.A.; Daeschlein, G.; Ahmed, R.; Pfeiffer, S.; Görlitz, A.; Simon, D.; Schon, M.P.; Wandke, D.; Emmert, S. Alleviation of chronic venous leg ulcers with a hand-held dielectric barrier discharge plasma generator (PlasmaDerm((R))VU-2010): Results of a monocentric, two-armed, open, prospective, randomized and controlled trial (NCT01415622). J. Eur. Acad. Dermatol. Venereol. 2015, 29, 148–155. [Google Scholar] [CrossRef]
- Chuangsuwanich, A.; Assadamongkol, T.; Boonyawan, D. The Healing Effect of Low-Temperature Atmospheric-Pressure Plasma in Pressure Ulcer. Int. J. Low. Extremity Wounds 2016, 15, 313–319. [Google Scholar] [CrossRef] [PubMed]
- Metelmann, H.-R.; Woedtke, T.; Bussiahn, R.; Weltmann, K.-D.; Rieck, M.; Khalili, R.; Podmelle, F.; Waite, P.D. Experimental Recovery of CO2-Laser Skin Lesions by Plasma Stimulation. Am. J. Cosmet. Surg. 2012, 29, 52–56. [Google Scholar] [CrossRef]
- Metelmann, H.-R.; Vu, T.T.; Do, H.T.; Le, T.N.B.; Hoang, T.H.A.; Phi, T.T.T.; Luong, T.M.L.; Van Doan, V.T.; Nguyen, T.T.H.; Nguyen, T.H.M.; et al. Scar formation of laser skin lesions after cold atmospheric pressure plasma (CAP) treatment: A clinical long term observation. Clin. Plasma Med. 2013, 1, 30–35. [Google Scholar] [CrossRef]
- Vandersee, S.; Richter, H.; Lademann, J.; Beyer, M.; Kramer, A.; Knorr, F.; Lange-Asschenfeldt, B. Laser scanning microscopy as a means to assess the augmentation of tissue repair by exposition of wounds to tissue tolerable plasma. Laser Phys. Lett. 2014, 11, 115701. [Google Scholar] [CrossRef]
- Heinlin, J.; Zimmermann, J.L.; Zeman, F.; Bunk, W.; Isbary, G.; Landthaler, M.; Maisch, T.; Monetti, R.; Morfill, G.; Shimizu, T.; et al. Randomized placebo-controlled human pilot study of cold atmospheric argon plasma on skin graft donor sites. Wound Repair Regen. 2013, 21, 800–807. [Google Scholar] [CrossRef]
- Assadian, O.; Ousey, K.J.; Daeschlein, G.; Kramer, A.; Parker, C.; Tanner, J.; Leaper, D.J. Effects and safety of atmospheric low-temperature plasma on bacterial reduction in chronic wounds and wound size reduction: A systematic review and meta-analysis. Int. Wound J. 2018, 16, 103–111. [Google Scholar] [CrossRef]
- Stratmann, B.; Costea, T.-C.; Nolte, C.; Hiller, J.; Schmidt, J.; Reindel, J.; Masur, K.; Motz, W.; Timm, J.; Kerner, W.; et al. Effect of Cold Atmospheric Plasma Therapy vs Standard Therapy Placebo on Wound Healing in Patients With Diabetic Foot Ulcers. JAMA Netw. Open 2020, 3, e2010411. [Google Scholar] [CrossRef]
- Mirpour, S.; Fathollah, S.; Mansouri, P.; Larijani, B.; Ghoranneviss, M.; Tehrani, M.M.; Amini, M.R. Cold atmospheric plasma as an effective method to treat diabetic foot ulcers: A randomized clinical trial. Sci. Rep. 2020, 10, 1–9. [Google Scholar] [CrossRef]
- Hoffmann, M.; Bruch, H.-P.; Kujath, P.; Limmer, S. Cold-plasma coagulation in the treatment of malignant pleural mesothelioma: Results of a combined approach. Interact. Cardiovasc. Thorac. Surg. 2010, 10, 502–505. [Google Scholar] [CrossRef][Green Version]
- Metelmann, H.-R.; Nedrelow, D.S.; Seebauer, C.; Schuster, M.; Von Woedtke, T.; Weltmann, K.-D.; Kindler, S.; Metelmann, P.H.; Finkelstein, S.E.; Von Hoff, D.D.; et al. Head and neck cancer treatment and physical plasma. Clin. Plasma Med. 2015, 3, 17–23. [Google Scholar] [CrossRef]
- Schuster, M.; Seebauer, C.; Rutkowski, R.; Hauschild, A.; Podmelle, F.; Metelmann, C.; Metelmann, B.; Von Woedtke, T.; Hasse, S.; Weltmann, K.-D.; et al. Visible tumor surface response to physical plasma and apoptotic cell kill in head and neck cancer. J. Cranio-Maxillofac. Surg. 2016, 44, 1445–1452. [Google Scholar] [CrossRef] [PubMed]
- Friedman, P.C.; Miller, V.; Fridman, G.; Lin, A.; Fridman, A. Successful treatment of actinic keratoses using nonthermal atmospheric pressure plasma: A case series. J. Am. Acad. Dermatol. 2017, 76, 349–350. [Google Scholar] [CrossRef] [PubMed]
- Metelmann, H.-R.; Seebauer, C.; Miller, V.; Fridman, A.; Bauer, G.; Graves, D.B.; Pouvesle, J.-M.; Rutkowski, R.; Schuster, M.; Bekeschus, S.; et al. Clinical experience with cold plasma in the treatment of locally advanced head and neck cancer. Clin. Plasma Med. 2018, 9, 6–13. [Google Scholar] [CrossRef]
- Wirtz, M.; Stoffels, I.; Dissemond, J.; Schadendorf, D.; Roesch, A. Actinic keratoses treated with cold atmospheric plasma. J. Eur. Acad. Dermatol. Venereol. 2017, 32, 37. [Google Scholar] [CrossRef]
- Privat-Maldonado, A.; Bengtson, C.; Razzokov, J.; Smits, E.; Bogaerts, A. Modifying the Tumour Microenvironment: Challenges and Future Perspectives for Anticancer Plasma Treatments. Cancers 2019, 11, 1920. [Google Scholar] [CrossRef]
- Dubuc, A.; Monsarrat, P.; Virard, F.; Merbahi, N.; Sarrette, J.-P.; Laurencin-Dalicieux, S.; Cousty, S. Use of cold-atmospheric plasma in oncology: A concise systematic review. Ther. Adv. Med Oncol. 2018, 10, 1758835918786475. [Google Scholar] [CrossRef]
- Calugaru, A.; Cremer, L.; Herold, A.; Lupu, A.; Szegli, G.; Lungu, C.; Lungu, A.; Georgescu, N. The effect of the plasma needle on tumoral cell lines apoptosis. Roum. Arch. Microbiol. Immunol. 2007, 64, 57. [Google Scholar]
- Chernets, N.; Kurpad, D.S.; Alexeev, V.; Rodrigues, D.B.; Freeman, T.A. Reaction Chemistry Generated by Nanosecond Pulsed Dielectric Barrier Discharge Treatment is Responsible for the Tumor Eradication in the B16 Melanoma Mouse Model. Plasma Process. Polym. 2015, 12, 1400–1409. [Google Scholar] [CrossRef]
- Keidar, M.; Walk, R.; Shashurin, A.; Srinivasan, P.; Sandler, A.; Dasgupta, S.; Ravi, R.; Guerrero-Preston, R.E.; Trink, B. Cold plasma selectivity and the possibility of a paradigm shift in cancer therapy. Br. J. Cancer 2011, 105, 1295–1301. [Google Scholar] [CrossRef]
- Bekeschus, S.; Clemen, R.; Nießner, F.; Sagwal, S.K.; Freund, E.; Schmidt, A. Medical Gas Plasma Jet Technology Targets Murine Melanoma in an Immunogenic Fashion. Adv. Sci. 2020, 7, 1903438. [Google Scholar] [CrossRef]
- Brullé, L.; Vandamme, M.; Riès, D.; Martel, É.; Robert, É.; Lerondel, S.; Trichet, V.; Richard, S.; Pouvesle, J.-M.; Le Pape, A. Effects of a Non Thermal Plasma Treatment Alone or in Combination with Gemcitabine in a MIA PaCa2-luc Orthotopic Pancreatic Carcinoma Model. PLoS ONE 2012, 7, e52653. [Google Scholar] [CrossRef] [PubMed]
- Semmler, M.L.; Bekeschus, S.; Schäfer, M.; Bernhardt, T.; Fischer, T.; Witzke, K.; Seebauer, C.; Rebl, H.; Grambow, E.; Vollmar, B.; et al. Molecular Mechanisms of the Efficacy of Cold Atmospheric Pressure Plasma (CAP) in Cancer Treatment. Cancers 2020, 12, 269. [Google Scholar] [CrossRef] [PubMed]
- Lademann, J.; Richter, H.; Alborova, A.; Humme, D.; Patzelt, A.; Kramer, A.; Weltmann, K.-D.; Hartmann, B.; Ottomann, C.; Fluhr, J.W.; et al. Risk assessment of the application of a plasma jet in dermatology. J. Biomed. Opt. 2009, 14, 054025. [Google Scholar] [CrossRef] [PubMed]
- Heinlin, J.; Isbary, G.; Stolz, W.; Morfill, G.; Landthaler, M.; Shimizu, T.; Steffes, B.; Nosenko, T.; Zimmermann, J.; Karrer, S. Plasma applications in medicine with a special focus on dermatology. J. Eur. Acad. Dermatol. Venereol. 2010, 25, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Bekeschus, S.; Schmidt, A.; Weltmann, K.-D.; Von Woedtke, T. The plasma jet kINPen – A powerful tool for wound healing. Clin. Plasma Med. 2016, 4, 19–28. [Google Scholar] [CrossRef]
- Recio, A.C.; Felter, C.E.; Schneider, A.C.; McDonald, J.W. High-voltage electrical stimulation for the management of Stage III and IV pressure ulcers among adults with spinal cord injury: Demonstration of its utility for recalcitrant wounds below the level of injury. J. Spinal Cord Med. 2012, 35, 58–63. [Google Scholar] [CrossRef][Green Version]
- Houghton, P.E.; Campbell, K.E.; Fraser, C.H.; Harris, C.; Keast, D.H.; Potter, P.J.; Hayes, K.C.; Woodbury, M.G. Electrical Stimulation Therapy Increases Rate of Healing of Pressure Ulcers in Community-Dwelling People With Spinal Cord Injury. Arch. Phys. Med. Rehabil. 2010, 91, 669–678. [Google Scholar] [CrossRef]
- Gardner, S.E.; Frantz, R.A.; Schmidt, F.L. Effect of electrical stimulation on chronic wound healing: A meta-analysis. Wound Repair Regen. 1999, 7, 495–503. [Google Scholar] [CrossRef]
- Cho, M.R.; Thatte, H.S.; Lee, R.C.; Golan, D.E. Integrin-dependent human macrophage migration induced by oscillatory electrical stimulation. Ann. Biomed. Eng. 2000, 28, 234–243. [Google Scholar] [CrossRef]
- Goldman, R.; Pollack, S. Electric fields and proliferation in a chronic wound model. Bioelectromagnetics 1996, 17, 450–457. [Google Scholar] [CrossRef]
- Pullar, C.E.; Baier, B.S.; Kariya, Y.; Russell, A.J.; Horst, B.A.; Marinkovich, M.P.; Isseroff, R.R. β4 Integrin and Epidermal Growth Factor Coordinately Regulate Electric Field-mediated Directional Migration via Rac1. Mol. Boil. Cell 2006, 17, 4925–4935. [Google Scholar] [CrossRef] [PubMed]
- Daeschlein, G.; Assadian, O.; Kloth, L.C.; Meinl, C.; Ney, F.; Kramer, A. Antibacterial activity of positive and negative polarity low-voltage pulsed current (LVPC) on six typical Gram-positive and Gram-negative bacterial pathogens of chronic wounds. Wound Repair Regen. 2007, 15, 399–403. [Google Scholar] [CrossRef] [PubMed]
- Wolff, C.M.; Steuer, A.; Stoffels, I.; Von Woedtke, T.; Weltmann, K.-D.; Bekeschus, S.; Kolb, J.F. Combination of cold plasma and pulsed electric fields – A rationale for cancer patients in palliative care. Clin. Plasma Med. 2019, 16, 100096. [Google Scholar] [CrossRef]
- Von Woedtke, T.; Schmidt, A.; Bekeschus, S.; Wende, K.; Weltmann, K.-D. Plasma Medicine: A Field of Applied Redox Biology. In Vivo 2019, 33, 1011–1026. [Google Scholar] [CrossRef]
- Roy, J.; Galano, J.-M.; Durand, T.; Le Guennec, J.-Y.; Lee, J.C.-Y. Physiological role of reactive oxygen species as promoters of natural defenses. FASEB J. 2017, 31, 3729–3745. [Google Scholar] [CrossRef] [PubMed]
- Dröge, W. Free Radicals in the Physiological Control of Cell Function. Physiol. Rev. 2002, 82, 47–95. [Google Scholar] [CrossRef]
- Graves, D.B. The emerging role of reactive oxygen and nitrogen species in redox biology and some implications for plasma applications to medicine and biology. J. Phys. D Appl. Phys. 2012, 45, 263001. [Google Scholar] [CrossRef]
- Broughton, G.; Janis, J.E.; Attinger, C.E. The Basic Science of Wound Healing. Plast. Reconstr. Surg. 2006, 117, 12S–34S. [Google Scholar] [CrossRef]
- Pastar, I.; Stojadinovic, O.; Yin, N.C.; Ramírez, H.; Nusbaum, A.G.; Sawaya, A.; Patel, S.B.; Khalid, L.; Isseroff, R.R.; Tomic-Canic, M. Epithelialization in Wound Healing: A Comprehensive Review. Adv. Wound Care 2014, 3, 445–464. [Google Scholar] [CrossRef]
- Soneja, A.; Drews, M.; Malinski, T. Role of nitric oxide, nitroxidative and oxidative stress in wound healing. Pharmacol. Rep. 2005, 57, 108–119. [Google Scholar]
- Nathan, C.; Ding, A. Nonresolving Inflammation. Cell 2010, 140, 871–882. [Google Scholar] [CrossRef] [PubMed]
- Thomas, D.D.; Ridnour, L.A.; Isenberg, J.S.; Flores-Santana, W.; Switzer, C.H.; Donzelli, S.; Hussain, P.; Vecoli, C.; Paolocci, N.; Ambs, S.; et al. The chemical biology of nitric oxide: Implications in cellular signaling. Free. Radic. Biol. Med. 2008, 45, 18–31. [Google Scholar] [CrossRef] [PubMed]
- Sen, C.K.; Roy, S. Redox signals in wound healing. Biochim. et Biophys. Acta Gen. Subj. 2008, 1780, 1348–1361. [Google Scholar] [CrossRef] [PubMed]
- Witte, M.B.; Barbul, A. Role of nitric oxide in wound repair. Am. J. Surg. 2002, 183, 406–412. [Google Scholar] [CrossRef]
- Luo, J.-D.; Chen, A.F. Nitric oxide: A newly discovered function on wound healing. Acta Pharmacol. Sin. 2005, 26, 259–264. [Google Scholar] [CrossRef]
- Roy, S.; Khanna, S.; Nallu, K.; Hunt, T.K.; Sen, C.K. Dermal Wound Healing Is Subject to Redox Control. Mol. Ther. 2006, 13, 211–220. [Google Scholar] [CrossRef]
- Schmidt, A.; Bekeschus, S. Redox for Repair: Cold Physical Plasmas and Nrf2 Signaling Promoting Wound Healing. Antioxidants 2018, 7, 146. [Google Scholar] [CrossRef]
- Schmidt, A.; Wende, K.; Bekeschus, S.; Bundscherer, L.; Barton, A.; Ottmüller, K.; Weltmann, K.-D.; Masur, K. Non-thermal plasma treatment is associated with changes in transcriptome of human epithelial skin cells. Free. Radic. Res. 2013, 47, 577–592. [Google Scholar] [CrossRef]
- Arndt, S.; Unger, P.; Wacker, E.; Shimizu, T.; Heinlin, J.; Li, Y.-F.; Thomas, H.M.; Morfill, G.E.; Zimmermann, J.L.; Bosserhoff, A.-K.; et al. Cold Atmospheric Plasma (CAP) Changes Gene Expression of Key Molecules of the Wound Healing Machinery and Improves Wound Healing In Vitro and In Vivo. PLoS ONE 2013, 8, e79325. [Google Scholar] [CrossRef]
- Arndt, S.; Landthaler, M.; Zimmermann, J.L.; Unger, P.; Wacker, E.; Shimizu, T.; Li, Y.-F.; Morfill, G.E.; Bosserhoff, A.-K.; Karrer, S. Effects of Cold Atmospheric Plasma (CAP) on ß-Defensins, Inflammatory Cytokines, and Apoptosis-Related Molecules in Keratinocytes In Vitro and In Vivo. PLoS ONE 2015, 10, e0120041. [Google Scholar] [CrossRef]
- Duchesne, C.; Banzet, S.; Lataillade, J.; Rousseau, A.; Frescaline, N. Cold atmospheric plasma modulates endothelial nitric oxide synthase signalling and enhances burn wound neovascularisation. J. Pathol. 2019, 249, 368–380. [Google Scholar] [CrossRef] [PubMed]
- Arndt, S.; Unger, P.; Berneburg, M.; Bosserhoff, A.-K.; Karrer, S. Cold atmospheric plasma (CAP) activates angiogenesis-related molecules in skin keratinocytes, fibroblasts and endothelial cells and improves wound angiogenesis in an autocrine and paracrine mode. J. Dermatol. Sci. 2018, 89, 181–190. [Google Scholar] [CrossRef] [PubMed]
- Arjunan, K.P.; Clyne, A.M. A Nitric Oxide Producing Pin-to-Hole Spark Discharge Plasma Enhances Endothelial Cell Proliferation and Migration. Plasma Med. 2011, 1, 279–293. [Google Scholar] [CrossRef]
- Kalghatgi, S.; Friedman, G.; Fridman, A.; Clyne, A.M. Endothelial Cell Proliferation is Enhanced by Low Dose Non-Thermal Plasma Through Fibroblast Growth Factor-2 Release. Ann. Biomed. Eng. 2009, 38, 748–757. [Google Scholar] [CrossRef] [PubMed]
- Shome, D.; Von Woedtke, T.; Riedel, K.; Masur, K. The HIPPO Transducer YAP and Its Targets CTGF and Cyr61 Drive a Paracrine Signalling in Cold Atmospheric Plasma-Mediated Wound Healing. Oxidative Med. Cell. Longev. 2020, 2020, 4910280. [Google Scholar] [CrossRef]
- Schmidt, A.; Dietrich, S.; Steuer, A.; Weltmann, K.-D.; Von Woedtke, T.; Masur, K.; Wende, K. Non-thermal Plasma Activates Human Keratinocytes by Stimulation of Antioxidant and Phase II Pathways. J. Boil. Chem. 2015, 290, 6731–6750. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, A.; Bekeschus, S.; Jablonowski, H.; Barton, A.; Weltmann, K.-D.; Wende, K. Role of Ambient Gas Composition on Cold Physical Plasma-Elicited Cell Signaling in Keratinocytes. Biophys. J. 2017, 112, 2397–2407. [Google Scholar] [CrossRef]
- Schmidt, A.; Von Woedtke, T.; Bekeschus, S. Periodic Exposure of Keratinocytes to Cold Physical Plasma: An In Vitro Model for Redox-Related Diseases of the Skin. Oxidative Med. Cell. Longev. 2016, 2016, 1–17. [Google Scholar] [CrossRef]
- Schmidt, A.; Bekeschus, S.; Wende, K.; Vollmar, B.; Von Woedtke, T. A cold plasma jet accelerates wound healing in a murine model of full-thickness skin wounds. Exp. Dermatol. 2017, 26, 156–162. [Google Scholar] [CrossRef]
- Lou, B.-S.; Hsieh, J.-H.; Chen, C.-M.; Hou, C.-W.; Wu, H.-Y.; Chou, P.-Y.; Lai, C.-H.; Lee, J.-W. Helium/Argon-Generated Cold Atmospheric Plasma Facilitates Cutaneous Wound Healing. Front. Bioeng. Biotechnol. 2020, 8, 683. [Google Scholar] [CrossRef]
- Bekeschus, S.; Schmidt, A.; Niessner, F.; Gerling, T.; Weltmann, K.-D.; Wende, K. Basic Research in Plasma Medicine—A Throughput Approach from Liquids to Cells. J. Vis. Exp. 2017, e56331. [Google Scholar] [CrossRef] [PubMed]
- Sies, H. On the history of oxidative stress: Concept and some aspects of current development. Curr. Opin. Toxicol. 2018, 7, 122–126. [Google Scholar] [CrossRef]
- Holmstrom, K.; Finkel, T. Cellular mechanisms and physiological consequences of redox-dependent signalling. Nat. Rev. Mol. Cell Boil. 2014, 15, 411–421. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, A.; Von Woedtke, T.; Vollmar, B.; Hasse, S.; Bekeschus, S. Nrf2 signaling and inflammation are key events in physical plasma-spurred wound healing. Theranostics 2019, 9, 1066–1084. [Google Scholar] [CrossRef]
- Pan, J.-S.; Hong, M.-Z.; Ren, J.-L. Reactive oxygen species: A double-edged sword in oncogenesis. World J. Gastroenterol. 2009, 15, 1702–1707. [Google Scholar] [CrossRef]
- Glasauer, A.; Chandel, N.S. Targeting antioxidants for cancer therapy. Biochem. Pharmacol. 2014, 92, 90–101. [Google Scholar] [CrossRef]
- Hole, P.S.; Zabkiewicz, J.; Munje, C.; Newton, Z.; Pearn, L.; White, P.; Marquez, N.; Hills, R.; Burnett, A.K.; Tonks, A.; et al. Overproduction of NOX-derived ROS in AML promotes proliferation and is associated with defective oxidative stress signaling. Blood 2013, 122, 3322–3330. [Google Scholar] [CrossRef]
- Kong, Q.; Beel, J.; Lillehei, K. A threshold concept for cancer therapy. Med. Hypotheses 2000, 55, 29–35. [Google Scholar] [CrossRef]
- Fridman, G.; Shereshevsky, A.; Jost, M.M.; Brooks, A.D.; Fridman, A.; Gutsol, A.; Vasilets, V.N.; Friedman, G. Floating Electrode Dielectric Barrier Discharge Plasma in Air Promoting Apoptotic Behavior in Melanoma Skin Cancer Cell Lines. Plasma Chem. Plasma Process. 2007, 27, 163–176. [Google Scholar] [CrossRef]
- Lupu, A.-R.; Georgescu, N. Cold atmospheric plasma jet effects on V79-4 cells. Roum. Arch. Microbiol. Immunol. 2011, 69, 67–74. [Google Scholar]
- Regulski, M.J. Cellular Senescence: What, Why, and How. Wounds 2017, 29, 168–174. [Google Scholar] [PubMed]
- Arndt, S.; Wacker, E.; Li, Y.-F.; Shimizu, T.; Thomas, H.M.; Morfill, G.E.; Karrer, S.; Zimmermann, J.L.; Bosserhoff, A.-K. Cold atmospheric plasma, a new strategy to induce senescence in melanoma cells. Exp. Dermatol. 2013, 22, 284–289. [Google Scholar] [CrossRef] [PubMed]
- Schneider, C.; Gebhardt, L.; Arndt, S.; Karrer, S.; Zimmermann, J.L.; Fischer, M.J.M.; Bosserhoff, A.-K. Cold atmospheric plasma causes a calcium influx in melanoma cells triggering CAP-induced senescence. Sci. Rep. 2018, 8, 10048. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Wang, X.; Vikash, V.; Ye, Q.; Wu, D.; Liu, Y.; Dong, W. ROS and ROS-Mediated Cellular Signaling. Oxidative Med. Cell. Longev. 2016, 2016, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Mitra, S.; Nguyen, L.N.; Akter, M.; Park, G.; Choi, E.; Choi, E. Impact of ROS Generated by Chemical, Physical, and Plasma Techniques on Cancer Attenuation. Cancers 2019, 11, 1030. [Google Scholar] [CrossRef]
- Kolev, K.; Skopál, J.; Simon, L.; Csonka, É.; Nagy, Z.; Machovich, R. Matrix metalloproteinase-9 expression in post-hypoxic human brain capillary endothelial cells: H2O2 as a trigger and NF-κB as a signal transducer. Thromb. Haemost. 2003, 90, 528–537. [Google Scholar] [CrossRef]
- Tobar, N.; Villar, V.; Santibañez, J.F. ROS-NFκΒ mediates TGF-β1-induced expression of urokinase-type plasminogen activator, matrix metalloproteinase-9 and cell invasion. Mol. Cell. Biochem. 2010, 340, 195–202. [Google Scholar] [CrossRef]
- Van Der Heiden, K.; Cuhlmann, S.; Luong, L.A.; Zakkar, M.; Evans, P.C. Role of nuclear factor κB in cardiovascular health and disease. Clin. Sci. 2010, 118, 593–605. [Google Scholar] [CrossRef]
- Jansen, P.L.; Rosch, R.; Jansen, M.; Binnebösel, M.; Junge, K.; Alfonso-Jaume, A.; Klinge, U.; Lovett, D.H.; Mertens, P.R. Regulation of MMP-2 Gene Transcription in Dermal Wounds. J. Investig. Dermatol. 2007, 127, 1762–1767. [Google Scholar] [CrossRef]
- Montesinos, M.C.; Desai-Merchant, A.; Cronstein, B.N. Promotion of Wound Healing by an Agonist of Adenosine A2A Receptor Is Dependent on Tissue Plasminogen Activator. Inflammation 2015, 38, 2036–2041. [Google Scholar] [CrossRef]
- Kim, C.H.; Lee, J.H.; Won, J.-H.; Cho, M.K. Mesenchymal Stem Cells Improve Wound Healing In Vivo via Early Activation of Matrix Metalloproteinase-9 and Vascular Endothelial Growth Factor. J. Korean Med Sci. 2011, 26, 726–733. [Google Scholar] [CrossRef] [PubMed]
- Zou, Z.; Zhang, Y.; Hao, L.; Wang, F.; Liu, D.; Su, Y.; Sun, H. More insight into mesenchymal stem cells and their effects inside the body. Expert Opin. Boil. Ther. 2010, 10, 215–230. [Google Scholar] [CrossRef] [PubMed]
- Amar, S.; Smith, L.; Fields, G.B. Matrix metalloproteinase collagenolysis in health and disease. Biochim. Biophys. Acta Bioenerg 2017, 1864, 1940–1951. [Google Scholar] [CrossRef] [PubMed]
- Dreymüller, D.; Theodorou, K.; Donners, M.M.; Ludwig, A. Fine Tuning Cell Migration by a Disintegrin and Metalloproteinases. Mediat. Inflamm. 2017, 2017, 1–22. [Google Scholar] [CrossRef]
- Yan, D.; Xiao, H.; Zhu, W.; Nourmohammadi, N.; Zhang, L.G.; Bian, K.; Keidar, M. The role of aquaporins in the anti-glioblastoma capacity of the cold plasma-stimulated medium. J. Phys. D Appl. Phys. 2017, 50, 055401. [Google Scholar] [CrossRef]
- Van Der Paal, J.; Neyts, E.C.; Verlackt, C.C.W.; Bogaerts, A. Effect of lipid peroxidation on membrane permeability of cancer and normal cells subjected to oxidative stress† †Electronic supplementary information (ESI) available. See doi:10.1039/c5sc02311d Click here for additional data file. Chem. Sci. 2015, 7, 489–498. [Google Scholar] [CrossRef] [PubMed]
- Wolff, C.M.; Kolb, J.F.; Weltmann, K.-D.; Von Woedtke, T.; Bekeschus, S. Combination Treatment with Cold Physical Plasma and Pulsed Electric Fields Augments ROS Production and Cytotoxicity in Lymphoma. Cancers 2020, 12, 845. [Google Scholar] [CrossRef]
- Schmidt, A.; Von Woedtke, T.; Stenzel, J.; Lindner, T.; Polei, S.; Vollmar, B.; Bekeschus, S. One Year Follow-Up Risk Assessment in SKH-1 Mice and Wounds Treated with an Argon Plasma Jet. Int. J. Mol. Sci. 2017, 18, 868. [Google Scholar] [CrossRef]
- Wende, K.; Bekeschus, S.; Schmidt, A.; Jatsch, L.; Hasse, S.; Weltmann, K.; Masur, K.; Von Woedtke, T. Risk assessment of a cold argon plasma jet in respect to its mutagenicity. Mutat. Res. Toxicol. Environ. Mutagen. 2016, 798, 48–54. [Google Scholar] [CrossRef]
| Plasma Source | Treatment of | Number of Participants | Reference (Year) |
|---|---|---|---|
| MicroPlaSter α | Chronic wounds | 36 | [6] (2010) |
| CPC 1500 System (jet) | Pleural mesothelioma | 8 | [20] (2010) |
| MicroPlaSter α/β | Chronic wounds | 24 | [7] (2012) |
| kINPen | Acute wounds | 5 | [13] (2012) |
| MicroPlaSter α/β | Chronic wounds | 70 | [10] (2013) |
| MicroPlaSter β | Acute wounds | 34 | [16] (2013) |
| kINPen | Acute wounds | 6 | [15] (2014) |
| kINPen | Chronic wounds | 34 | [8] (2015) |
| PlasmaDerm | Chronic wounds | 14 | [11] (2015) |
| kINPen | Chronic wounds | 16 | [9] (2015) |
| kINPen | Advanced squamous cell carcinoma of the head and neck | 12 | [21] (2015) |
| BIOPlasma jet | Chronic wounds | 50 | [12] (2016) |
| kINPen | Advanced squamous cell carcinoma of the head and neck | Group I: 12 Group II: 9 | [22] (2016) |
| Custom-made device with hand-held electrode (FPG10-01NM10) | Actinic keratosis | 5 | [23] (2017) |
| kINPen | Locally advanced head and neck cancers | 6 | [24] (2018) |
| Adtec Steri-Plas | Actinic keratosis | 7 | [25] (2018) |
| kINPen | Chronic wounds | 45 | [18] (2020) |
| plasma jet | Chronic wounds | 44 | [19] (2020) |
| Reference | Device | Type | Feed Gas | Treatment Time | Repetition | Distance | slm |
|---|---|---|---|---|---|---|---|
| [6] | MicroPlaSter α | Torch | Argon | 5 min | daily (on average 7.86 treatments) | 20 mm | 2.2 |
| [20] | CPC 1500 System (jet) | Jet | Helium | 15–46 min | once | ||
| [7] | MicroPlaSter α/β | Torch | Argon | 2 min | daily (on average 11.75 treatments) | 2.2 | |
| [13] | kINPen MED | Jet | Argon | 10 s, 30 s, or 3×10 s | once | 10 mm | |
| [10] | MicroPlaSter α/β | Torch | Argon | 3–7 min | daily (on average 7.90 treatments) | 20 mm | 2.2 |
| [16] | MicroPlaSter β | Torch | Argon | 2 min | daily except for weekend | 20 mm | 2.2 |
| [15] | kINPen MED | Jet | Argon | 1 min (8 mm/s) | once | 10 mm | 5.0 |
| [8] | kINPen 09 | Jet | Argon | 1 min/cm | once | 7–8 mm | 5.0 |
| [11] | PlasmaDerm | DBD | n/a (air) | 45 s/cm2 | 3 × week for 8 weeks | n/a | |
| [9] | kINPen MED | Jet | Argon | 1 min/cm | 3 × week for 2 weeks | 7–8 mm | 5.0 |
| [21] | kINPen MED | Jet | Argon | 1 min/cm2 | 3 × week for 1 week | 8 mm | between 3–6 |
| [12] | BIOPlasma jet | Jet | Argon | 1 min/cm2 | 1 × week for 8 weeks | 1–3 mm | |
| [22] | kINPen MED | Jet | Argon | Group I: 1 min spot exposure Group II: 3 min spot exposure | Group I: 3 × week for 1 weeks Group II: once | 8 mm | |
| [23] | Custom-made device with hand-held electrode (FPG10-01NM10) | FE-DBD | n/a (air) | 1–2 min | once | 2.7 mm | n/a |
| [24] | kINPen MED | Jet | Argon | 1 min/cm2 | 3 × week for 1 week followed by an intermittence of 1 week without CAP | 8 mm | 5.0 |
| [25] | Adtec Steri-Plas | Jet | 2 min | 2 × week (7 treatments) | |||
| [18] | kINPen MED | Jet | Argon | 30 s/cm2 | 5 × daily, then 3 × every second day | ||
| [19] | plasma jet | Jet | Helium | 5 min | 3 × week for 3 weeks | 10 mm |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boeckmann, L.; Schäfer, M.; Bernhardt, T.; Semmler, M.L.; Jung, O.; Ojak, G.; Fischer, T.; Peters, K.; Nebe, B.; Müller-Hilke, B.; et al. Cold Atmospheric Pressure Plasma in Wound Healing and Cancer Treatment. Appl. Sci. 2020, 10, 6898. https://doi.org/10.3390/app10196898
Boeckmann L, Schäfer M, Bernhardt T, Semmler ML, Jung O, Ojak G, Fischer T, Peters K, Nebe B, Müller-Hilke B, et al. Cold Atmospheric Pressure Plasma in Wound Healing and Cancer Treatment. Applied Sciences. 2020; 10(19):6898. https://doi.org/10.3390/app10196898
Chicago/Turabian StyleBoeckmann, Lars, Mirijam Schäfer, Thoralf Bernhardt, Marie Luise Semmler, Ole Jung, Gregor Ojak, Tobias Fischer, Kirsten Peters, Barbara Nebe, Brigitte Müller-Hilke, and et al. 2020. "Cold Atmospheric Pressure Plasma in Wound Healing and Cancer Treatment" Applied Sciences 10, no. 19: 6898. https://doi.org/10.3390/app10196898
APA StyleBoeckmann, L., Schäfer, M., Bernhardt, T., Semmler, M. L., Jung, O., Ojak, G., Fischer, T., Peters, K., Nebe, B., Müller-Hilke, B., Seebauer, C., Bekeschus, S., & Emmert, S. (2020). Cold Atmospheric Pressure Plasma in Wound Healing and Cancer Treatment. Applied Sciences, 10(19), 6898. https://doi.org/10.3390/app10196898









