Potential Use of Environmental Biological Samples for Retrospective Electron Paramagnetic Resonance Dosimetry of Radiation Accidents
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Biological Sample
2.2. EPR Measurement
2.3. Experimental Design and Sample Irradiation
3. Results
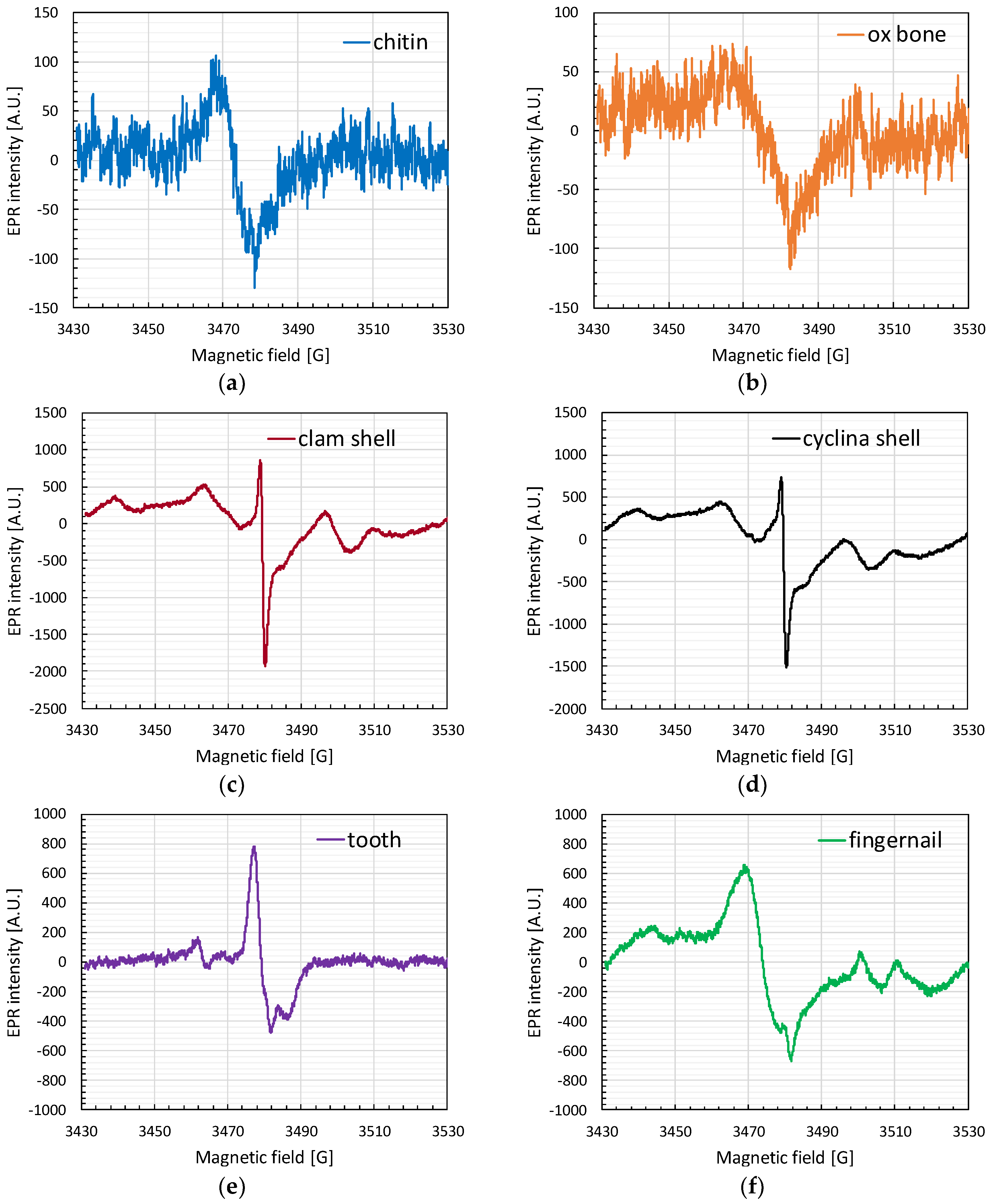
3.1. EPR Spectra
3.2. Dose-Response Curve
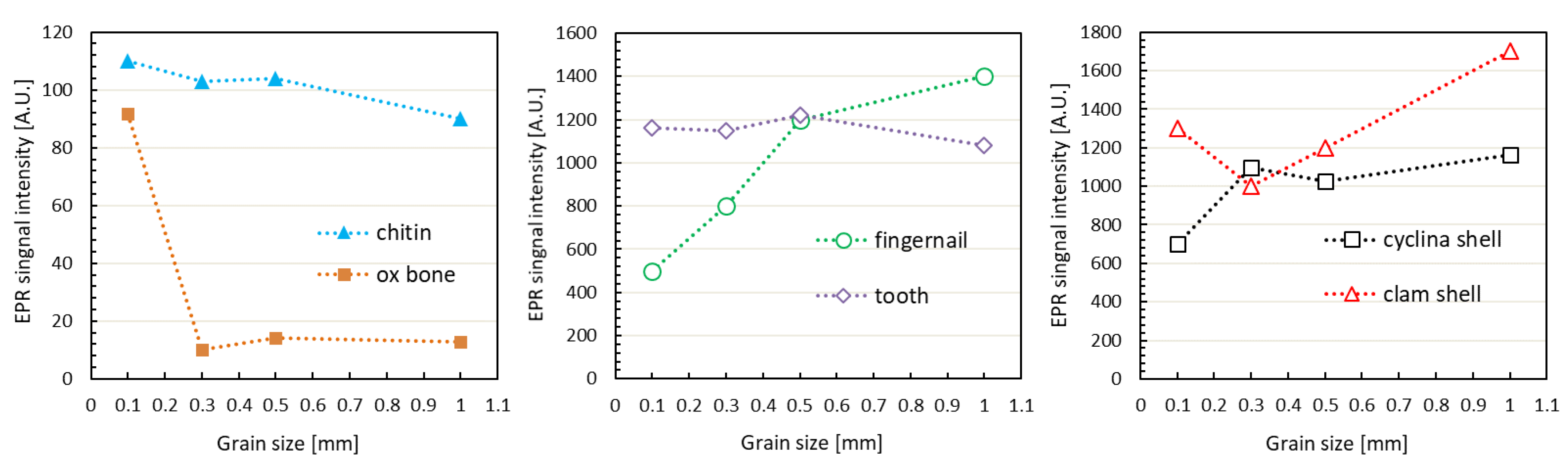
3.3. Grain Size
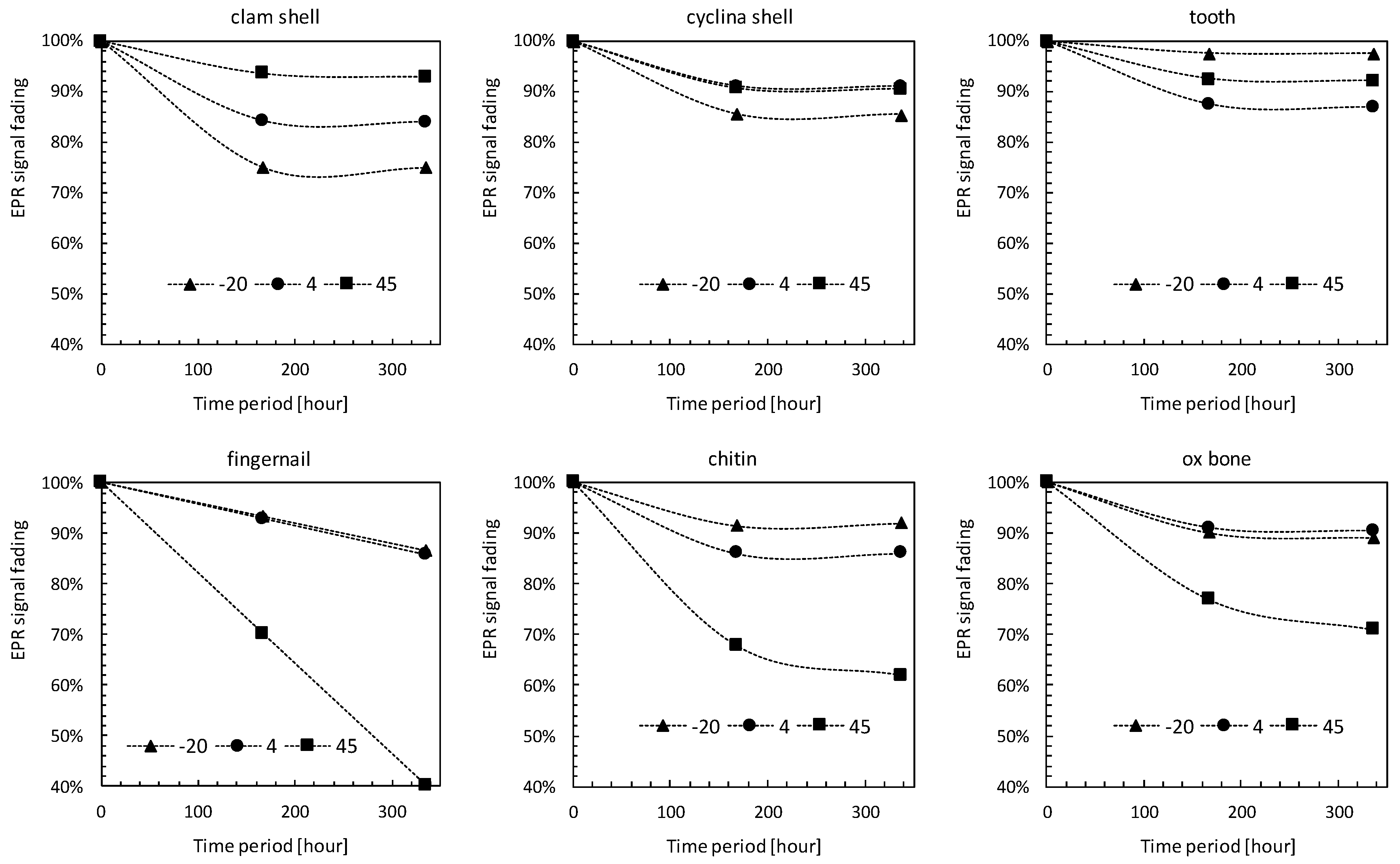
3.4. Storage Temperature
4. Discussion and Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Liu, C.Y.; Lai, L.H.; Wang, C.F.; Chuang, K.S.; Lu, C.C.; Lin, J.P.; Lin, H.H. Establishment of conversion coefficient of whole body effective dose by human tissue of electron paramagnetic resonance (EPR). Radiat. Phys. Chem. 2019, 155, 82–88. [Google Scholar] [CrossRef]
- Desrosiers, M.F.; Puhl, J.M. Absorbed-dose/dose-rate dependence studies for the alanine-EPR dosimetry system. Radiat. Phys. Chem. 2009, 78, 461–463. [Google Scholar] [CrossRef]
- Ikeya, M.; Miyajima, J.; Okajima, S. ESR dosimetry for atomic bomb survivors using shell buttons and tooth enamel. Jpn. J. Appl. Phys. 1984, 23, L697. [Google Scholar] [CrossRef]
- Sudprasert, W.; Monthonwattana, S.; Vitittheeranon, A. Identification of irradiated rice noodles by electron spin resonance spectroscopy. Radiat. Meas. 2012, 47, 640–643. [Google Scholar] [CrossRef]
- Trompier, F.; Kornak, L.; Calas, C.; Romanyukha, A.; LeBlanc, B.; Mitchell, C.A.; Swartz, H.M.; Clairand, I. Protocol for emergency EPR dosimetry in fingernails. Radiat. Meas. 2007, 42, 1085–1088. [Google Scholar] [CrossRef][Green Version]
- Yordanov, N.D.; Aleksieva, K. Preparation and applicability of fresh fruit samples for the identification of radiation treatment by EPR. Radiat. Phys. Chem. 2009, 78, 213–216. [Google Scholar] [CrossRef]
- Yordanov, N.D.; Mladenova, B. EPR studies on gamma-irradiated snails hard tissues. Radiat. Phys. Chem. 2001, 60, 191–193. [Google Scholar] [CrossRef]
- Soliman, Y.; Ali, L.I.; Moustafa, H.; Tadros, S.M. EPR dosimetric properties of 2-methylalanine pellet for radiation processing application. Radiat. Phys. Chem. 2014, 102, 11–15. [Google Scholar] [CrossRef]
- Desrosiers, M.F. gamma.Irradiated seafoods: Identification and dosimetry by electron paramagnetic resonance spectroscopy. J. Agric. Food Chem. 1989, 37, 96–100. [Google Scholar] [CrossRef]
- Stachowicz, W.; Burlinska, G.; Michalik, J. EPR detection of foods preserved with ionizing radiation. Radiat. Phys. Chem. 1998, 52, 157–160. [Google Scholar] [CrossRef]
- Anton, M. Uncertainties in alanine/ESR dosimetry at the Physikalisch-Technische Bundesanstalt. Phys. Med. Biol. 2006, 51, 5419–5440. [Google Scholar] [CrossRef] [PubMed]
- Jacob, P.; Bailiff, I.K.; Balonov, M.A.; Bauchinger, M.; Bouville, A.; Haskell, E.; Nakamura, N.; Romanyukha, A. Retrospective assessment of exposures to ionising radiation: Abstract. ICRU 2002, 2, 9. [Google Scholar] [CrossRef]
- Cano, N.F.; Munita, C.S.; Watanabe, S.; Barbosa, R.F.; Chubaci, J.F.; Tatumi, S.H.; Neves, E.G. OSL and EPR dating of pottery from the archaeological sites in Amazon Valley, Brazil. Quat. Int. 2014, 352, 176–180. [Google Scholar] [CrossRef]
- Egersdörfer, S.; Wieser, A.; Müller, A. Tooth enamel as a detector material for retrospective EPR dosimetry. Appl. Radiat. Isot. 1996, 47, 1299–1303. [Google Scholar] [CrossRef]
- Fattibene, P.; Callens, F. EPR dosimetry with tooth enamel: A review. Appl. Radiat. Isot. 2010, 68, 2033–2116. [Google Scholar] [CrossRef]
- Ivannikov, A.I.; Sanin, D.; Nalapko, M.; Skvortsov, V.F.; Stepanenko, V.F.; Tsyb, A.F.; Trompier, F.; Zhumadilov, K.; Hoshi, M. Dental enamel EPR dosimetry: Comparative testing of the spectra processing methods for determination of radiation-induced signal amplitude. Health Phys. 2010, 98, 345–351. [Google Scholar] [CrossRef]
- Chandra, H.; Symons, M.C. Sulphur radicals formed by cutting α-keratin. Nature 1987, 328, 833–834. [Google Scholar] [CrossRef]
- Sholom, S.; McKeever, S. Stability of X-band EPR signals from fingernails under vacuum storage. Radiat. Phys. Chem. 2017, 141, 78–87. [Google Scholar] [CrossRef]
- Chumak, V.; Bailiff, I.; Baran, N.; Bugai, A.; Dubovsky, S.; Fedosov, I.; Finin, V.; Haskell, E.; Hayes, R.; Ivannikov, A.; et al. The first international intercomparison of EPR-dosimetry with teeth: First results. Appl. Radiat. Isot. 1996, 47, 1281–1286. [Google Scholar] [CrossRef]
- Haskell, E.H.; Hayes, R.B.; Kenner, G.H.; Wieser, A.; Aragno, D.; Fattibene, P.; Onori, S. Achievable precision and accuracy in EPR dosimetry of tooth enamel. Radiat. Prot. Dosim. 1999, 84, 527–535. [Google Scholar] [CrossRef]
- Swartz, H.M.; Flood, A.B.; Williams, B.B.; Dong, R.; Swarts, S.G.; He, X.; Grinberg, O.; Sidabras, J.; Demidenko, E.; Gui, J.; et al. Electron paramagnetic resonance dosimetry for a large-scale radiation incident. Health Phys. 2012, 103, 255–267. [Google Scholar] [CrossRef] [PubMed]
- Symons, M.C.R.; Chandra, H.; Wyatt, J.L. Electron paramagnetic resonance spectra of irradiated finger-nails: A possible measure of accidental exposure. Radiat. Prot. Dosim. 1995, 58, 11–15. [Google Scholar]
- Sadło, J.; Michalik, J.; Stachowicz, W.; Strzelczak, G.; Dziedzic-Gocławska, A.; Ostrowski, K. EPR study on biominerals as materials for retrospective dosimetry. Nukleonika 2006, 51, 95–100. [Google Scholar]
- Trompier, F.; Romanyukha, A.; Reyes, R.; Vezin, H.; Queinnec, F.; Gourier, D. State of the art in nail dosimetry: Free radicals identification and reaction mechanisms. Biophysik 2014, 53, 291–303. [Google Scholar] [CrossRef]
- Hong, D.; Lee, K. Grain size effect of tooth enamel to electron paramagnetic resonance spectrum. Nucl. Sci. Technol. 2004, 41, 200–202. [Google Scholar]
- Liu, C.R.; Yin, G.M.; Han, F. Effects of grain size on quartz ESR dating of Ti–Li center in fluvial and lacustrine sediments. Quat. Geochronol. 2015, 30, 513–518. [Google Scholar] [CrossRef]
- Romanyukha, A.; Trompier, F.; LeBlanc, B.; Calas, C.; Clairand, I.; Mitchell, C.A.; Smirniotopoulos, J.G.; Swartz, H.M. EPR dosimetry in chemically treated fingernails. Radiat. Meas. 2007, 42, 1110–1113. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Blanchard, S.C.; Chasteen, N.D. Electron paramagnetic resonance spectrum of a sea shell. Mytilus edulis. J. Phys. Chem. 1976, 80, 1362–1367. [Google Scholar] [CrossRef]
- Viscomi, D.; Fattibene, P. Radiation-induced signals analysed by EPR spectrometry applied to fortuitous dosimetry. Annali dell’Istituto Superiore di Sanità 2009, 45, 287–296. [Google Scholar]
- Bougai, A.; Brik, A.; Chumak, V.; Desrosiers, M.; Dubovski, S.; Fattibene, P.; Romanyukha, A. Use of Electron Paramagnetic Resonance Dosimetry with Tooth Enamel for Retrospective Dose Assessment; TECDOC-1331; International Atomic Energy Agency: Vienna, Austria, 2002. [Google Scholar]


© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lu, C.-C.; Lin, H.-H.; Hsu, C.-H.; Wang, F.-N.; Lin, J.-P.; Lai, L.-H. Potential Use of Environmental Biological Samples for Retrospective Electron Paramagnetic Resonance Dosimetry of Radiation Accidents. Appl. Sci. 2020, 10, 6867. https://doi.org/10.3390/app10196867
Lu C-C, Lin H-H, Hsu C-H, Wang F-N, Lin J-P, Lai L-H. Potential Use of Environmental Biological Samples for Retrospective Electron Paramagnetic Resonance Dosimetry of Radiation Accidents. Applied Sciences. 2020; 10(19):6867. https://doi.org/10.3390/app10196867
Chicago/Turabian StyleLu, Chia-Chun, Hsin-Hon Lin, Ching-Han Hsu, Fu-Nien Wang, Jao-Perng Lin, and Lu-Han Lai. 2020. "Potential Use of Environmental Biological Samples for Retrospective Electron Paramagnetic Resonance Dosimetry of Radiation Accidents" Applied Sciences 10, no. 19: 6867. https://doi.org/10.3390/app10196867
APA StyleLu, C.-C., Lin, H.-H., Hsu, C.-H., Wang, F.-N., Lin, J.-P., & Lai, L.-H. (2020). Potential Use of Environmental Biological Samples for Retrospective Electron Paramagnetic Resonance Dosimetry of Radiation Accidents. Applied Sciences, 10(19), 6867. https://doi.org/10.3390/app10196867




