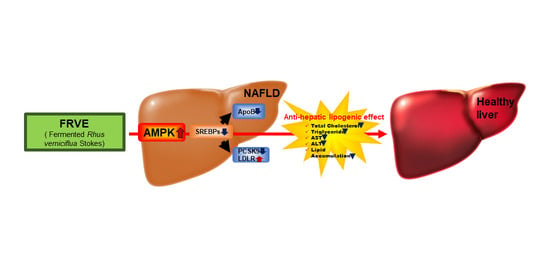
Fermented Rhus Verniciflua Stokes Extract Alleviates Nonalcoholic Fatty Liver through the AMPK/SREBP1/PCSK9 Pathway in HFD-Induced Nonalcoholic Fatty Liver Animal Model
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of FRVE
2.2. High-Performance Liquid Chromatography
2.3. Ethics Statement
2.4. Animal Experiment
2.5. Biochemical Analyses
2.6. Histological Examination
2.7. Oil Red O Staining
2.8. RNA Isolation and Real-Time RT-PCR
2.9. Immunohistochemistry
2.10. Statistical Analysis
3. Results
3.1. Confirmation of FRVE Detoxification and Main Components (Fustin and Fisetin) of FRVE
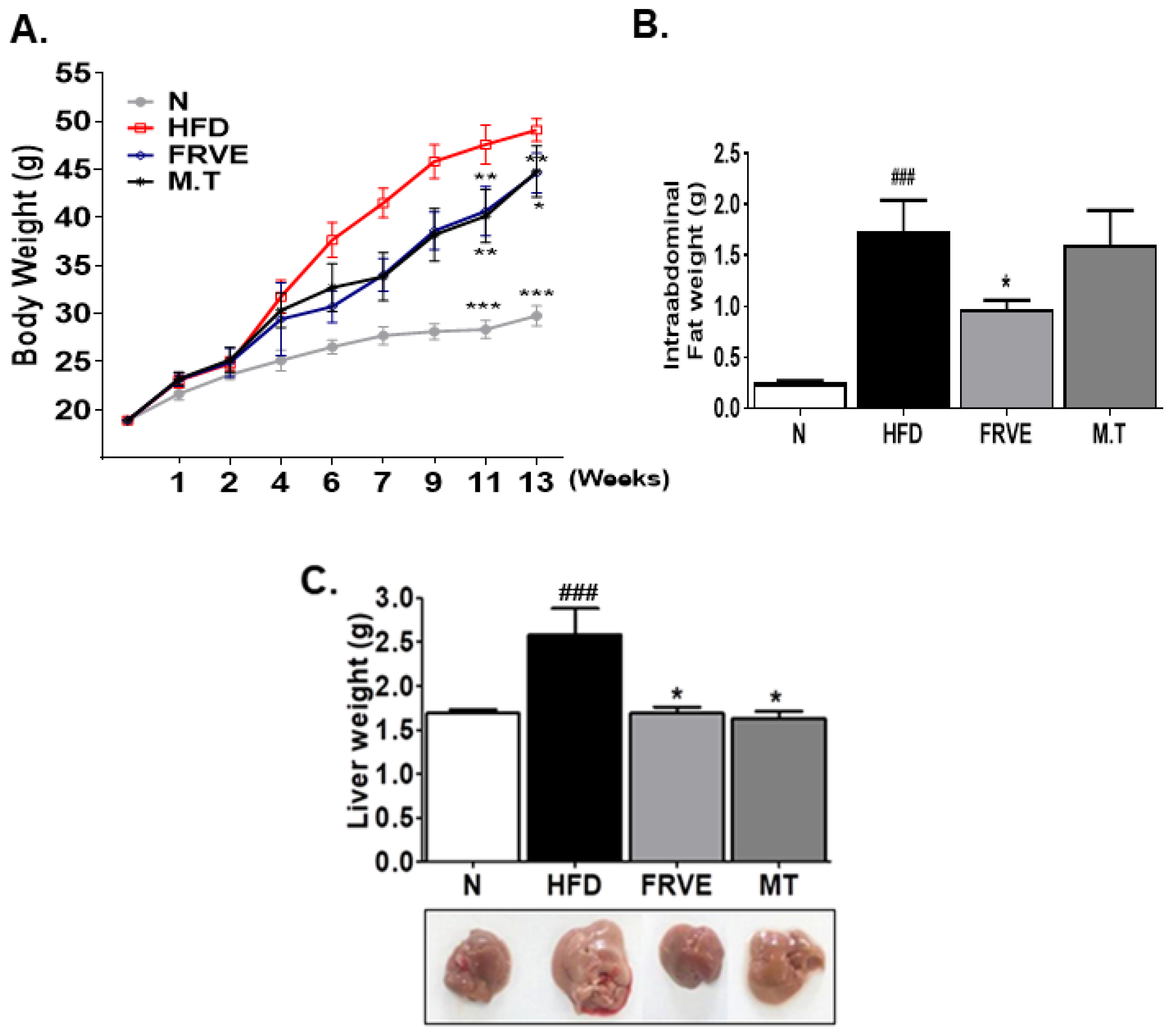
3.2. FRVE Decreased Body Weight, Intra-Abdominal Fat Weight, and Liver Weight in the HFD-Induced NAFLD Model
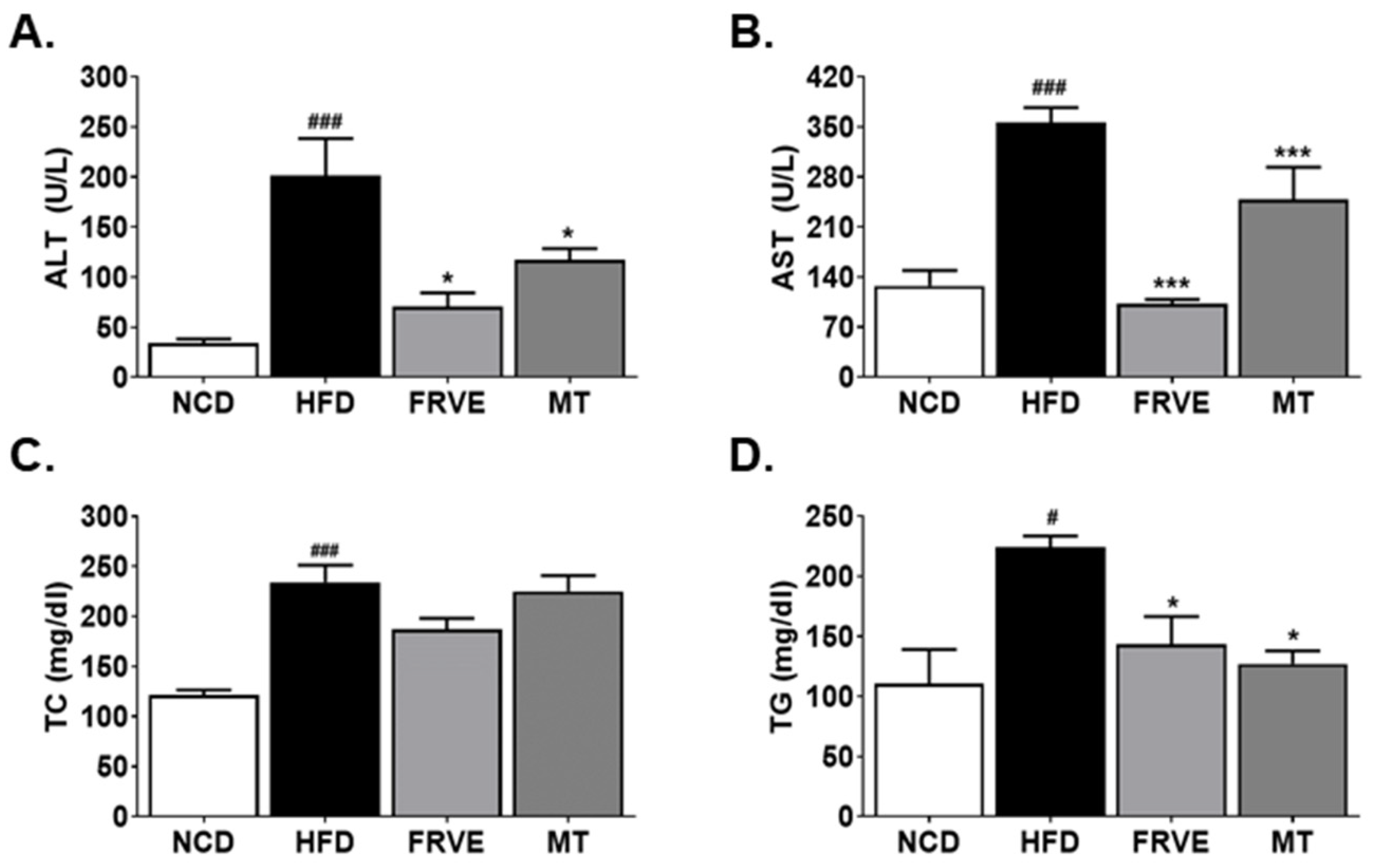
3.3. FRVE Downregulated Serum Liver Function and Lipid Markers in an HFD-Induced Nonalcoholic Fatty Liver Model
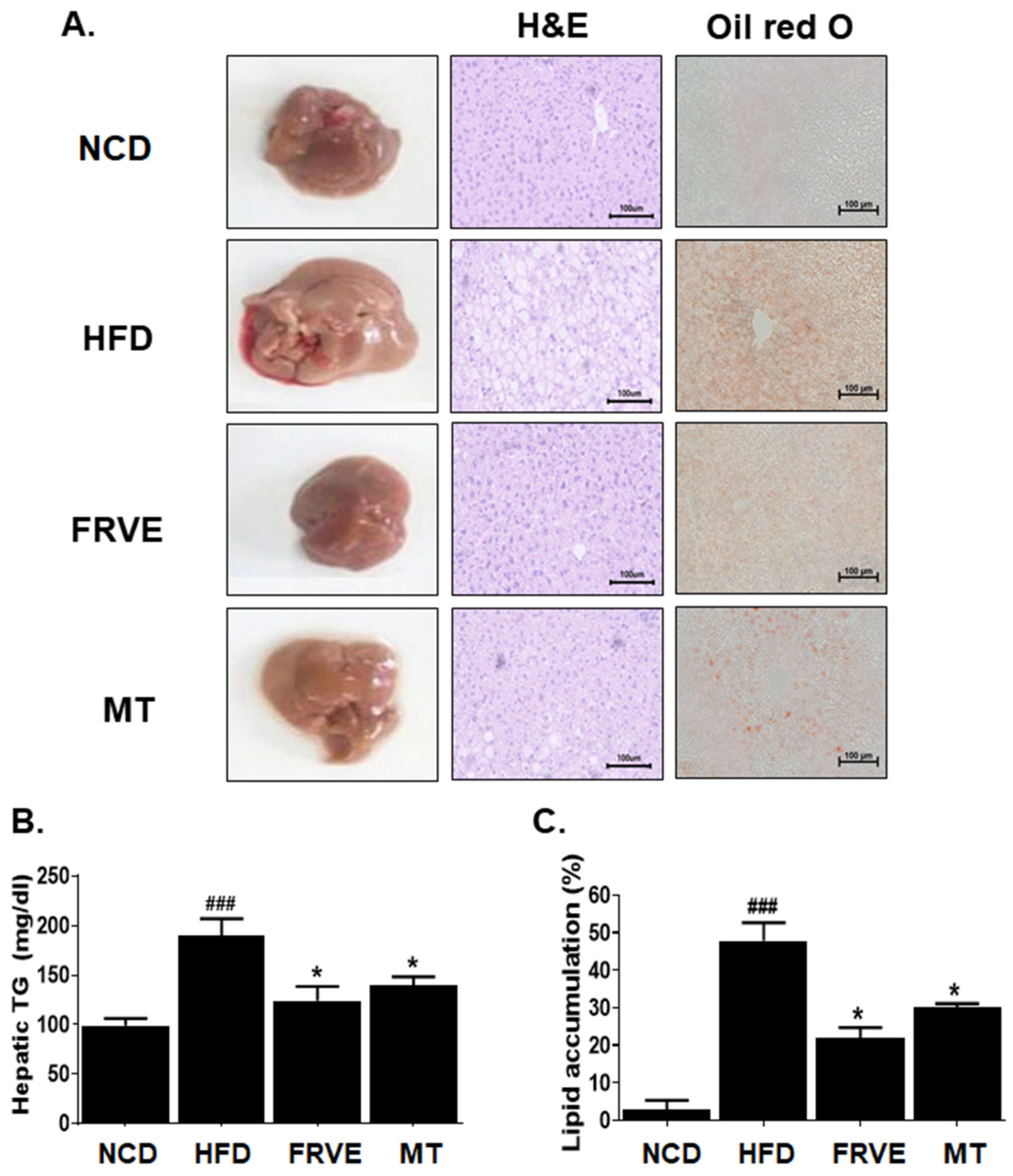
3.4. FRVE Inhibited Hepatic Steatosis and Lipid Accumulation in an HFD-Induced Nonalcoholic Fatty Liver Model
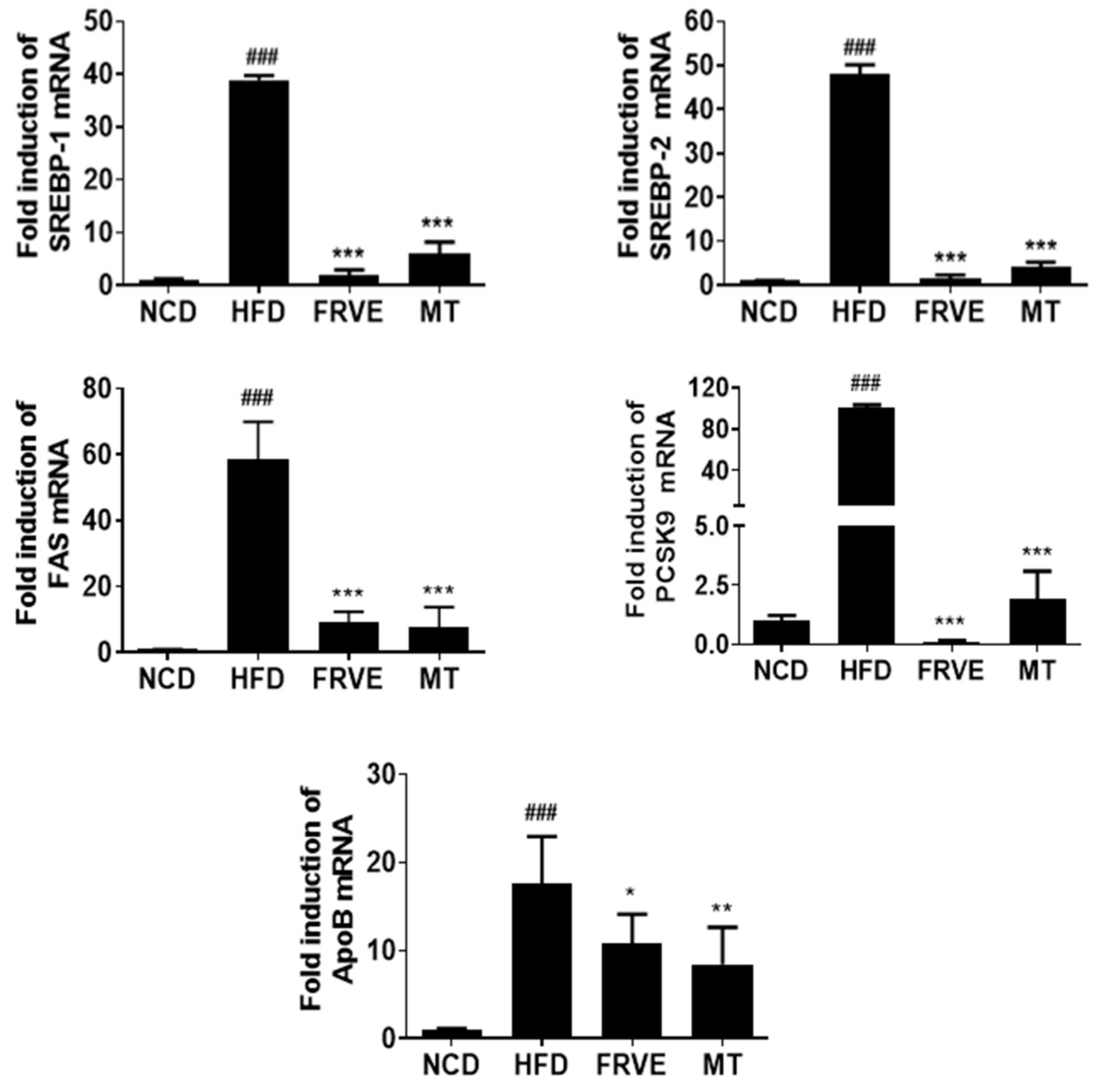
3.5. FRVE Downregulated the mRNA Levels of Lipogenesis-Related Genes in HFD-Induced Nonalcoholic Fatty Liver Model
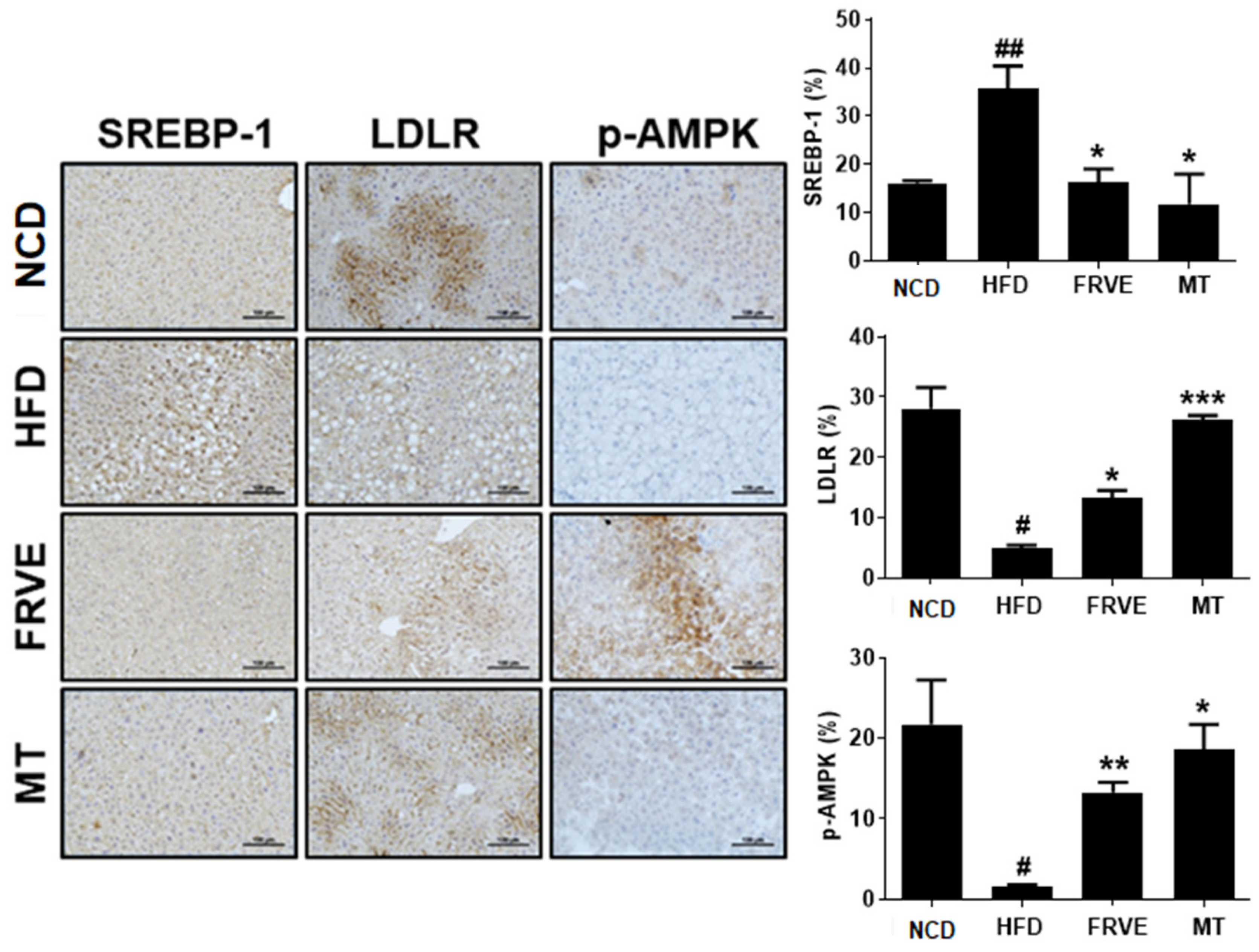
3.6. FRVE Inhibited HFD-Induced Hepatic Steatosis via AMPK Activation
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Younossi, Z.M.; Stepanova, M.; Younossi, Y.; Golabi, P.; Mishra, A.; Rafiq, N.; Henry, L. Epidemiology of chronic liver diseases in the USA in the past three decades. Gut 2020, 69, 564–568. [Google Scholar] [CrossRef] [PubMed]
- Collaborators, G.B.D.O.; Afshin, A.; Forouzanfar, M.H.; Reitsma, M.B.; Sur, P.; Estep, K.; Lee, A.; Marczak, L.; Mokdad, A.H.; Moradi-Lakeh, M.; et al. Health Effects of Overweight and Obesity in 195 Countries over 25 Years. N. Engl. J. Med. 2017, 377, 13–27. [Google Scholar] [CrossRef] [PubMed]
- Assy, N.; Kaita, K.; Mymin, D.; Levy, C.; Rosser, B.; Minuk, G. Fatty infiltration of liver in hyperlipidemic patients. Dig. Dis. Sci. 2000, 45, 1929–1934. [Google Scholar] [CrossRef]
- Patil, R.; Sood, G.K. Non-alcoholic fatty liver disease and cardiovascular risk. World J. Gastrointest. Pathophysiol. 2017, 8, 51–58. [Google Scholar] [CrossRef]
- Lee, M.-S.; Kim, J.-S.; Cho, S.-M.; Lee, S.O.; Kim, S.-H.; Lee, H.-J. Fermented Rhus verniciflua Stokes extract exerts an Antihepatic Lipogenic effect in oleic-acid-induced HepG2 cells via Upregulation of AMP-activated protein kinase. J. Agric. Food Chem. 2015, 63, 7270–7276. [Google Scholar] [CrossRef] [PubMed]
- Smith, B.K.; Marcinko, K.; Desjardins, E.M.; Lally, J.S.; Ford, R.J.; Steinberg, G.R. Treatment of nonalcoholic fatty liver disease: Role of AMPK. Am. J. Physiol. Endocrinol. Metab. 2016, 311, E730–E740. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Xu, S.; Mihaylova, M.M.; Zheng, B.; Hou, X.; Jiang, B.; Park, O.; Luo, Z.; Lefai, E.; Shyy, J.Y.-J. AMPK phosphorylates and inhibits SREBP activity to attenuate hepatic steatosis and atherosclerosis in diet-induced insulin-resistant mice. Cell Metab. 2011, 13, 376–388. [Google Scholar] [CrossRef]
- Duff, C.J.; Hooper, N.M. PCSK9: An emerging target for treatment of hypercholesterolemia. Expert Opin. Ther. Targets 2011, 15, 157–168. [Google Scholar] [CrossRef]
- Kang, Y.; Yoon, S.W.; Park, B. Allergen-removed Rhus verniciflua Stokes suppresses invasion and migration of pancreatic cancer cells through downregulation of the JAK/STAT and Src/FAK signaling pathways. Oncol. Rep. 2018, 40, 3060–3068. [Google Scholar] [CrossRef]
- Lee, K.-W.; Um, E.-S.; Jung, B.-B.; Choi, E.-S.; Kim, E.-Y.; Lee, S.; Jang, E.; Lee, J.-H.; Kim, Y. Rhus verniciflua Stokes extract induces inhibition of cell growth and apoptosis in human chronic myelogenous leukemia K562 cells. Oncol. Rep. 2018, 39, 1141–1147. [Google Scholar] [CrossRef]
- Choi, H.S.; Kim, M.K.; Choi, Y.K.; Shin, Y.C.; Cho, S.-G.; Ko, S.-G. Rhus verniciflua Stokes (RVS) and butein induce apoptosis of paclitaxel-resistant SKOV-3/PAX ovarian cancer cells through inhibition of AKT phosphorylation. BMC Complement. Altern. Med. 2016, 16, 122. [Google Scholar] [CrossRef] [PubMed]
- Chae, J.; Lee, S.; Lee, S. Potential efficacy of allergen removed Rhus verniciflua Stokes extract to maintain progression-free survival of patients with advanced hepatobiliary cancer. Explore 2018, 14, 300–304. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.S.; Lee, C.W.; Kim, J.-H.; Lee, J.-C.; An, W.G. Extract of Rhus verniciflua Stokes Induces p53-Mediated Apoptosis in MCF-7 Breast Cancer Cells. Evid.-Based Complement. Altern. Med. 2019, 2019, 9407340. [Google Scholar] [CrossRef] [PubMed]
- Lamichhane, R.; Kim, S.-G.; Kang, S.; Lee, K.-H.; Pandeya, P.R.; Jung, H.-J. Exploration of underlying mechanism of anti-adipogenic activity of sulfuretin. Biol. Pharm. Bull. 2017, 40, 1366–1373. [Google Scholar] [CrossRef]
- Song, N.-J.; Yoon, H.-J.; Kim, K.H.; Jung, S.-R.; Jang, W.-S.; Seo, C.-R.; Lee, Y.M.; Kweon, D.-H.; Hong, J.-W.; Lee, J.-S.; et al. Butein is a novel anti-adipogenic compound. J. Lipid Res. 2013, 54, 1385–1396. [Google Scholar] [CrossRef]
- Choi, D.R.; Jeong, J.H.; Yu, K.S.; Lee, N.S.; Jeong, Y.G.; Kim, D.K.; Na, C.S.; Na, D.S.; Hwang, W.M.; Han, S.; et al. Extract of Rhus verniciflua stokes protects against renal ischemia-reperfusion injury by enhancing Nrf2-mediated induction of antioxidant enzymes. Exp. Ther. Med. 2018, 15, 3827–3835. [Google Scholar]
- Kim, B.-G.; Song, Y.; Lee, M.-G.; Ku, J.-M.; Jin, S.-J.; Hong, J.-W.; Lee, S.; Kang, H. Macrophages from mice administered rhus verniciflua stokes extract show selective anti-inflammatory activity. Nutrients 2018, 10, 1926. [Google Scholar] [CrossRef]
- Kim, J.H.; Shin, Y.C.; Ko, S.-G. Integrating traditional medicine into modern inflammatory diseases care: Multitargeting by Rhus verniciflua Stokes. Mediat. Inflamm. 2014, 2014, 154561. [Google Scholar] [CrossRef]
- Moon, J.E.; Shin, J.-H.; Kwon, O.; Kim, J.Y. A standardized extract of rhus verniciflua stokes protects wistar rats against lipopolysaccharide-induced acute inflammation. J. Med. Food 2015, 18, 1223–1230. [Google Scholar] [CrossRef]
- Oh, P.-S.; Lee, S.-J.; Lim, K.-T. Glycoprotein Isolated from Rhus verniciflua S TOKES Inhibits Inflammation-Related Protein and Nitric Oxide Production in LPS-Stimulated RAW 264.7 Cells. Biol. Pharm. Bull. 2007, 30, 111–116. [Google Scholar] [CrossRef]
- Jung, C.H.; Kim, J.H.; Kim, J.H.; Chung, J.H.; Choi, H.S.; Seo, J.B.; Shin, Y.C.; Kim, S.H.; Ko, S.G. Anti-inflammatory effect of Rhus verniviflua Stokes by suppression of iNOS-mediated Akt and ERK pathways: In-vitro and in-vivo studies. J. Pharm. Pharmacol. 2011, 63, 679–687. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.-S.; Yeo, S.-H.; Jeong, S.-T.; Choi, J.-H.; Park, H.-S.; Kim, M.-K. Preparation and characterization of urushiol free fermented Rhus verniciflua stem bark (FRVSB) extracts. Korean J. Food Sci. Technol. 2012, 44, 173–178. [Google Scholar] [CrossRef]
- Choi, H.-S.; Kim, M.-K.; Park, H.-S.; Yun, S.-E.; Mun, S.-P.; Kim, J.-S.; Sapkota, K.; Kim, S.; Kim, T.-Y.; Kim, S.-J.; et al. Biological detoxification of lacquer tree (Rhus verniciflua Stokes) stem bark by mushroom species. Food Sci. Biotechnol. 2007, 16, 935–942. [Google Scholar]
- Jeong, H.Y.; Lee, T.H.; Lee, H.J.; Cho, J.-Y.; Moon, J.-H. Ionization Neutralizes the Allergy-Inducing Property of 3-Pentadecylcatechol: A Urushiol Derivative. J. Med. Food 2020. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.-O.; Kim, S.-J.; Kim, J.-S.; Ji, H.; Lee, E.-O.; Lee, H.-J. Comparison of the main components and bioactivity of Rhus verniciflua Stokes extracts by different detoxification processing methods. BMC Complement. Altern. Med. 2018, 18, 242. [Google Scholar] [CrossRef]
- Czaja, A.J. Hepatic inflammation and progressive liver fibrosis in chronic liver disease. World J. Gastroenterol. 2014, 20, 2515. [Google Scholar] [CrossRef]
- Sun, B.; Karin, M. Obesity, inflammation, and liver cancer. J. Hepatol. 2012, 56, 704–713. [Google Scholar] [CrossRef]
- Takahashi, Y.; Soejima, Y.; Fukusato, T. Animal models of nonalcoholic fatty liver disease/nonalcoholic steatohepatitis. World J. Gastroenterol. 2012, 18, 2300. [Google Scholar] [CrossRef]
- Buettner, R.; Schölmerich, J.; Bollheimer, L.C. High-fat diets: Modeling the metabolic disorders of human obesity in rodents. Obesity 2007, 15, 798–808. [Google Scholar] [CrossRef]
- Stranges, S.; Dorn, J.M.; Muti, P.; Freudenheim, J.L.; Farinaro, E.; Russell, M.; Nochajski, T.H.; Trevisan, M. Body fat distribution, relative weight, and liver enzyme levels: A population-based study. Hepatology 2004, 39, 754–763. [Google Scholar] [CrossRef]
- Fraulob, J.C.; Ogg-Diamantino, R.; Fernandes-Santos, C.; Aguila, M.B.; Mandarim-de-Lacerda, C.A. A mouse model of metabolic syndrome: Insulin resistance, fatty liver and non-alcoholic fatty pancreas disease (NAFPD) in C57BL/6 mice fed a high fat diet. J. Clin. Biochem. Nutr. 2010, 46, 212–223. [Google Scholar] [CrossRef] [PubMed]
- Jeong, S.-J.; Park, J.-G.; Kim, S.; Kweon, H.Y.; Seo, S.; Na, D.-S.; Lee, D.; Hong, C.Y.; Na, C.-S.; Dong, M.-S. Extract of Rhus verniciflua stokes protects the diet-induced hyperlipidemia in mice. Arch. Pharmacal Res. 2015, 38, 2049–2058. [Google Scholar] [CrossRef] [PubMed]
- Edwards, P.A.; Tabor, D.; Kast, H.R.; Venkateswaran, A. Regulation of gene expression by SREBP and SCAP. Biochim. Biophys. Acta 2000, 1529, 103–113. [Google Scholar] [CrossRef]
- Sakakura, Y.; Shimano, H.; Sone, H.; Takahashi, A.; Inoue, K.; Toyoshima, H.; Suzuki, S.; Yamada, N. Sterol regulatory element-binding proteins induce an entire pathway of cholesterol synthesis. Biochem. Biophys. Res. Commun. 2001, 286, 176–183. [Google Scholar] [CrossRef]
- Guo, D.; Bell, E.H.; Mischel, P.; Chakravarti, A. Targeting SREBP-1-driven lipid metabolism to treat cancer. Curr. Pharm. Des. 2014, 20, 2619–2626. [Google Scholar] [CrossRef]
- Madison, B.B. Srebp2: A master regulator of sterol and fatty acid synthesis. J. Lipid Res. 2016, 57, 333–335. [Google Scholar] [CrossRef]
- Wang, S.-L.; Du, E.Z.; Martin, T.D.; Davis, R.A. Coordinate regulation of lipogenesis, the assembly and secretion of apolipoprotein B-containing lipoproteins by sterol response element binding protein 1. J. Biol. Chem. 1997, 272, 19351–19358. [Google Scholar] [CrossRef]
- Lin, M.-S.; Guo, S.-E.; Lin, H.-S.; Hsu, J.-T.; Lin, Y.-S.; Lin, T.-H.; Huang, T.-J.; Chen, M.-Y.; Chung, C.-M. Impact of apolipoprotein B on hepatosteatosis in a population infected with Hepatitis C virus: A cross-sectional observational study. Obes. Facts 2016, 9, 101–111. [Google Scholar] [CrossRef]
- Bayly, G.R. Lipids and disorders of lipoprotein metabolism. In Clinical Biochemistry: Metabolic and Clinical Aspects; Elsevier: Amsterdam, The Netherlands, 2014; pp. 702–736. [Google Scholar]
- Ruscica, M.; Ferri, N.; Macchi, C.; Meroni, M.; Lanti, C.; Ricci, C.; Maggioni, M.; Fracanzani, A.L.; Badiali, S.; Fargion, S. Liver fat accumulation is associated with circulating PCSK9. Ann. Med. 2016, 48, 384–391. [Google Scholar] [CrossRef]
- Lakoski, S.G.; Lagace, T.A.; Cohen, J.C.; Horton, J.D.; Hobbs, H.H. Metabolism. Genetic and metabolic determinants of plasma PCSK9 levels. J. Clin. Endocrinol. Metab. 2009, 94, 2537–2543. [Google Scholar] [CrossRef]
- Zhao, Y.; Peng, L.; Yang, L.-C.; Xu, X.-D.; Li, W.-J.; Luo, X.-M.; Jin, X. Wedelolactone regulates lipid metabolism and improves hepatic steatosis partly by AMPK activation and up-regulation of expression of PPARα/LPL and LDLR. PLoS ONE 2015, 10, e0132720. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.Y.; Yuan, H.D.; Chung, I.K.; Chung, S.H. Compound K, intestinal metabolite of ginsenoside, attenuates hepatic lipid accumulation via AMPK activation in human hepatoma cells. J. Agric. Food Chem. 2009, 57, 1532–1537. [Google Scholar] [CrossRef] [PubMed]
- Kahn, B.B.; Alquier, T.; Carling, D.; Hardie, D.G. AMP-activated protein kinase: Ancient energy gauge provides clues to modern understanding of metabolism. Cell Metab. 2005, 1, 15–25. [Google Scholar] [CrossRef] [PubMed]

| Ingredient | (g/kg) |
|---|---|
| Casein | 265.0 |
| l-Cystine | 4.0 |
| Maltodextrin | 160.0 |
| Sucrose | 90.0 |
| Lard | 310.0 |
| Soybean Oil | 30.0 |
| Cellulose | 65.5 |
| Mineral mix, AIN-93-VX (94047) | 48.0 |
| Calcium Phosphate, dibasic | 3.4 |
| Vitamin Mix, AIN-93-VX (94047) | 21.0 |
| Choline Bitartrate | 3.0 |
| Blue Food Color | 0.1 |
| Primer | Forward Primer (5′-3′) | Reverse Primer |
|---|---|---|
| SREBP-1 | GGAGCCATGGATTGCACATT | GGCCCGGGAAGTCACTGT |
| SREBP-2 | GCGTTCTGGAGACCATGGA | ACAAAGTTGCTCTGAAAACAAATCA |
| FAS | GCGATGAAGAGCATGGTTTAG | GGCTCAAGGGTTCCATGTT |
| PCSK9 | TTGCAGCAGCTGGGAACTT | CCGACTGTGATGACCTCTGGA |
| ApoB | AAGCACCTCCGAAAGTACGTG | CTCCAGCTCTACCTTACAGTTGA |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, S.-O.; Xu, Y.; Han, H.; Jeong, S.-T.; Lee, Y.-K.; Paik, J.K.; Cha, J.-S.; Lee, H.-J. Fermented Rhus Verniciflua Stokes Extract Alleviates Nonalcoholic Fatty Liver through the AMPK/SREBP1/PCSK9 Pathway in HFD-Induced Nonalcoholic Fatty Liver Animal Model. Appl. Sci. 2020, 10, 6833. https://doi.org/10.3390/app10196833
Lee S-O, Xu Y, Han H, Jeong S-T, Lee Y-K, Paik JK, Cha J-S, Lee H-J. Fermented Rhus Verniciflua Stokes Extract Alleviates Nonalcoholic Fatty Liver through the AMPK/SREBP1/PCSK9 Pathway in HFD-Induced Nonalcoholic Fatty Liver Animal Model. Applied Sciences. 2020; 10(19):6833. https://doi.org/10.3390/app10196833
Chicago/Turabian StyleLee, Seon-Ok, Yinzhu Xu, Hengmin Han, Seok-Tae Jeong, You-Kyung Lee, Jean Kyung Paik, Jin-Sol Cha, and Hyo-Jeong Lee. 2020. "Fermented Rhus Verniciflua Stokes Extract Alleviates Nonalcoholic Fatty Liver through the AMPK/SREBP1/PCSK9 Pathway in HFD-Induced Nonalcoholic Fatty Liver Animal Model" Applied Sciences 10, no. 19: 6833. https://doi.org/10.3390/app10196833
APA StyleLee, S.-O., Xu, Y., Han, H., Jeong, S.-T., Lee, Y.-K., Paik, J. K., Cha, J.-S., & Lee, H.-J. (2020). Fermented Rhus Verniciflua Stokes Extract Alleviates Nonalcoholic Fatty Liver through the AMPK/SREBP1/PCSK9 Pathway in HFD-Induced Nonalcoholic Fatty Liver Animal Model. Applied Sciences, 10(19), 6833. https://doi.org/10.3390/app10196833