Abstract
The purpose of this study resides in the design and deposition of several types of bioactive interfaces with complex composition, targeting a superior osseointegration of bone implants. The experimental approach is framed by two oxide systems, SiO2‒CaO‒P2O5‒ZnO‒MgO and SiO2‒CaO‒P2O5‒ZnO‒SrO, while the percentage values were established as optimised solutions for ensuring wear resistance, bioactivity and beneficial effects on cell metabolism and reproduction. Moreover, two methods dedicated to fils growth (pulsed laser deposition and spin coating) were explored as potential variants for coating the bioinert materials and providing a transitional anchoring layer between the artificial substitute and host tissue. The obtained layers were evaluated as vitroceramic in nature, nanostructured in morphology and bioactive in relation to the physiological environment. The response of human fetal osteoblasts placed in contact with the new engineered surfaces was characterized by a significant proliferation from 1 to 4 days, which validates their suitability for hard tissue applications.
1. Introduction
Accidents resulting in bone trauma, degenerative diseases at the bone level and failed implantation of bone substitutes are generating the highest demand in terms of artificial materials with suitable properties for hard tissue engineering [1]. As a consequence, modern medicine must respond to the challenges imposed by the need to increase the quality of life for the patients involved in such scenarios [2,3].
Beyond the basic features of an implant, namely size, shape, mechanical strength, lack of cytotoxicity [4], current concerns are directed towards the design and fabrication of devices with superior characteristics, among which bioactivity and osseointegration potential are the most important [5]. By combining well-known and long-tested materials with bioactive coatings [6,7], antibacterial agents [8,9], antioxidant compounds [10] or biologically active molecules [11], novel systems that mimic the means of healing proper to the living body can be proposed, subsequently leading to bone regeneration and remodelling.
If, to date, the focus has been on the quality and durability up to 15 years of medical implants, the real and actual stakes are defined in the activity of metallic substitutes, namely absence of bioactivity and low chemical stability [12]. Poor bioactivity leads to an incorrect fixation of the substitute, requiring a later replacement through surgery, while rapid corrosion favours the spread of toxic ions in the body, endangering patient’s health [13]. Surface engineering is absolutely necessary to contribute to the optimization of the implant–tissue interface and overall physical condition.
In other words, a successful replacement operation implies the emergence of a strong bond between the implantable entity and host tissue, followed by an active bioconnection at the surface level. The identified solution consists in covering the orthopaedic implants with ceramics [14], glasses [15] or vitroceramics [16,17] in order to functionally modify their external side and provide bioactivity and increased biocompatibility, as well as a specific framework for performant integration and osseointegration [18,19]. Among the multitude of artificial materials that enhance the efficiency of metallic implants (demineralized bone matrix, coralline hydroxyapatite, calcium phosphate-based ceramics, etc.), bioglasses are the only ones which favour osteoinduction, besides osteoconduction and osseointegration. Contrariwise, their disadvantages begin with a short lifespan and continue with low mechanical properties [20]. However, the process of ion doping allowed the improvement in bioglass performances: magnesium, strontium, iron, zinc and silver are just a few of the tackled metallic cations [21,22]. Moreover, doping with targeted elements can stimulate bone regeneration, cells differentiation and finally healing [23]. In conclusion, the symbiosis between a brittle bioactive glass and a resistant bioinert metal, in the form of a coating attached to a support, means a winning approach that ensures a high level of bioactivity and excellent mechanical strength.
Bioactive glasses are inorganic materials with a high ability to react to surface stimuli, triggering biological effects in contact with physiological fluids or living cells. The ionic exchange and further apatite formation on their surface represent mechanisms that are extensively studied at present [24]. In addition, the unlimited compositional possibilities make a little explored field available to the researchers, where exceptional findings can arise based on synergistic implications in complex systems [25].
Zinc (Zn) is an element with great potential for cellular growth, proliferation and differentiation, also having a particularly important role in DNA replication and enzyme production; additionally, it is a well-known antibacterial agent [26]. Sergi et al. [27] studied Zn-containing bioglasses synthesized by the melt-quenching route, within SiO2‒P2O5‒Na2O‒K2O‒CaO‒MgO‒SrO‒ZnO system, ZnO substituting MgO; they concluded that Zn induces an antibacterial effect, hinders glass crystallization and retards apatite precipitation. Magnesium (Mg) is a very interesting element as a component of bioglasses, manifesting its effects at the level of glass processability (crystallization avoidance or delay), mechanical properties’ enhancement, bioactivity improvement and angiogenesis stimulation [28]. Souza et al. [29] reported obtaining Mg-including bioglasses through the conventional approach, within the Na2O‒CaO‒MgO‒SiO2‒P2O5 system, with MgO replacing CaO; the findings can be summarised as follows: Mg-rich compositions are characterized by a lower glass transition temperature, a higher crystallization temperature and attenuated rate of conversion of the initially emerged amorphous calcium phosphate to a crystalline apatite layer. Strontium (Sr) is an element that has enjoyed special attention recently due to its beneficial influence on cell proliferation and differentiation, bacteria apoptosis, osteogenesis and bone reconstruction speed [30]. Solgi et al. [31] prepared Sr-containing bioglasses by the sol-gel method, within the SiO2‒CaO‒SrO‒P2O5 system, with SrO substituting CaO; the results showed that Sr does not structurally alter the glass network and stimulates bone cells’ production of alkaline phosphatase. Moreover, Taherkhani and Moztarzadeh [32] demonstrated that the replacement of CaO with SrO in relation to the previous oxide system brings significant changes: enhanced tendency towards crystallization, accelerated formation of apatite and increased metabolic activity of osteoblasts as Sr content increases.
The sol-gel method is an appropriate experimental means of tissue engineering applications, with the positive effects being multiple: versatile composition, facile processing and improved bioactivity [33,34]. Through this type of approach, all kinds of dimensionalities (particles, fibres, coatings, scaffolds, massive bodies, etc.) can be built [35,36]. A careful control of the determining parameters is enough to adjust the final properties and define the functional performances. Afterwards, the deposition of thin films on metallic substrates can be done by different techniques: spraying, sputtering, ablation, centrifugation, etc. [37,38,39,40]. Pulsed laser deposition (PLD) is such an alternative, involving the ablation of one or more targets with a pulsed laser beam, followed by material transfer via plasma at the surface of a support; stoichiometry preservation and lack of contamination are the most representative assets [41]. Spin coating (SC) is another option for growing one-dimensional structures from a precursor solution that is rotated at high speed on the surface of a substrate; experimental simplicity and thickness control constitute its gains [42].
Our research group reported on several complex oxide systems, which were processed by sol-gel, spin coating and pulsed laser deposition as bioactive and biocompatible coatings [6,8,16,41,42], leading to considerable expertise in the field. In this work, novel compositions including Mg or Sr were tested in the form of thin films for providing suitable materials to coat the bioinert ceramic or metallic bone implants, ensure a biologically active interface and subsequently triggering speeded and superior osseointegration of them within the afflicted body.
2. Materials and Methods
2.1. Targets Synthesis
The growth of bioactive interfaces to trigger osseointegration for the bioinert implants started from the design of the oxide composition and continued with material processing either directly from the precursor solutions or indirectly from previously synthesized targets; in both cases, the desired coatings were deposited through appropriate techniques. The selected preparation method was sol-gel, chosen mainly at the expense of the conventional melt-quenching one; it is used very often in tissue engineering applications because of a number of indisputable advantages, such as the possibility of controlled crystallization, generation of mesoporous structures and presence of silanol groups on the surface (which favours the growth of hydroxyapatite when introduced in fluids reproducing the physiological environment) [33]. The two established oxide compositions are presented in Table 1. Basically, the influence of two cations (Mg2+ and Sr2+) on the mineralogical composition and subsequent biological response was the objective of the present study.

Table 1.
Oxide composition of the designed targets.
Thus, tetraethyl orthosilicate (Si(OC2H5)4, TEOS, Aldrich, St. Louis, MO, USA), calcium nitrate tetrahydrate (Ca(NO3)2·4H2O, Merck, Kenilworth, NJ, USA), triethyl phosphate (PO(OC2H5)3, TEP, Merck, Kenilworth, NJ, USA), zinc nitrate hexahydrate (Zn(NO3)2·6H2O, Sigma-Aldrich, St. Louis, MO, USA), magnesium nitrate hexahydrate (Mg(NO3)2·6H2O, Merck, Kenilworth, NJ, USA) and strontium nitrate (Sr(NO3)2, Sigma-Aldrich, St. Louis, MO, USA) were employed as reagents with high purity. Briefly, for each composition, two colourless and clear solutions were prepared, one of alkoxides and one of nitrates, which were then mixed and transformed over time (12 h) into gel. In the next step, the gel was converted into powder by aging for 24 h, drying at 80 °C for 12 h and calcining at 600 °C for 2 h, with the latter being granulated with polymeric binder, shaped in the form of disc and finally sintered at 800 °C for 6 h. This last stage of thermal treatment is a very important one, because it leads to the emergence of connections between the primary particles, offering, in this way, the desired compaction and densification to the targets (called Target-Mg and Target-Sr, in correlation with the described compositions), from which the layers are to be deposited; the recorded shrinkage was of about 32% for Target-Mg and 26% for Target-Sr.
2.2. Interfaces Deposition
The obtaining of bioactive interfaces on silicon substrate was performed using the spin coating (SC) or pulsed laser deposition (PLD) techniques, as they ensure a stoichiometric transfer by means of the homogenised precursor solution or generated plasma plume. In the first case, the solutions described within the section, dedicated to target synthesis, were spread by centrifugation as films at the substrate surface; 10,000 rpm for 1 min was applied and three successive layers were overlapped, with intermediate calcination at 200 °C for 4 min. In the second case, the coatings were achieved with the help of a 532 nm nanosecond laser (73–74 mJ/pulse, 29,100 pulses, 10 Hz), in the experimental situation of a target-substrate distance of 4 cm and working oxygen pressure of 100 mTorr. Other important and variable parameters are included in Table 2, where the codes of the resulting samples can also be found.

Table 2.
Deposition details of the deposited interfaces.
2.3. Materials Characterization
The microstructure of the targets and morphology of the interfaces, together with their elemental composition, were investigated through scanning electron microscopy (SEM) coupled with energy-dispersive X-ray spectroscopy (EDX), employing a FEI Quanta Inspect F electron microscope, operated at 30 kV; the specimens were coated with a thin layer of gold by DC magnetron sputtering in order to ensure surface conductivity. The phase composition and crystalline structure of the targets and films were evaluated with the help of X-ray diffraction (XRD), using a Shimadzu XRD 6000 diffractometer (targets) or a PANalytical Empyrean diffractometer (films), 2θ ranging between 10 and 70°.
2.4. Biological Evaluation
The biological properties of the deposited interfaces were assessed through in vitro tests. In order to investigate the bioactivity, a testing solution (simulated biological fluid, SBF, pH = 7.3) was prepared according to the recipe described by Kokubo et al. [43]; the samples were immersed in this solution and kept at 37 °C for 28 days, after which they were gently washed with distilled water and dried at 50 °C for 12 h in order to perform surface imaging.
As far as biocompatibility is concerned, human fetal osteoblast cell line (hFOB 1.19, ATCC, Manassas, VA, USA) was cultivated in MEM α (A1049001, Gibco, Gaithersburg, MD, USA) supplemented with 10% Fetal Bovine Serum (FBS, F7524, Sigma, St. Louis, MO, USA) and 1% Penicillin-Streptomycin (P4333, Sigma, St. Louis, MO, USA), seeded via pipetting a drop of cell suspension on the top surface of the obtained coatings, and left to adhere for 30 min. Consequently, the cell culture medium was added and the samples were incubated at 37 °C in 5% CO2 for 24 or 96 h. The cellular viability was estimated with LIVE/DEAD Viability/Cytotoxicity Kit, for mammalian cells (L3224, Life Technologies, Carlsbad, CA, USA) [44]. The cells were seeded on the coated samples placed in 48-well plates at a density of 1 × 105 cells/well. After 1 or 4 days in culture, the samples were rinsed with 1× Phosphate Buffered Saline (PBS, P3813, Sigma, St. Louis, MO, USA) to remove serum esterase activity and incubated for 30 min at room temperature with 2 µM Calcein-AM and 4 µM Ethidium Homodimer-1, reagents belonging to the LIVE/DEAD kit. After incubation, 1X PBS was added and the viability was evaluated with a Zeiss LSM 880 confocal microscope, employing 488 and 514 nm lasers and processing with ZEN 2.3 software.
3. Results and Discussion
3.1. Targets Characterization
The most complex part was represented by the target production, since they must meet a set of general requirements in terms of composition, homogeneity, densification and size. Within the physical approach (PLD), based on the thermal analyses, the gels were calcined at 600 °C to almost completely remove the components with low thermal stability and retain the inorganic part in a form with as little crystallinity as possible. This low degree of ordering is mandatory when aiming to ensure the premises for the generation of bioactive materials. Further, the targets were sintered at 800 °C; thus, an increase of 200 °C in temperature was selected, based on the idea that high-quality films are deposited from well-compacted sources. The effects of the second thermal treatment in terms of morphology and phase composition can be observed in Figure 1. Achieved in fracture, the SEM images reveal important differences between Target-Mg and Target-Sr. The two samples are heterogenous, alternating more densified areas, which appeared as a result of grains coalescence, with the agglomerations of grains, not strongly connected yet and crossed by a network of pores or channels; therefore, an open interconnected porosity may be appraised (Figure 1a,b). Moreover, massive blocks can be identified, either with a continuous aspect (Target-Mg) or crossed by micrometric cracks (Target-Sr), which probably occurred during crystallization processes, as a way to eliminate internal tensions. The specificity of the Mg-containing composition resides in the existence of honeycomb-like entities, which leads to a larger proportion of pores, with higher maximum diameter and wide size distribution.
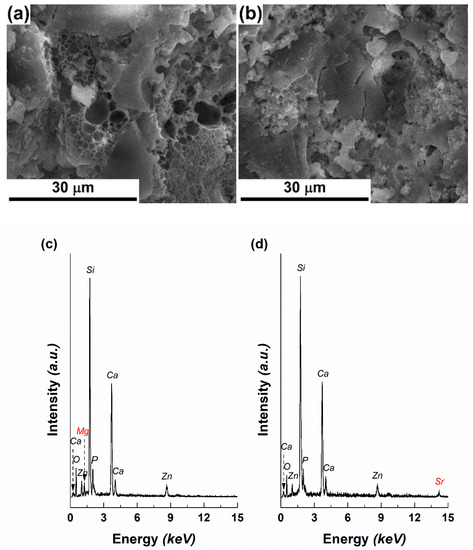
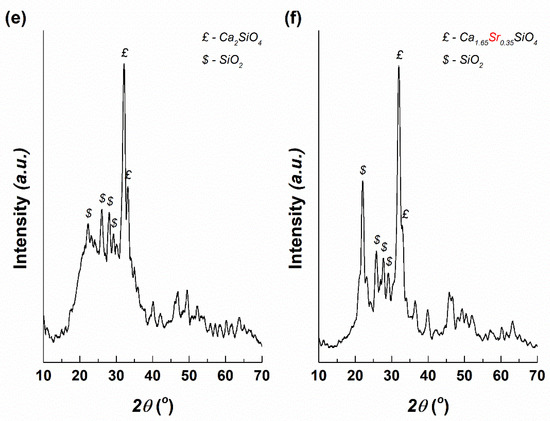
Figure 1.
SEM images of: (a) Target-Mg and (b) Target-Sr, EDX spectra of: (c) Target-Mg and (d) Target-Sr and XRD patterns of: (e) Target-Mg and (f) Target-Sr.
The EDX spectra recorded on both targets are similar, the elements of interest looming with intensities proportional to the designed compositions, as follows: Si, Ca, P, Zn, Mg/Sr, O (Figure 1c,d). It is well known that Mg2+ contributes to mechanical strength and bioactivity [28], while Sr2+ improves cellular proliferation and osteogenesis [30]; the other oxides constitute the background matrix that differently embeds the mentioned alkaline earth elements.
The target’s vitroceramic nature was demonstrated by the XRD patterns (Figure 1e,f), through the characteristic shape: a broad halo peak in the range 15–40° and several narrow intense peaks emerging from it. For Target-Mg, two main crystalline phases were identified: calcium silicate (Ca2SiO4) with hexagonal structure (ICDD-00-023-1042 and ICDD-00-077-0420) and silicon dioxide (SiO2) with different symmetries (ICDD-00-081-1665, ICDD-00-082-1410 and ICDD-00-083-0541). Going to Target-Sr, two main crystalline phases were also detected: calcium strontium silicate (Ca1.65Sr0.35SiO4) with orthorhombic structure (00-077-0399) and SiO2 with different symmetries (ICDD-00-081-1665, ICDD-00-082-1410, ICDD-00-083-0541 and ICDD 00-083-1833). Ca2SiO4-based structures represent the major crystalline component within both targets, being surrounded by a vitreous matrix that includes the other elements (P, Zn, Mg). As can be noticed, Sr penetrates the ordered network of Ca2SiO4 and settles on Ca sites, leading to a partial substitution. Starting from the ionic radii of Ca, Mg and Sr (Ca2+(VI)—1.00 Å, Mg2+(VI)—0.72 Å, Sr2+(VI)—1.18 Å), differences of 28% appear between Ca and Mg, respectively, and 18% between Ca and Sr; such a theoretical background sustains the preferential incorporation of Sr.
3.2. Interfaces Characterization
Using the formerly described targets, four films were deposited on silicon substrate, two from each target, changing the temperature at which the substrate was maintained during the experiment. Their appearance, together with the visualization of the bare substrate, is available in Figure 2. On one hand, the silicon plate exhibits a clean and smooth surface, without defects even at high magnification (Figure 2a), while on the other hand, all grown interfaces are composed of grains with dimensions below 50 nm and covered by a family of droplets of a maximum of 3 μm (Figure 2b–e). An obvious difference can be noticed between the two approached compositions, especially at low magnification, namely a surface more crowded with droplets, but also pieces of material of irregular shape for Sr-including coatings (Figure 2d,e). Additionally, the elevations seem to be more flattened in the case of Mg-containing layers, for which the SEM images show a smaller number of bright areas, namely, fewer heights for electron accumulation (Figure 2b,c). This ascertainment can be translated into a higher roughness for PLD-Sr-1 and PLD-Sr-2, which can be further considered as a matter of low-quality deposition from a target that does not fully meet the requirements of densification. However, this morphology could have positive implications on cells adhesion, as usually they are much more compatible with those materials of suited roughness. For example, de Bonis et al. [45] deposited bioactive glass-ceramic films by nanosecond PLD and obtained the typical morphology: a surface sprinkled with droplets, which supposes an inherent roughness, suitable for cell attachment. Sanz et al. [46] also employed PLD to obtain bioactive glass films; the increase in the laser pulse energy was correlated with better surface properties and cellular behaviour.
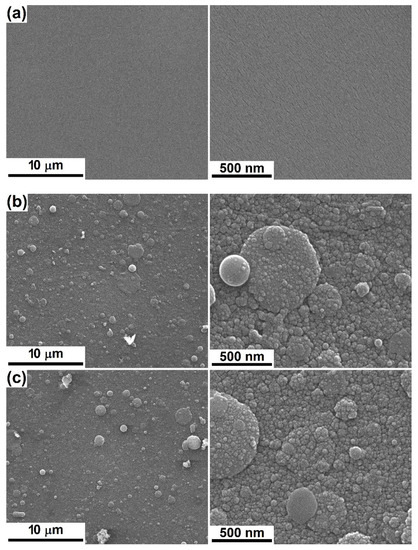
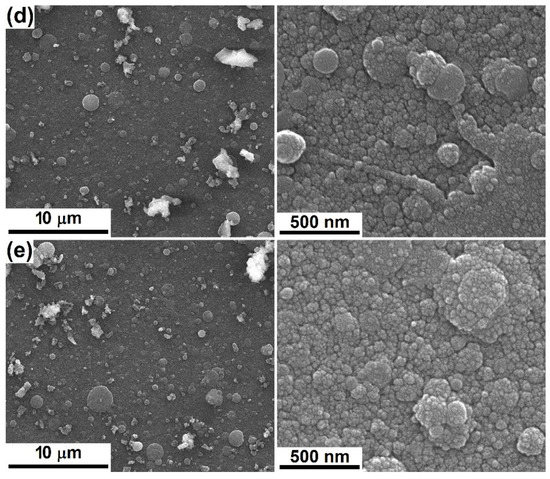
Figure 2.
SEM images at different magnifications of: (a) bare substrate and (b) PLD-Mg-1, (c) PLD-Mg-2, (d) PLD-Sr-1 and (e) PCL-Sr-2 interfaces.
The next two samples were prepared through the chemical route (SC) on silicon substrate with different features. Since very thin films are expected, a rougher substrate was necessary in order to ensure an adherence that will last during the immersion in physiological fluids or subjection to mechanical stresses. Figure 3 comparatively presents the aspect of the bare silicon plate, as well as coated ones. If the first one is crossed by mixed hills and valleys with sharp edges and corners, in other words, a coarse and faceted roughness (Figure 3a), the next two display surfaces within which the primary irregularities were partially alleviated by the deposited interfaces, proving their reduced thickness, but also their homogeneity and continuity (Figure 3b,c). Moreover, at high magnification, a visible nanostructuring (grains with dimensions below 20 nm) can be seen, interrupted by branched cracks prevalently for SC-Mg, likely a consequence of the drying and calcining processes, when the solvents and thermally degradable components are removed, leaving behind a discontinuous layer due to the high dilution of the precursor solution and subsequently insufficient amount of solid material. However, the covering degree is quite high, exceeding 95%; the value was estimated by relating the area covered by cracks to the total investigated area.
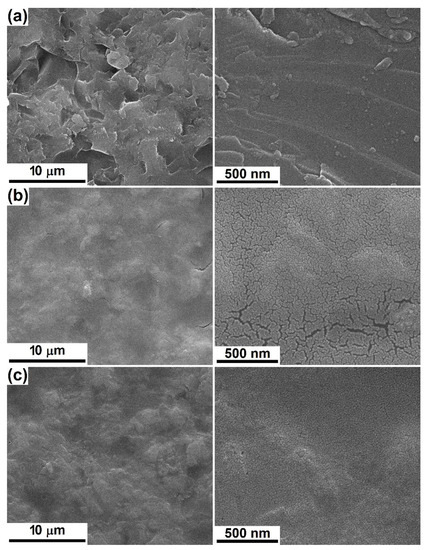
Figure 3.
SEM images at different magnifications of: (a) bare substrate and (b) SC-Mg and (c) SC-Sr interfaces.
The EDX spectra corresponding to the grown coatings are illustrated in Figure 4a in comparison with those of the substrates used for each set of samples. To the signals of Si, mainly identified for the bare silicon plates, supplementary maxima assigned to the elements considered in the primary compositions (Ca, P, Zn, Mg/Sr) emerge, similar to the targets. Due to the higher thickness of PLD-derived interfaces, the Sr maximum placed at high energy is entirely discernible, while its existence in the spectrum of SC-Sr is ambiguous, even though the associated quantitative estimation proposes a value of several percent.
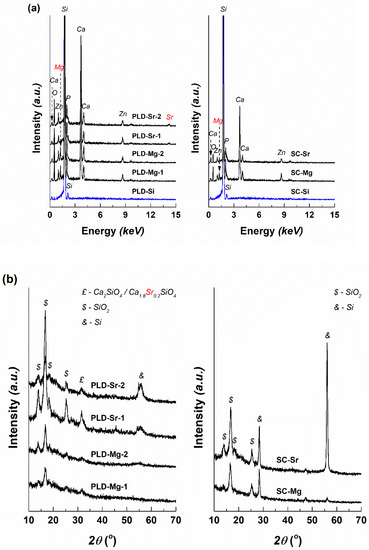
Figure 4.
(a) Energy-dispersive X-ray spectroscopy (EDX) spectra and (b) XRD patterns of the deposited interfaces.
Regarding the crystallinity of the deposited layers, the XRD patterns were recorded at grazing incidence to reduce the substrate contribution, which, even under this configuration sometimes generates separate peaks (silicon, Si, cubic structure, ICDD-00-075-0589), as can be observed in Figure 4b. The results are correlated, to a certain extent, with those presented for the targets, in the sense that a vitroceramic character is confirmed, with a larger maxima width this time, indicating the formation of smaller crystallites. Moreover, PLD-Sr-1 and PLD-Sr-2 include an important proportion of the Ca2SiO4-based phase (Ca1.8Sr0.2SiO4) with orthorhombic structure (ICDD 00-077-1621), in addition to various types of SiO2-ordered networks (ICDD-00-080-2147, ICDD-00-082-1567, ICDD-00-082-1569 and ICDD-00-083-1339). On a closer look, a changed substitution ratio can be seen in the case of calcium strontium silicate when comparing its formula before and after the laser ablation experiments. Thus, if a sintering temperature of 800 °C applied for the targets favours the penetration of Sr into Ca2SiO4 crystalline structure, the coatings obtained at 25 or 300 °C are characterized by a higher Ca2+/Sr2+ ratio, namely, a lower substitution degree. It can also be stated that the presence of Sr increases the tendency towards crystallization [32]. For instance, Wang et al. [39] demonstrated that the crystallinity and adhesive strength of the films grown by PLD increase with substrate temperature raising. In another train of thought, compared to the Ar atmosphere, the O2 atmosphere seems to be beneficial for the PLD preparing of films with excellent osteoinductivity [47].
In conclusion, the obtained interfaces are suitable for biological applications, sustained especially by the structural characteristics. A vitreous matrix which encapsulates specific crystalline phases, easily attackable when placed in contact with the physiological fluids and prone to apatite formation, represents a material with bioactive potential. The identified crystalline silicate compounds (Ca2SiO4, Ca1.8Sr0.2SiO4) also play important roles in emphasizing the biocompatible and bioactive character, while SiO2 can act as a reinforcing agent, improving the mechanical properties [41].
3.3. Biological Evaluation
According to the SEM images captured after 28 days of immersion in SBF, the PLD-engineered layers present on their surface apatitic formations composed of spherical entities with diameters below 200 nm, lightly gathered in favoured areas; occasionally, hollow structures can be identified (Figure 5a–d). These types of appearance are typical of the products that arise during a mineralization process [6,8,36,42]. It is not clear if, apart from these micrometric and undefined agglomerations, a thin and continuous layer of apatite is nucleated on the entire exposed area. SC-Mg and SC-Sr seem to be less prone to mineralization (Figure 5e,f), probably because of the increased calcination temperature (600 °C) that leads to a more compact material, which is harder to degrade. The amorphous structures have faster dissolution and reprecipitation rates, which implies a better bioactivity than the crystalline ones; however, new bone tissue seems to grow better on crystalline surfaces, maybe due to the stimulation effect exerted by some ordered domains [39]. Anyway, shallow signs of bioactivity can be noticed in the form of lacy regions or quasi-spherical growths; even a discontinuous apatite-forming ability is acceptable for the targeted applications, based on the idea that the bioactive areas will effectively ensure a network of anchoring sites in relation to the surrounding hard tissue. It is likely that the mineralization capacity and rate can be tuned through the processing parameters and, subsequently, through films thickness and crystallinity.
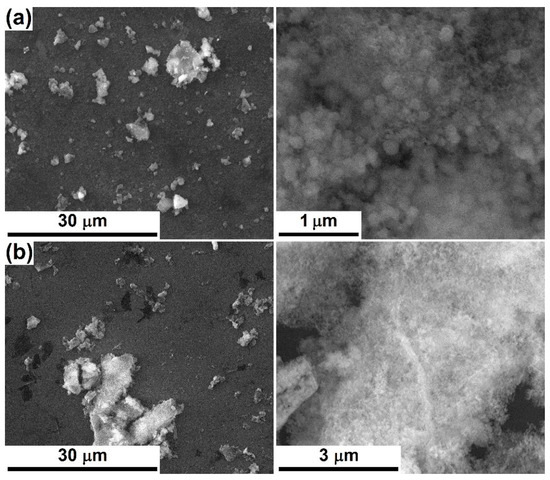
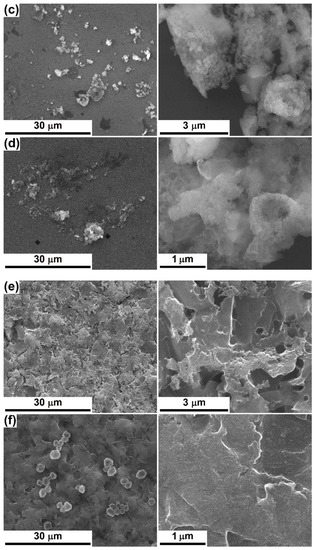
Figure 5.
SEM images at different magnifications, after 28 days of immersion in SBF, of: (a) PLD-Mg-1, (b) PLD-Mg-2, (c) PLD-Sr-1, (d) PLD-Sr-2, (e) SC-Mg and (f) SC-Sr interfaces.
Cellular viability and proliferation testing was performed through the LIVE/DEAD assay and fluorescence microscopy after 1 or 4 days of culturing the human fetal osteoblasts in the presence of the developed coatings. The corresponding images are comparatively displayed in Figure 6, where the viable cells are coloured in green and the nuclei of dead cells in red. Reported to the Control (represented here by a bare silicon plate), the deposited interfaces support cellular adhesion and significantly promote osteoblasts proliferation at longer periods. Additionally, the rounded shape turns into an elongated one and the cells cover, in a very compact and organized way, the whole available surface. No important differences were detected between the two oxide compositions and deposition techniques. However, on a closer look, the PLD layers deposited at room temperature (Figure 6b,d) seem to be more suitable supports for osteoblasts culturing, at least after 1 day, maybe due to an easier release of beneficial ions from the vitreous matrix. A comparative analysis between the SC-derived films reveals a better cellular adhesion and viability for SC-Sr in the short term (1 day), while in the long term (4 days), the behaviour of SC-Mg and SC-Sr is balanced. Overall, the cells cultured for 4 days on the surface of the coated samples display similar features in terms of proliferation, which confirms the appropriateness of such compositions for biomedical applications.
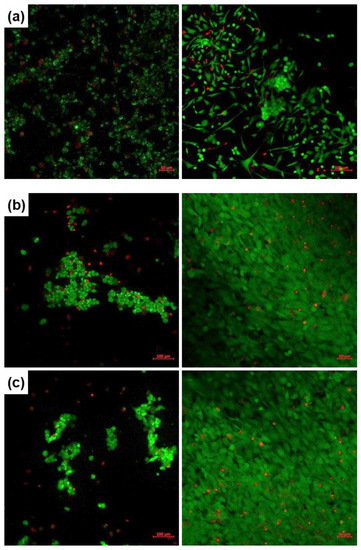
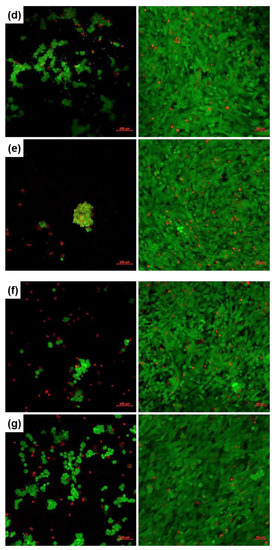
Figure 6.
Fluorescence images recorded on human fetal osteoblasts, cultured for 1 (left) or 4 days (right), on: (a) Control, (b) PLD-Mg-1, (c) PLD-Mg-2, (d) PLD-Sr-1, (e) PLD-Sr-2, (f) SC-Mg and (g) SC-Sr interfaces.
4. Conclusions
Bioactive interfaces belonging to SiO2‒CaO‒P2O5‒ZnO‒MgO/SrO oxide systems were produced by two different techniques: pulsed laser deposition and spin coating. The final objective was represented by the achievement of an induced bioactivity at the level of bioinert implants’ surface at the time of their integration into the living body. The experimental results showed the presence of several crystalline phases embedded in vitreous matrices within both the targets and films. From a morphological point of view, the deposited layers are continuous, reduced in thickness and composed of nanograins. Their bioactivity was validated by the formation of sparse apatite agglomerations after immersion in a simulated biological fluid for 28 days, while the cellular adhesion and proliferation assay using osteoblast cells demonstrated the biocompatibility. In this way, the potential of all coated samples to generate improved devices dedicated to bone tissue engineering was confirmed. To the best of our knowledge, such compositions have not been reported in the scientific literature to date as either oxide ratios or two-dimensional structures.
Author Contributions
Conceptualization, C.B.; Methodology, I.C., C.B. and A.B.; Validation, S.-I.J.; Investigation, V.-P.T., I.C., A.B., D.-D.B. and C.B.; Resources, S.-I.J., A.B. and C.B.; Writing—original draft preparation, C.B.; Writing—review and editing, C.B.; Supervision, S.-I.J. and C.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Acknowledgments
The fluorescence microscopy images acquisition was possible due to European Regional Development Fund through Competitiveness Operational Program 2014–2020, Priority axis 1, Project no. P_36_611, MySMIS code 107066, Innovative Technologies for Materials Quality Assurance in Health, Energy and Environmental-Center for Innovative Manufacturing Solutions of Smart Biomaterials and Biomedical Surfaces -INOVABIOMED. We greatly appreciate Habil. Cristiana TANASE, Head of Biochemistry-Proteomics Department, “Victor Babes” National Institute of Pathology, for providing the hFOB cell line.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Kumar, P.; Sindhu, A. Materials for tissue engineering. In Advances in Animal Biotechnology and Its Applications; Gahlawat, S., Duhan, J., Salar, R., Siwach, P., Kumar, S., Kaur, P., Eds.; Springer: Berlin/Heidelberg, Germany, 2018; pp. 357–370. [Google Scholar]
- Chen, Y.; Li, W.; Zhang, C.; Wu, Z.; Liu, J. Recent developments of biomaterials for additive manufacturing of bone scaffolds. Adv. Healthc. Mater. 2020. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Huang, J.; Narayan, R.J. Gradient scaffolds for osteochondral tissue engineering and regeneration. J. Mater. Chem. B 2020. [Google Scholar] [CrossRef] [PubMed]
- Han, F.; Zhu, C.; Chen, L.; Wicks, J.; Li, B. Bio-instructive scaffolds for bone regeneration. In Bio-Instructive Scaffolds for Musculoskeletal Tissue Engineering and Regenerative Medicine; Brown, J.L., Kumbar, S.G., Banik, B.L., Eds.; Academic Press: Waltham, MA, USA, 2017; pp. 55–84. [Google Scholar]
- Zhang, K.; Wang, S.; Zhou, C.; Cheng, L.; Gao, X.; Xie, X.; Sun, J.; Wang, H.; Weir, M.D.; Reynolds, M.A.; et al. Advanced smart biomaterials and constructs for hard tissue engineering and regeneration. Bone Res. 2018, 6, 31. [Google Scholar] [CrossRef] [PubMed]
- Schitea, R.I.; Nitu, A.; Ciobota, A.A.; Munteanu, A.L.; David, I.M.; Miu, D.; Raileanu, M.; Bacalum, M.; Busuioc, C. Pulsed laser deposition derived bioactive glass-ceramic coatings for enhancing the biocompatibility of scaffolding materials. Materials 2020, 13, 2615. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.G.X.; Myers, D.E.; Wallace, G.G.; Brandt, M.; Choong, P.F.M. Bioactive coatings for orthopaedic implants—Recent trends in development of implant coatings. Int. J. Mol. Sci. 2014, 15, 11878–11921. [Google Scholar] [CrossRef]
- Prefac, G.A.; Milea, M.L.; Vadureanu, A.M.; Muraru, S.; Dobrin, D.I.; Isopencu, G.O.; Jinga, S.I.; Raileanu, M.; Bacalum, M.; Busuioc, C. CeO2 containing thin films as bioactive coatings for orthopaedic implants. Coatings 2020, 10, 642. [Google Scholar] [CrossRef]
- van Hengel, I.A.J.; Tierolf, M.W.A.M.; Valerio, V.P.M.; Minneboo, M.; Fluit, A.C.; Fratila-Apachitei, L.E.; Apachiteia, I.; Zadpoor, A.A. Self-defending additively manufactured bone implants bearing silver and copper nanoparticles. J. Mater. Chem. B 2020, 8, 1589–1602. [Google Scholar] [CrossRef]
- Marrazzo, P.; O’Leary, C. Repositioning natural antioxidants for therapeutic applications in tissue engineering. Bioengineering 2020, 7, 104. [Google Scholar] [CrossRef]
- Schliephake, H.; Rublack, J.; Aeckerle, N.; Fooster, A.; Schwenzer, B.; Reichert, J.; Scharnweber, D. In vivo effect of immobilisation of bone morphogenic protein 2 on titanium implants through nano-anchored oligonucleotides. Eur. Cell. Mater. 2015, 30, 28–40. [Google Scholar] [CrossRef]
- Prasad, S.; Ehrensberger, M.; Gibson, M.P.; Kim, H.; Monaco, E.A. Biomaterial properties of titanium in dentistry. J. Oral Biosci. 2015, 57, 192–199. [Google Scholar] [CrossRef]
- Chen, Q.; Thouas, G.A. Metallic implant biomaterials. Mater. Sci. Eng. R 2015, 87, 1–57. [Google Scholar] [CrossRef]
- Rafieerad, A.R.; Bushroa, A.R.; Nasiri-Tabrizi, B.; Baradaran, S.; Shahtalebi, S.; Khanahmadi, S.; Afshar-Mohajer, M.; Vadivelu, J.; Yusof, F.; Basirun, W.J. In-vitro bioassay of electrophoretically deposited hydroxyapatite-zirconia nanocomposite coating on Ti-6Al-7Nb implant. Adv. Appl. Ceram. 2017, 116, 293–306. [Google Scholar] [CrossRef]
- Bellucci, D.; Bianchi, M.; Graziani, G.; Gambardella, A.; Berni, M.; Russo, A.; Cannillo, V. Pulsed Electron Deposition of nanostructured bioactive glass coatings for biomedical applications. Ceram. Int. 2017, 43, 15862–15867. [Google Scholar] [CrossRef]
- Negrea, R.; Busuioc, C.; Constantinoiu, I.; Miu, D.; Enache, C.; Iordache, F.; Jinga, S.I. Akermanite based coatings grown by pulsed laser deposition for metallic implants employed in orthopaedics. Surf. Coat. Technol. 2019, 357, 1015–1026. [Google Scholar] [CrossRef]
- Marghussian, V. Biomedical applications of nano-glass ceramics. In Nano-Glass Ceramics: Processing, Properties and Applications; Marghussian, V., Ed.; William Andrew: Amsterdam, The Netherlands, 2015; pp. 225–241. [Google Scholar]
- Oliver, J.N.; Su, Y.; Lu, X.; Kuo, P.H.; Du, J.; Zhu, D. Bioactive glass coatings on metallic implants for biomedical applications. Bioact. Mater. 2019, 4, 261–270. [Google Scholar] [CrossRef]
- Ibrahim, M.Z.; Sarhan, A.A.D.; Yusuf, F.; Hamdi, M. Biomedical materials and techniques to improve the tribological, mechanical and biomedical properties of orthopedic implants—A review article. J. Alloys Compd. 2017, 714, 636–667. [Google Scholar] [CrossRef]
- Li, W.; Boccaccini, A.R. Bioactive glasses: Traditional and prospective applications in healthcare. In Hot Topics in Biomaterials; Ahmed, I., Ed.; Future Medicine: London, UK, 2014; pp. 56–68. [Google Scholar]
- Bellucci, D.; Cannillo, V. A novel bioactive glass containing strontium and magnesium with ultra-high crystallization temperature. Mater. Lett. 2018, 213, 67–70. [Google Scholar] [CrossRef]
- Guldiren, D.; Aydin, S. Antimicrobial property of silver, silver-zinc and silver-copper incorporated soda lime glass prepared by ion exchange. Mater. Sci. Eng. C 2017, 78, 826–832. [Google Scholar] [CrossRef]
- Nandi, S.K.; Mahatom, A.; Kundum, B.; Mukherjeem, P. Doped bioactive glass materials in bone regeneration. In Advanced Techniques in Bone Regeneration; Zorzi, A.R., de Miranda, J.B., Eds.; IntechOpen: London, UK, 2016; pp. 275–328. [Google Scholar]
- Rahaman, M.N.; Day, D.E.; Sonny Bal, B.; Fu, Q.; Jung, S.B.; Bonewald, L.F.; Tomsia, A.P. Bioactive glass in tissue engineering. Acta Biomater. 2011, 7, 2355–2373. [Google Scholar] [CrossRef]
- He, X.; Liu, Y.; Tan, Y.; Grover, L.M.; Song, J.; Duan, S.; Zhao, D.; Tan, X. Rubidium-containing mesoporous bioactive glass scaffolds support angiogenesis, osteogenesis and antibacterial activity. Mater. Sci. Eng. C 2019, 105, 110155. [Google Scholar] [CrossRef]
- Balasubramanian, P.; Strobel, L.A.; Kneser, U.; Boccaccini, A.R. Zinc-containing bioactive glasses for bone regeneration, dental and orthopedic applications. Biomed. Glasses 2015, 1, 51–69. [Google Scholar] [CrossRef]
- Sergi, R.; Bellucci, D.; Salvatori, R.; Maisetta, G.; Batoni, G.; Cannillo, V. Zinc containing bioactive glasses with ultra-high crystallization temperature, good biological performance and antibacterial effects. Mater. Sci. Eng. C 2019, 104, 109910. [Google Scholar] [CrossRef] [PubMed]
- Diba, M.; Tapia, F.; Boccaccini, A.R.; Strobel, L.A. Magnesium-containing bioactive glasses for biomedical applications. Int. J. Appl. Glass Sci. 2012, 3, 221–253. [Google Scholar] [CrossRef]
- Souza, M.T.; Crovace, M.C.; Schroder, C.; Eckert, H.; Peitl, O.; Zanotto, E.D. Effect of magnesium ion incorporation on the thermal stability, dissolution behavior and bioactivity in Bioglass-derived glasses. J. Non-Cryst. Solids 2013, 382, 57–65. [Google Scholar] [CrossRef]
- Kargozar, S.; Montazerian, M.; Fiume, E.; Baino, F. Multiple and promising applications of strontium (Sr)-containing bioactive glasses in bone tissue engineering. Front. Bioeng. Biotechnol. 2019, 7, 161. [Google Scholar] [CrossRef]
- Solgi, S.; Khakbiz, M.; Shahrezaee, M.; Zamanian, A.; Tahriri, M.; Keshtkari, S.; Raz, M.; Khoshroo, K.; Moghadas, S.; Rajabnejad, A. Synthesis, characterization and in vitro biological evaluation of sol-gel derived Sr-containing nano bioactive glass. Silicon 2015, 9, 535–542. [Google Scholar] [CrossRef]
- Taherkhani, S.; Moztarzadeh, F. Influence of strontium on the structure and biological properties of sol-gel-derived mesoporous bioactive glass (MBG) powder. J. Sol-Gel Sci. Technol. 2016, 78, 539–549. [Google Scholar] [CrossRef]
- Baino, F.; Fiume, E.; Miola, M.; Verne, E. Bioactive sol-gel glasses: Processing, properties, and applications. Int. J. Appl. Ceram. Technol. 2018, 15, 841–860. [Google Scholar] [CrossRef]
- Pereira, M.M.; Jones, J.R.; Hench, L.L. Bioactive glass and hybrid scaffolds prepared by sol-gel method for bone tissue engineering. Adv. Appl. Ceram. 2005, 104, 35–42. [Google Scholar] [CrossRef]
- Jinga, S.I.; Constantinoiu, I.; Surdu, V.A.; Iordache, F.; Busuioc, C. Sol-gel-derived mineral scaffolds within SiO2–P2O5–CaO–MgO–ZnO–CaF2 system. J. Sol-Gel Sci. Technol. 2019, 90, 411–421. [Google Scholar] [CrossRef]
- Voicu, G.; Ene, V.L.; Sava, D.F.; Surdu, V.A.; Busuioc, C. Sol-gel derived vitroceramic materials for biomedical applications. J. Non-Cryst. Solids 2016, 449, 75–82. [Google Scholar] [CrossRef]
- Saber-Samandari, S.; Baradaran, S.; Nasiri-Tabrizi, B.; Alamara, K.; Basirun, W.J. Microstructural evolution and micromechanical properties of thermally sprayed hydroxyapatite coating. Adv. Appl. Ceram. 2018, 117, 452–460. [Google Scholar] [CrossRef]
- Ivanova, A.A.; Surmeneva, M.A.; Surmenev, R.A.; Depla, D. Influence of deposition conditions on the composition, texture and microstructure of RF-magnetron sputter-deposited hydroxyapatite thin films. Thin Solid Films 2015, 591, 368–374. [Google Scholar] [CrossRef]
- Wang, D.G.; Chen, C.Z.; Ma, Q.S.; Jin, Q.P.; Li, H.C. A study on in vitro and in vivo bioactivity of HA/45S5 composite films by pulsed laser deposition. Appl. Surf. Sci. 2013, 270, 667–674. [Google Scholar] [CrossRef]
- Prodan, A.M.; Iconaru, S.L.; Predoi, M.V.; Predoi, D.; Motelica-Heino, M.; Turculet, C.S.; Beuran, M. Silver-doped hydroxyapatite thin layers obtained by sol-gel spin coating procedure. Coatings 2020, 10, 14. [Google Scholar] [CrossRef]
- Voicu, G.; Miu, D.; Dogaru, I.; Jinga, S.I.; Busuioc, C. Vitroceramic interface deposited on titanium substrate by pulsed laser deposition method. Int. J. Pharm. 2016, 510, 449–456. [Google Scholar] [CrossRef]
- Busuioc, C.; Constantinoiu, I.; Enculescu, M.; Beregoi, M.; Jinga, S.I. Ceramic thin films deposited by spin coating as coatings for metallic implants. Rom. J. Mater. 2018, 48, 401–406. [Google Scholar]
- Kokubo, T.; Kushitani, H.; Sakka, S.; Kitsugi, T.; Yamamuro, T. Solutions able to reproduce in vivo surface-structure changes in bioactive glass-ceramic A-W. J. Biomed. Mater. Res. 1990, 24, 721–734. [Google Scholar] [CrossRef]
- LIVE/DEAD™ Viability/Cytotoxicity Kit, for Mammalian Cells; Thermo Fisher Scientific: Waltham, MA, USA, 2005; p. L3224.
- de Bonis, A.; Curcio, M.; Fosca, M.; Cacciotti, I.; Santagata, A.; Teghil, R.; Rau, J.V. RBP1 bioactive glass-ceramic films obtained by Pulsed Laser Deposition. Mater. Lett. 2016, 175, 195–198. [Google Scholar] [CrossRef]
- Sanz, C.K.; dos Santos, A.R.; da Silva, M.H.P.; Marcal, R.; Tute, E.M.; Meza, E.L.; Mello, A.; Borghi, F.F.; de Souza Camargo, S.A. Niobo-phosphate bioactive glass films produced by pulsed laser deposition on titanium surfaces for improved cell adhesion. Ceram. Int. 2019, 45, 18052–18058. [Google Scholar] [CrossRef]
- Wang, D.G.; Zhang, W.L.; Li, H.J.; Zhang, J.H.; Chen, C.Z. HA/BG composite films deposited by pulse laser under O2 atmosphere. Ceram. Int. 2017, 43, 672–676. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).