Migration of the 3T3 Cell with a Lamellipodium on Various Stiffness Substrates—Tensegrity Model
Abstract
1. Introduction
2. Materials and Methods
2.1. Tensegrity Model
2.2. Material Properties of the Elements
2.3. Prestress
2.4. Simulation Procedure
2.5. Cell Movement Mapping
3. Results
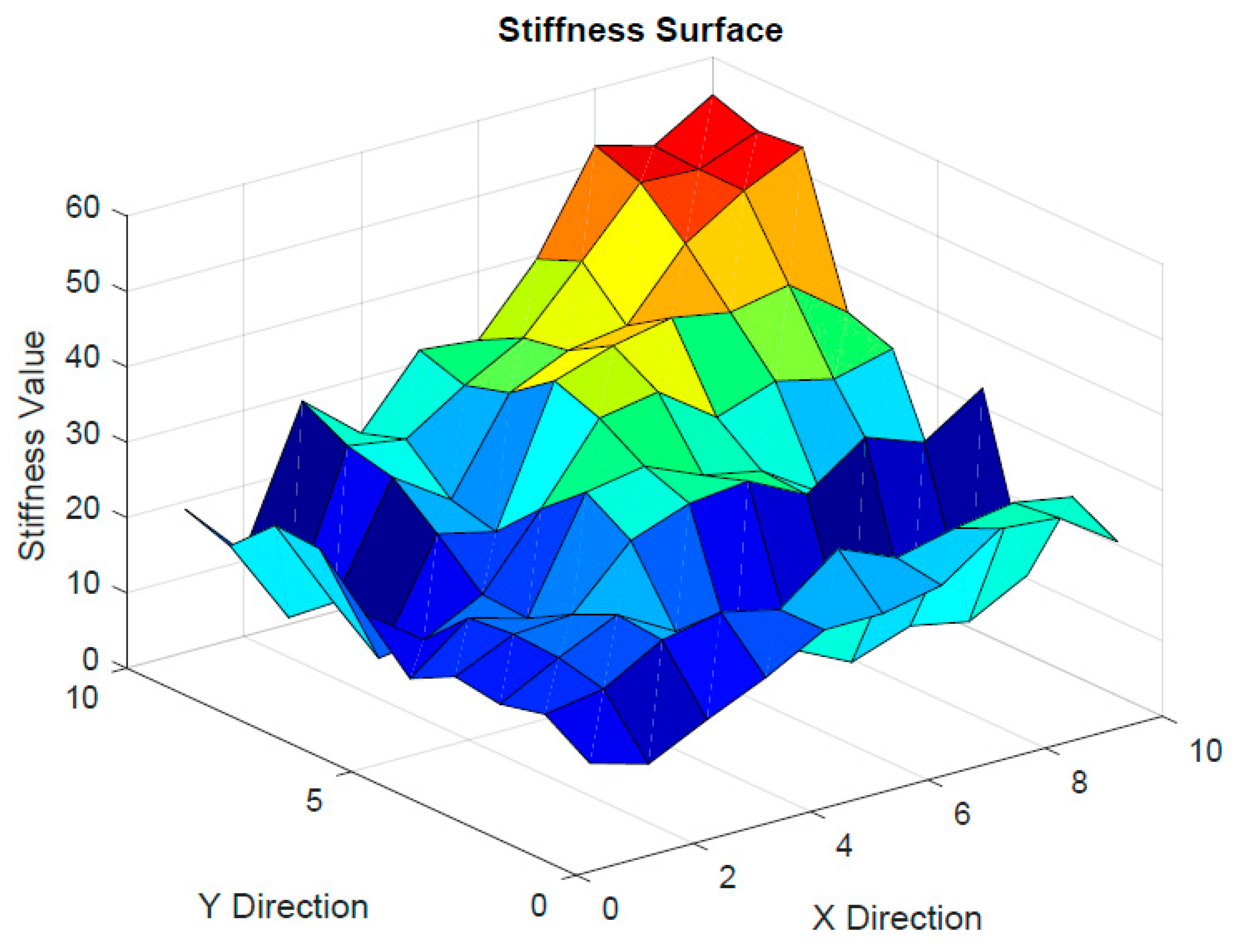
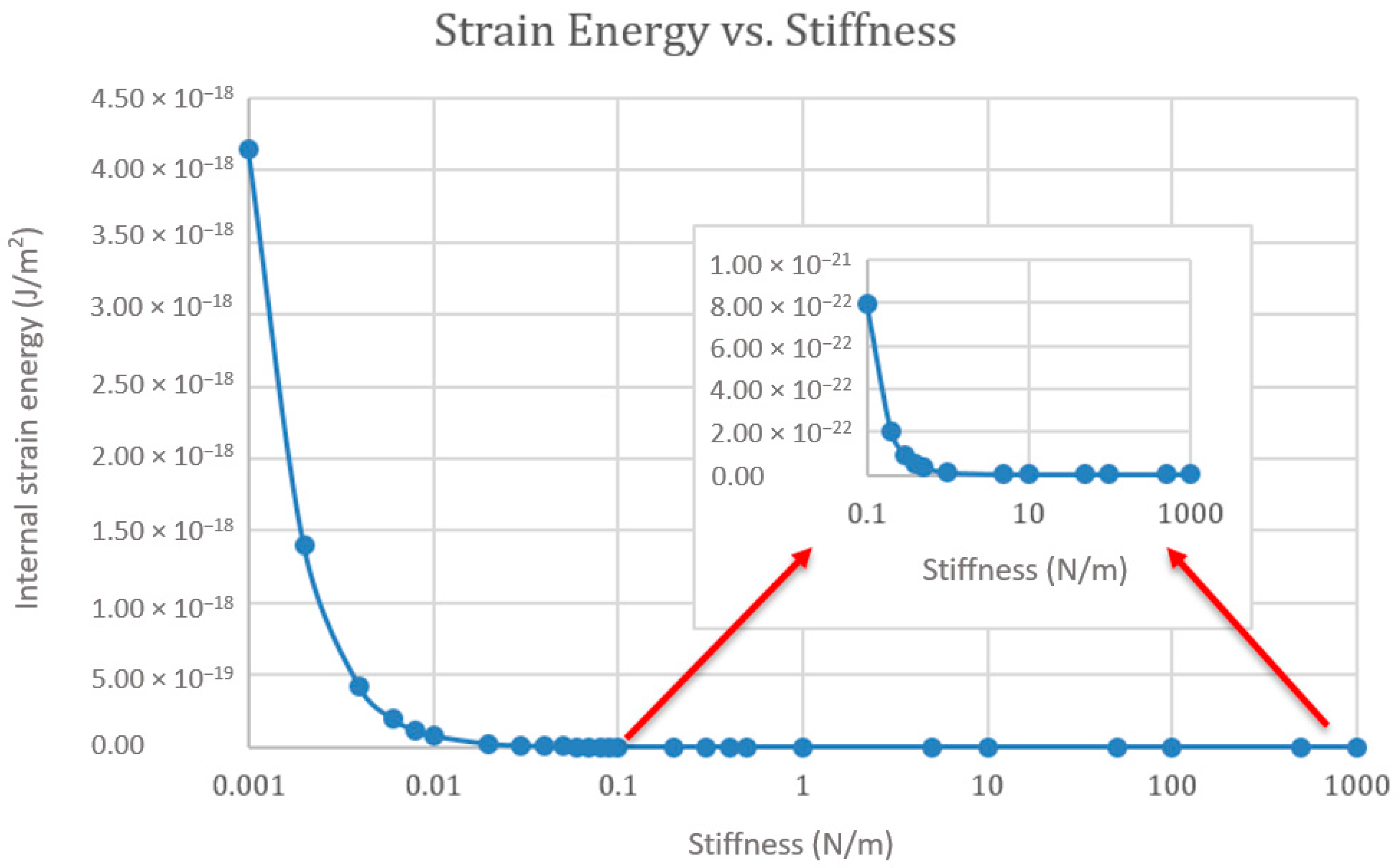
Effect of the Substrate Stiffness on the Cell’s Strain Energy
4. Discussion
5. Conclusions
Funding
Acknowledgments
Conflicts of Interest
References
- Lo, C.M.; Wang, H.B.; Dembo, M.; Wang, Y.L. Cell movement is guided by the rigidity of the substrate. Biophys. J. 2000, 79, 144–152. [Google Scholar] [CrossRef]
- Curtze, S.; Dembo, M.; Miron, M.; Jones, D.B. Dynamic changes in traction forces with DC electric field in osteoblast-like cells. J. Cell Sci. 2004, 117 Pt 13, 2721–2729. [Google Scholar] [CrossRef]
- Umemura, K.; Miyabayashi, T.; Taira, H.; Suzuki, A.; Kumashiro, Y.; Okano, T.; Mayama, S. Use of a microchamber for analysis of thermal variation of the gliding phenomenon of single Navicula pavillardii cells. Eur. Biophys. J. 2015, 44, 113–119. [Google Scholar] [CrossRef] [PubMed]
- Yalcintas, E.P.; Hu, J.; Liu, Y.; Voloshin, A. Modeling cell spreading and alignment on micro-wavy surfaces. Comput. Modeling Eng. Sci. 2014, 98, 151–180. [Google Scholar] [CrossRef]
- Park, J.S.; Kim, D.-H.; Levchenko, A. Topotaxis: A new mechanism of directed cell migration in topographic ecm gradients. Biophys. J. 2018, 114, 1257–1263. [Google Scholar] [CrossRef] [PubMed]
- Walsh, D.I., 3rd; Lalli, M.L.; Kassas, J.M.; Asthagiri, A.R.; Murthy, S.K. Cell chemotaxis on paper for diagnostics. Anal. Chem. 2015, 87, 5505–5510. [Google Scholar] [CrossRef]
- Stamenović, D.; Ingber, D.E. Tensegrity-guided self assembly: From molecules to living cells. Soft Matter 2009, 5, 1137–1145. [Google Scholar] [CrossRef]
- Discher, D.E.; Janmey, P.; Wang, Y.L. Tissue cells feel and respond to the stiffness of their substrate. Science 2005, 310, 1139–1143. [Google Scholar] [CrossRef]
- Georges, P.C.; Janmey, P.A. Cell type-specific response to growth on soft materials. J. Appl. Physiol. 2005, 98, 1547–1553. [Google Scholar] [CrossRef]
- Wang, N.; Butler, J.P.; Ingber, D.E. Mechanotransduction across the cell surface and through the cytoskeleton. Science 1993, 260, 1124–1127. [Google Scholar] [CrossRef]
- Chicurel, M.E.; Chen, C.S.; Ingber, D.E. Cellular control lies in the balance of forces. Curr. Opin. Cell Biol. 1998, 10, 232–239. [Google Scholar] [CrossRef]
- Ghosh, K.; Pan, Z.; Guan, E.; Ge, S.; Liu, Y.; Nakamura, T.; Ren, X.D.; Rafailovich, M.; Clark, R.A. Cell adaptation to a physiologically relevant ECM mimic with different viscoelastic properties. Biomaterials 2007, 28, 671–679. [Google Scholar] [CrossRef] [PubMed]
- Wong, S.; Guo, W.H.; Wang, Y.L. Fibroblasts probe substrate rigidity with filopodia extensions before occupying an area. PNAS 2014, 111, 17176–17181. [Google Scholar] [CrossRef]
- Sochol, R.D.; Higa, A.T.; Janairo, R.R.; Li, S.; Lin, L. Unidirectional mechanical cellular stimuli via micropost array gradients. Soft Matter 2011, 7, 4606–4609. [Google Scholar] [CrossRef]
- Zhang, H.; Lin, F.; Huang, J.; Xiong, C. Anisotropic stiffness gradient-regulated mechanical guidance drives directional migration of cancer cells. Acta Biomater. 2020, 106, 181–192. [Google Scholar] [CrossRef] [PubMed]
- Flaherty, B.; McGarry, J.P.; McHugh, P.E. Mathematical models of cell motility. Cell Biochem. Biophys. 2007, 49, 14–28. [Google Scholar] [CrossRef] [PubMed]
- McGarry, J.G.; Prendergast, P.J. A three-dimensional finite element model of an adherent eukaryotic cell. Eur. Cell Mater. 2004, 7, 27–33. [Google Scholar] [CrossRef]
- Chen, T.J.; Wu, C.C.; Tang, M.J.; Huang, J.S.; Su, F.C. Complexity of the tensegrity structure for dynamic energy and force distribution of cytoskeleton during cell spreading. PLoS ONE 2010, 5, e14392. [Google Scholar] [CrossRef]
- Parameswaran, H.; Lutchen, K.R.; Suki, B. A computational model of the response of adherent cells to stretch and changes in substrate stiffness. J. Appl. Physiol. 2014, 116, 825–834. [Google Scholar] [CrossRef]
- Wendling, S.; Oddou, C.; Isabey, D. Stiffening response of a cellular tensegrity model. J. Theor. Biol. 1999, 196, 309–325. [Google Scholar] [CrossRef]
- Coughlin, M.F.; Stamenovic, D. A tensegrity model of the cytoskeleton in spread and round cells. J. Biomech. Eng. 1998, 120, 770–777. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.S.; Mrksich, M.; Huang, S.; Whitesides, G.M.; Ingber, D.E. Geometric control of cell life and death. Science 1997, 276, 1425–1428. [Google Scholar] [CrossRef] [PubMed]
- Bernstein, L.R.; Liotta, L.A. Molecular mediators of interactions with extracellular matrix components in metastasis and angiogenesis. Curr. Opin. Oncol. 1994, 6, 106. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Gonzalez, D.; Muñoz-Barrutia, A. Computational insights into the influence of substrate stiffness on collective cell migration. Extrem. Mech. Lett. 2020, 40, 100928. [Google Scholar] [CrossRef]
- Yeung, T.; Georges, P.C.; Flanagan, L.A.; Marg, B.; Ortiz, M.; Funaki, M.; Zahir, N.; Ming, W.Y.; Weaver, V.; Janmey, P.A. Effects of substrate stiffness on cell morphology, cytoskeletal structure, and adhesion. Cell Motil. Cytoskelet. 2005, 60, 24–34. [Google Scholar] [CrossRef]
- Li, S.; Guan, J.L.; Chien, S. Biochemistry and biomechanics of cell motility. Annu. Rev. Biomed. Eng. 2005, 7, 105–150. [Google Scholar] [CrossRef]
- Sheetz, M.P.; Felsenfeld, D.P.; Galbraith, C.G. Cell migration: Regulation of force on extracellular-matrix-integrin complexes. Trends Cell Biol. 1998, 8, 51–54. [Google Scholar] [CrossRef]
- Canver, A.C.; Ngo, O.; Urbano, R.L.; Clyne, A.M. Endothelial directed collective migration depends on substrate stiffness via localized myosin contractility and cell-matrix interactions. J. Biomech. 2016, 49, 1369–1380. [Google Scholar] [CrossRef]
- Saez, A.; Ghibaudo, M.; Buguin, A.; Silberzan, P.; Ladoux, B. Rigidity-driven growth and migration of epithelial cells on microstructured anisotropic substrates. Proc. Natl. Acad. Sci. USA 2007, 104, 8281–8286. [Google Scholar] [CrossRef]
- Yeung, A.; Evans, E. Cortical shell-liquid core model for passive flow of liquid-like spherical cells into micropipets. Biophys. J. 1989, 56, 139–149. [Google Scholar] [CrossRef]
- Coughlin, M.F.; Stamenović, D. A prestressed cable network model of the adherent cell cytoskeleton. Biophys. J. 2003, 84, 1328–1336. [Google Scholar] [CrossRef]
- Karcher, H.; Lammerding, J.; Huang, H.; Lee, R.T.; Kamm, R.D.; Kaazempur-Mofrad, M.R. A three-dimensional viscoelastic model for cell deformation with experimental verification. Biophys. J. 2003, 85, 3336–3349. [Google Scholar] [CrossRef]
- Lim, C.T.; Zhou, E.H.; Quek, S.T. Mechanical models for living cells—A review. J. Biomech. 2006, 39, 195–216. [Google Scholar] [CrossRef] [PubMed]
- Shieh, A.C.; Athanasiou, K.A. Principles of cell mechanics for cartilage tissue engineering. Ann. Biomed. Eng. 2003, 31, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Fuller, B. Tensegrity. Portf. Artnews Annu. 1961, 4, 112–127. [Google Scholar]
- Ingber, D.E. Cellular tensegrity: Defining new rules of biological design that govern the cytoskeleton. J. Cell Sci. 1993, 104, 613–627. [Google Scholar]
- Sander, E.A.; Stylianopoulos, T.; Tranquillo, R.T.; Barocas, V.H. Image-based multiscale modeling predicts tissue-level and network-level fiber reorganization in stretched cell-compacted collagen gels. Proc. Natl. Acad. Sci. USA 2009, 106, 17675–17680. [Google Scholar] [CrossRef]
- Cukierman, E.; Pankov, R.; Stevens, D.R.; Yamada, K.M. Taking cell-matrix adhesions to the third dimension. Science 2001, 294, 1708–1712. [Google Scholar] [CrossRef]
- Ni, Y.; Chiang, M.Y. Cell morphology and migration linked to substrate rigidity. Soft Matter 2007, 3, 1285–1292. [Google Scholar] [CrossRef]
- Zanotelli, M.R.; Rahman-Zaman, A.; VanderBurgh, J.A.; Taufalele, P.V.; Jain, A.; Erickson, D.; Bordeleau, F.; Reinhart-King, C.A. Energetic costs regulated by cell mechanics and confinement are predictive of migration path during decision-making. Nat. Commun. 2019, 10, 4185. [Google Scholar] [CrossRef]
- Mattila, P.K.; Lappalainen, P. Filopodia: Molecular architecture and cellular functions. Nature 2008, 9, 446–454. [Google Scholar] [CrossRef] [PubMed]
- Ingber, D.E.; Tensegrity, I. Cell structure and hierarchical systems biology. J. Cell Sci. 2003, 116, 1157–1173. [Google Scholar] [CrossRef] [PubMed]
- Ingber, D.E. Tensegrity and mechanotransduction. J. Bodyw. Mov. Ther. 2008, 12, 198–200. [Google Scholar] [CrossRef] [PubMed]
- Todaro, G.J.; Green, H. Quantitative studies of the growth of mouse embryo cells in culture and their development into established lines. J. Cell Biol. 1963, 17, 299–313. [Google Scholar] [CrossRef]
- Mousavi, S.J.; Doweidar, M.H.; Doblaré, M. Computational modelling and analysis of mechanical conditions on cell locomotion and cell-cell interaction. Computer Methods in Biomechanics and Biomedical Engineering 2012, 17, 678–693. [Google Scholar] [CrossRef]
- Wang, N.; Stamenovic, D. Contribution of intermediate filaments to cell stiffness, stiffening, and growth. Am. J. Physiol. Cell Physiol. 2000, 279, C188–C194. [Google Scholar] [CrossRef]
- Voloshin, A. Modeling cell movement on a substrate with variable rigidity. Int. J. Biomed. Eng. Sci. 2016, 3, 19–36. [Google Scholar] [CrossRef]
- Gittes, F.; Mickey, B.; Nettleton, J.; Howard, J. Flexural rigidity of microtubules and actin filaments measured from thermal fluctuations in shape. J. Cell Biol. 1993, 120, 923–934. [Google Scholar] [CrossRef]
- Ingber, D.E. Tensegrity II. How structural networks influence cellular information processing networks. J. Cell Sci. 2003, 116, 1397–1408. [Google Scholar] [CrossRef]
- Mehrbod, M.; Mofrad, M.R.K. On the significance of microtubule flexural behavior in cytoskeletal mechanics. PLoS ONE 2011, 6, e25627. [Google Scholar] [CrossRef]
- Trichet, L.; Le Digabel, J.; Hawkins, R.J.; Vedula, S.R.K.; Gupta, M.; Ribrault, C.; Hersen, P.; Voituriez, R.; Ladoux, B. Evidence of a large-scale mechanosensing mechanism for cellular adaptation to substrate stiffness. Proc. Natl. Acad. Sci. USA 2012, 109, 6933–6938. [Google Scholar] [CrossRef] [PubMed]
- Gray, D.S.; Tien, J.; Chen, C.S. Repositioning of cells by mechanotaxis on surfaces with micropatterned Young’s modulus. J. Biomed. Mater. Res. Part A 2003, 66, 605–614. [Google Scholar] [CrossRef] [PubMed]
- Stamenovic, D.; Liang, Z.; Chen, J.; Wang, N. Effect of the cytoskeletal prestress on the mechanical impedance of cultured airway smooth muscle cells. J. Appl. Physiol. 2002, 92, 1443–1450. [Google Scholar] [CrossRef] [PubMed]
- Gardel, M.L.; Nakamura, F.; Hartwig, J.H.; Crocker, J.C.; Stossel, T.P.; Weitz, D.A. Prestressed F-actin networks cross-linked by hinged filamins replicate mechanical properties of cells. Proc. Natl. Acad. Sci. USA 2006, 103, 1762–1767. [Google Scholar] [CrossRef]
- Pelham, R.J.; Wang, Y.L. High resolution detection of mechanical forces exerted by locomoting fibroblasts on the substrate. Mol. Biol. Cell 1999, 10, 935–945. [Google Scholar] [CrossRef]
- Galbraith, C.G.; Yamada, K.M.; Galbraith, J.A. Polymerizing actin fibers position integrins primed to probe for adhesion sites. Science 2007, 315, 992–995. [Google Scholar] [CrossRef]
- Hadjipanayi, E.; Mudera, V.; Brown, R.A. Guiding cell migration in 3D: A collagen matrix with graded directional stiffness. Cell Motil. Cytoskelet. 2009, 66, 121–128. [Google Scholar] [CrossRef]
- Lazopoulos, K.A.; Stamenović, D. Durotaxis as an elastic stability phenomenon. J. Biomech. 2008, 41, 1289–1294. [Google Scholar] [CrossRef]






| Microtubules | Microfilaments | Substrate | |
|---|---|---|---|
| ANSYS Element | Link180 | Beam188 | Combin14 |
| Cross Sectional Area (m2) | 45.2 × 10−17 | 45.2 × 10−17 | N/A |
| Length (m) | 2.40 × 10−5 | 1.467 × 10−5 | 2.26 × 10−5 |
| Young’s Modulus (Pa) | 1.20 × 109 | 2.6 × 109 | N/A |
| v | 0.3 | 0.3 | N/A |
| Stiffness Range (N/m) | N/A | N/A | 10−3–1000 |
© 2020 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Voloshin, A. Migration of the 3T3 Cell with a Lamellipodium on Various Stiffness Substrates—Tensegrity Model. Appl. Sci. 2020, 10, 6644. https://doi.org/10.3390/app10196644
Voloshin A. Migration of the 3T3 Cell with a Lamellipodium on Various Stiffness Substrates—Tensegrity Model. Applied Sciences. 2020; 10(19):6644. https://doi.org/10.3390/app10196644
Chicago/Turabian StyleVoloshin, Arkady. 2020. "Migration of the 3T3 Cell with a Lamellipodium on Various Stiffness Substrates—Tensegrity Model" Applied Sciences 10, no. 19: 6644. https://doi.org/10.3390/app10196644
APA StyleVoloshin, A. (2020). Migration of the 3T3 Cell with a Lamellipodium on Various Stiffness Substrates—Tensegrity Model. Applied Sciences, 10(19), 6644. https://doi.org/10.3390/app10196644





