1. Introduction
The common cold is the world’s most common respiratory disease among people of all ages [
1]. It is an acute infection of the upper respiratory tract (URTI) with symptoms, such as a sore throat, cough, cold, and discomfort, lasting up to 14 days [
2,
3]. In a given year, each adult suffers from a cold one to three times, while children, experiencing up to eleven colds per year, are much more frequently affected [
4,
5].
URTIs are usually of viral origin and can be caused by a variety of different viruses, such as adenoviruses, coronaviruses, and, above all, human rhinoviruses (RV) [
6]. Rhinoviruses are responsible for up to two-thirds of all infections of the upper respiratory tract and cause up to 80% of all URTIs during the high season in autumn [
7]. Regardless of the season, rhinoviruses are the most frequently detected viruses in the upper respiratory tract and, thus, the most widespread infectious agents in humans worldwide [
8,
9]. Although this is a self-limiting disease that requires symptomatic treatment at most and it is usually of viral origin, up to 30% of these visits to the doctor result in incorrect prescription of antibiotics due to inadequate diagnostic procedures, which contributes to the development of microbial resistance [
6].
Human rhinoviruses are 30 nm in diameter and non-enveloped viruses of the genus
Enterovirus within the family of
Picornaviridae [
10]. They possess a single-stranded ribonucleic acid genome comprising about 7.2 kilobases and is surrounded by an icosahedral capsid. Its genome consists of only one gene whose expressed polyprotein is cleaved by virally encoded proteases into smaller proteins following translation in host cells [
11]. Four of these proteins (VP1, VP2, VP3, and VP4) are structural proteins and form the capsid, while the remaining non-structural proteins are involved in viral replication [
3]. Currently, more than 100 rhinovirus serotypes are known, which are divided into three different species (RV-A, RV-B, and RV-C) [
11]. Their classification is primarily based on the receptor used for cell entry and genetic differences. More than 90% of the species of RV-A and RV-B use the membrane-bound intercellular cell adhesion molecule-1 (ICAM-1) to enter a host cell [
12,
13]. Therefore, ICAM-1 is considered the main receptor for rhinoviruses, whose expression in nasal and bronchial epithelial cells is additionally enhanced by the viral infection [
14,
15].
RV is mainly transmitted between humans via direct contact with infected persons or indirect contact with contaminated surfaces [
16,
17]. Since rhinoviruses can survive on the skin for several hours, both types of transmission are due to contamination of the hands with nasal secretions of the infected person [
18]. The viruses are then introduced into the body via the fingertips and contact with the eyes or nose [
19]. They can also be transmitted via aerosols caused by coughs, which primarily consist of saliva and are about 30 times less contaminated with virus particles than nasal secretions [
18]. The transmission rate is strongly dependent on the duration of exposure [
20].
Although respiratory infections have a bacterial origin in only 10% of all cases, they are frequently treated with antibiotics, which contribute enormously to the formation of antibiotic resistance [
6]. In addition to the mostly harmless URTIs, rhinoviruses are associated with more severe infections of the lower respiratory tract, including severe bronchiolitis in infants and children, pneumonia in immunosuppressed adults, and the development of asthma [
3]. Furthermore, RV infections can lead to exacerbations of chronic obstructive pulmonary disease and asthma [
21]. Nevertheless, to date, there are hardly any drugs or medical devices on the market that are specifically designed to treat or prevent rhinovirus infections. In this work, the potential of a hydrogel complex, represented by isla
® medic lozenges (Engelhard Arzneimittel, Niederdorfelden, Germany), and two hydrogels based on xanthan gum or sodium hyaluronate are investigated to prevent and treat rhinovirus infections. The hydrogel complex of isla
® medic consists of the polymers sodium hyaluronate, carbomer, xanthan gum, and an aqueous extract of
Cetraria islandica, which is rich in the mucilages lichenin and isolichenin [
22].
2. Materials and Methods
2.1. Cell Culture and Virus Propagation
H1-HeLa cells obtained from the American Type Culture Collection (ATCC® CRL-1958™, Manassas, VA, USA) were cultured at 37 °C and 0% CO2 in Leibovitz’s L-15 Medium supplemented with 10% fetal bovine serum and 1% L-glutamine (Capricorn Scientific, Ebsdorfergrund, Germany).
To propagate the human rhinovirus 14 (RV-14) (ATCC
® VR-284™, Manassas, VA, USA), H1-HeLa-monolayers with 80% confluence were inoculated with the virus at a multiplicity of infection of 0.01 [
23]. After 1 h at 33 °C and 5% CO
2, the remaining inoculum was withdrawn and the cells were covered with Eagle’s Minimum Essential Medium (EMEM) supplemented with 2% fetal bovine serum, 1%
l-glutamine (200 mM), 1% nonessential amino acids (100×), and 1% sodium pyruvate (100 mM) (RV-14-Medium) (Capricorn Scientific, Ebsdorfergrund, Germany). The cells were incubated until a cytopathic effect was observed and then lysed in three freeze/thaw cycles at −20 °C. RV-14 was harvested from the suspension after 5 min of centrifugation at 500× g and stored at −80 °C.
2.2. Virus Titer Determination Using an Endpoint Dilution Assay
H1-HeLa cells were seeded in 96-well plates (30,000 cells per well) and incubated overnight at 37 °C and 0% CO
2. Ten-fold dilutions (10
−2–10
−10) of the virus were inoculated in the wells (six replicates for each dilution, 200 µL per well) for 1 h at 33 °C and 5% CO
2. Afterward, the inoculum was replaced by RV-14 medium and the plates were incubated at 33 °C and 5% CO
2 for 72 h. The titers were determined as a tissue culture infection dose (TCID
50/0.2 mL), according to Reed and Muench [
24].
2.3. Preparation of Hydrogels
To prepare the sample, the hydrogel complex from isla
® medic (marketed in Germany as isla
® med voice) lozenges (Engelhard Arzneimittel, Niederdorfelden, Germany) was diluted with artificial saliva or RV-14 medium in that the lozenges were dissolved while being stirred at 400 rpm and 33 °C. The artificial saliva formulation contains 1.2 g potassium chloride, 0.83 g sodium chloride, 2.5 g sodium hydrogen phosphate dodecahydrate, 0.15 g calcium chloride dihydrate, 0.05 g magnesium chloride hexahydrate (Carl Roth GmbH & Co. KG, Karlsruhe, Germany), 1 g sorbic acid (Caesar & Loretz GmbH, Hilden, Germany), 5 g carmellose sodium 600 (Fagron GmbH & Co. KG, Glinde, Germany), and 43 g 70% sorbitol solution in 1000 mL of distilled water [
25]. Three different solvent volumes were chosen for isla
® medic, resulting in three different concentrations (Conc.) (
Table 1).
The solvent volumes were determined with an estimated sucking time for one lozenge of 8.6 ± 1.5 min and a stimulated salivary flow rate of 1–3 mL/min [
26]. The sample was prepared for 24 h before an experiment was conducted. In order to obtain indications regarding the function of the hydrogel complex, additional hydrogels based on 0.35% xanthan gum or 0.65% sodium hyaluronate were analyzed in this work. These two polymers were selected because they are components of the isla
® medic hydrogel complex. However, the concentrations of sodium hyaluronate and xanthan gum used in the experiments differ significantly from those in the samples of the lozenges. The concentrations were chosen with the intention that the viscosities of the hydrogels resemble the viscosities of the hydrogel complex samples.
2.4. Cell Viability Assay
To determine the concentrations of the hydrogel samples suitable for the in vitro experiments, a neutral red uptake assay was used to determine the cell viability of the H1-HeLa cells after incubation with the different samples. It was performed using the protocol of ISO 10993-5:2009 [
27]. In this assay, the neutral red dye accumulates in the lysosomes of viable cells and can be quantified spectroscopically after cell lysis. The higher the concentration of neutral red in the lysosomes, the more viable cells are present. H1-HeLa cells were seeded in 96-well plates (30,000 cells per well) and incubated overnight at 37 °C and 0% CO
2. After exposure to the samples, cells were incubated for 2 h with a neutral red dye (Carl Roth GmbH & Co. KG, Karlsruhe, Germany) in serum-free medium (50 µg/mL) at 37 °C. Cells were then washed with phosphate-buffered saline (PBS) and lysed with desorption solution (50% ethanol, 49% water, 1% acetic acid) while shaking at 200 rpm for 1 h. The absorbance was measured at 540 nm and 630 nm (reference wavelength) using a Synergy™ HTX Multi-Mode Microplate Reader with the software Gen5™ 2.07 (BioTek Instruments Inc., Winooski, VT, USA). Results are shown in relation to the untreated control (incubated with cell culture medium). All experiments were performed three times with at least six repetitions.
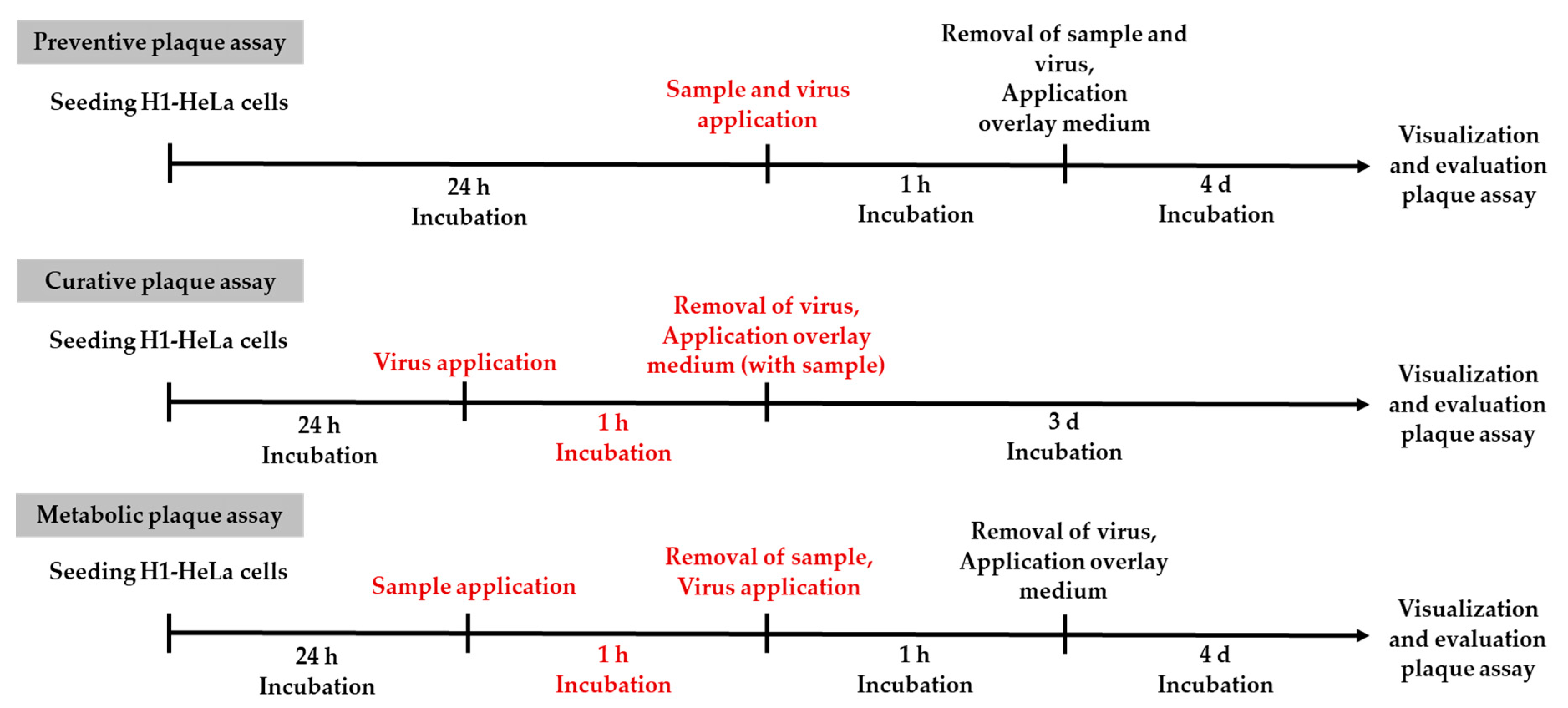
2.5. Plaque Assay for Testing the Protective and Curative Effect of the Hydrogels In Vitro
H1-HeLa cells (600,000 cells per well) were seeded into six-well plates and incubated overnight at 37 °C and 0% CO2. In order to test the protective barrier function of the samples, the cell monolayers were covered with 1517 µL of the hydrogel sample. The inoculum containing a dilution (10−5) of RV-14 was placed on top of the sample and incubated for 1 h at 33 °C and 5% CO2. After incubation, the sample and the inoculum were removed and an overlay medium (49.5% 2× concentrated EMEM, 49.5% 2%-carboxymethylcellulose solution, 1% penicillin/streptomycin) was added. The plates were incubated at 33 °C and 5% CO2 for 96 h. Then the cells were fixed with 10% (v/v) formaldehyde in ultrapure water for 1 h, stained with 0.2% (w/v) crystal violet (Sigma-Aldrich, St. Louis, USA) in 20% (v/v) ethanol for 10 minutes, and washed with phosphate buffered saline (PBS) until the plaques became visible. Plaques were counted 24 h after staining. For the control, the inoculum was applied directly onto the cell monolayer.
In order to test the curative effect of the samples on a rhinovirus infection, cells were first inoculated with RV-14 and then incubated with an overlay medium containing 40% (v/v) isla Conc. 3 for 72 h. The controls were incubated with an overlay medium containing 40% (v/v) 2x concentrated EMEM.
To ensure that the incubation of the cells with the hydrogel complex did not affect plaque formation in the preventive plaque assay on a metabolic level, an alternative plaque assay was performed. H1-HeLa cells were exposed to the samples for 1 h at 33 °C and 5% CO
2. After incubation, the cells were washed with PBS and inoculated with RV-14 for 1 h at 33 °C and 5% CO
2. Then the inoculum was withdrawn. The cells were covered with the regular overlay medium and incubated for 96 h at 33 °C and 5% CO
2. The plaques were visualized as described above. All results were compared to the untreated control. All experiments were performed three times with at least three repetitions.
Figure 1 provides an overview of all plaque assays performed in this work.
2.6. Diffusion of Sodium Fluorescein across the Hydrogels
To investigate the physical barrier function of the hydrogel samples, diffusion studies were performed through a mixed cellulose ester membrane (pore size of 0.2 µm, diffusion area of 1.76 cm2, Whatman, GE Healthcare, Chalfont St Giles, United Kingdom) in Franz diffusion cells (Gauer Glas, Püttligen, Germany). The acceptor chamber was filled with 12 mL of PBS. After 1 h of equilibration of the membrane in phosphate-buffered saline, it was placed in the acceptor chamber and the donor chamber was attached. Then 300 µL of a hydrogel sample (prepared in artificial saliva) was applied to the membrane, which resulted in a layer thickness of 0.17 cm. The control diffusion cell was filled with artificial saliva. A 0.01% (w/v) sodium fluorescein solution in phosphate-buffered saline was used to visualize the diffusion through the sample. For this purpose, 200 µL of the sodium fluorescein solution (20 µg) was placed onto the sample. Throughout the experiment, the temperature of the diffusion cells was kept at 33 °C and the PBS in the acceptor chamber was stirred with a magnetic stirrer at 500 rpm. Samples of the acceptor medium were taken at certain points in time (20 min, 40 min, 1 h, 1.5 h, 2 h, 2.5 h, 3 h, 4 h, 5 h, and 6 h) to determine the amount of sodium fluorescein present. Each time, the withdrawn acceptor medium was replaced with fresh phosphate-buffered saline. It was determined in triplicate for each sample.
To quantify the sodium fluorescein, the fluorescence signal was measured using a Synergy™ HTX Multi-Mode Microplate Reader with the software Gen5™ 2.07 (BioTek Instruments, Winooski, VT, USA), (absorbance: 485/20 nm, emission: 528/20 nm, gain: 35). The concentration of sodium fluorescein was then calculated with four-parameter logistic regression using the software MyAssays Desktop Version R3.2 1.0.995.570 (MyAssays, Brighton, UK).
2.7. Diffusion of RV-14 across the Hydrogels
Diffusion experiments to evaluate the RV-14 permeability of the samples were performed using BRANDplates® Insert System (BRAND GmbH & Co. KG, Wertheim, Germany) with a polycarbonate membrane (pore size 8.0 µm, diffusion area 59 mm2). RV-14 medium was used as an acceptor medium. 100 µL of the hydrogel sample (prepared in RV-14 medium) was applied to the membrane and equilibrated for 30 min at 33 °C. This was followed by applying 33 µL of the virus (1:10 dilution) and 1 h of incubation at 33 °C. The amount of virus in the acceptor medium was quantified by determining the virus titer as described above (Section 2.2).
2.8. Viscosity Measurements
The viscosity of the samples was determined with an oscillation and rotation viscometer (Modular Compact Rheometer MCR-102), a cone plate measuring system (CP 40-1), and Rheocompass software v1.20 (Anton Paar, Graz, Austria). The measuring gap was loaded with 300 µL of the hydrogels and an excess sample was removed once the measuring position was reached. This was followed by a holding time of 10 seconds to equilibrate the sample. A viscosity curve was recorded at a shear rate range of 1–100 s−1 and 33 °C (20 data points, linear ramp). Each sample was measured in triplicate. To evaluate the measured data, the mean values and standard deviations of the viscosities were calculated. Additionally, the average viscosity of the last five measurement points in the shear rate range of 79.2–100 s−1 was calculated.
2.9. Study of Mucoadhesive Properties of the Hydrogels
The relative viscosity increase in the porcine mucin and the polymers (sodium hyaluronate and xanthan gum) was determined in accordance with the publications of Thirawong et al. [
28] and Sriamornsak et al. [
29] to obtain indications regarding the muco-adhesive properties of isla
® medic and the thickened saliva in vivo. Dried mucin from porcine stomach type II (Sigma-Aldrich, St. Louis, MO, USA) was dispersed (10%
w/w) in artificial saliva while being stirred at 400 rpm for three hours at room temperature. To assess the mucoadhesive properties, the hydrogels (0.65% sodium hyaluronate, 0.35% xanthan gum) and mucin were each mixed in the ratio of 1:1. The mixtures were stirred for 10 min at 300 rpm and, after an incubation period of 10 and 30 minutes, respectively, their viscosities were determined as described above (2.8). To determine the synergism of mucin and the polymers, the viscosities of 0.65% sodium hyaluronate, 0.35% xanthan gum, and 10%
w/w mucin, mixed with artificial saliva in the ratio 1:1, were also determined. Lastly, the expected viscosity and the relative viscosity increase were calculated based on Thirawong et al. [
28] (Equations (1) and (2)).
First, the expected viscosity (
ηexp) was calculated by adding up the individual viscosities of the polymer (
ηp) and the porcine mucin (
ηm). The relative viscosity enhancement (
ηrel) was calculated by dividing the measured viscosity of the mixture (
ηobs) with the expected viscosity. The mean value of all data obtained for the relative viscosity enhancement over the entire shear rate range 1–100 s
−1 was calculated. Furthermore, the yield point, defined as the lowest shear stress above which a sample behaves like a liquid, is calculated from the viscosity measurements using the Bingham model [
30,
31]. All measurements were taken in duplicate with at least two repetitions.
2.10. Data Analysis and Statistics
Data are given as arithmetic mean values ± standard deviation. To verify statistically significant differences between the mean values of different groups, a two-way ANOVA, followed by a Tukey test, was carried out. Thus, p ≤ 0.05 is considered to indicate a difference. A statistical analysis was performed using GraphPad Prism® v8.3.0 and GraphPad Software (San Diego, CA, USA).
3. Results
3.1. isla® medic Lozenges Cause Artificial Saliva to Thicken and Form a Hydrogel
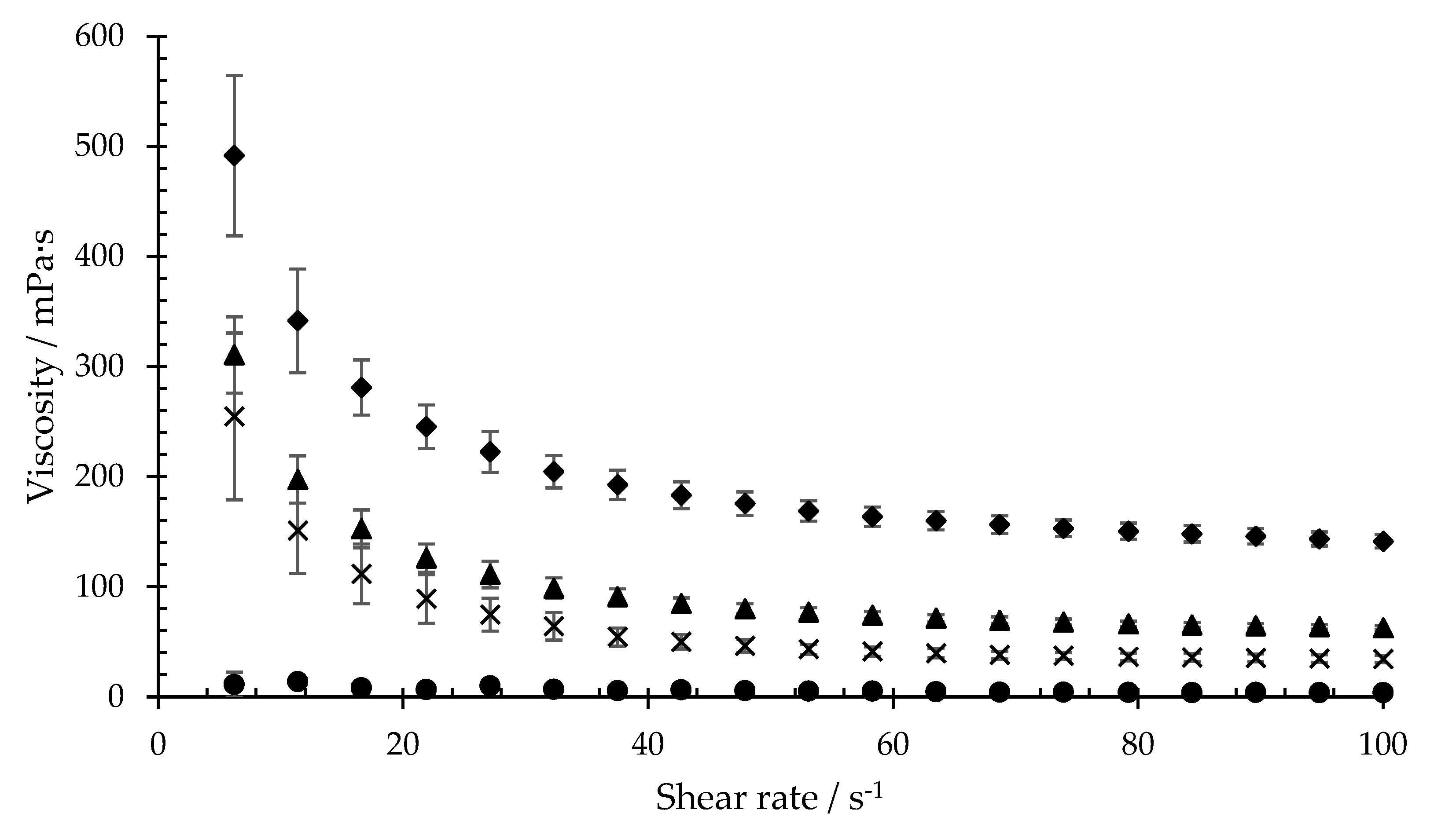
The viscosity measurements were taken to determine whether isla
® medic lozenges cause artificial saliva to thicken and form a hydrogel. Since rhinoviruses can be most efficiently replicated at 33 °C [
32], ideal conditions for adsorption and replication of the virus should be created for in vitro experiments. All viscosity measurements were performed at 33 °C. Based on the literature data for oral shear rates caused by chewing and swallowing, the viscosity of the formulations was determined within a shear rate range of 1100 s
−1 [
33]. To ensure a clear arrangement, all viscosity curves in
Figure 2 start at the second measuring point (6.21 s
−1).
All three concentrations of isla
® medic in artificial saliva led to an increase in viscosity when compared to the pure artificial saliva. A concentration-dependent increase in the viscosity of the saliva was determined. Higher concentration of the dissolved product in artificial saliva is associated with higher viscosity (
Table 2).
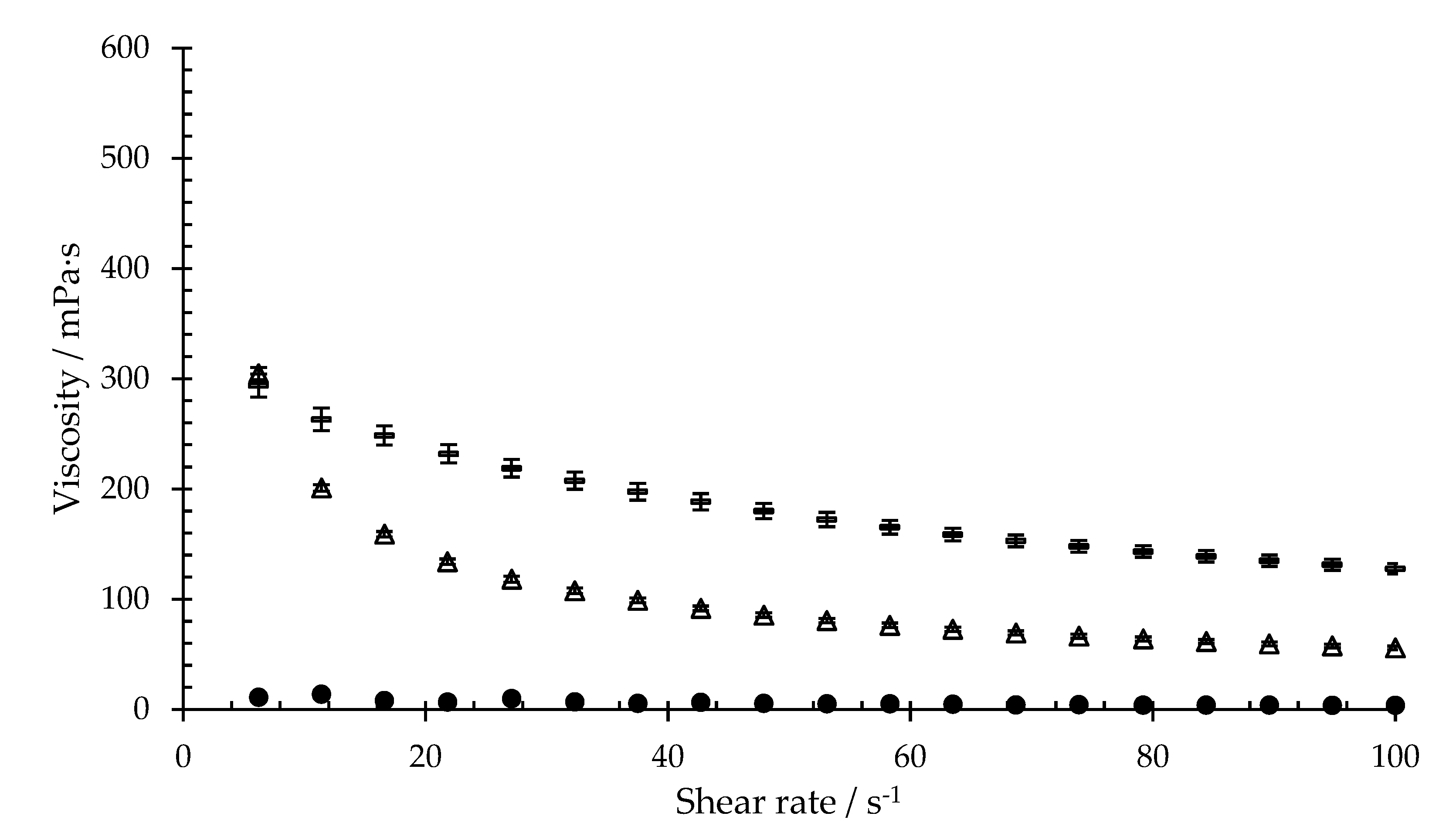
Figure 3 shows the flow curves of 0.35% xanthan gum and 0.65% sodium hyaluronate when compared to artificial saliva. These polymers also result in an increase in the viscosity of artificial saliva. Comparing the mean viscosities in the shear range from 79.2 s
−1 and 100 s
−1, the viscosities of xanthan gum and sodium hyaluronate are between the viscosities of isla Conc. 1 and isla Conc. 3.
3.2. Hydrogels Reduce the Permeation of Sodium Flourescein and Rhinovirus 14
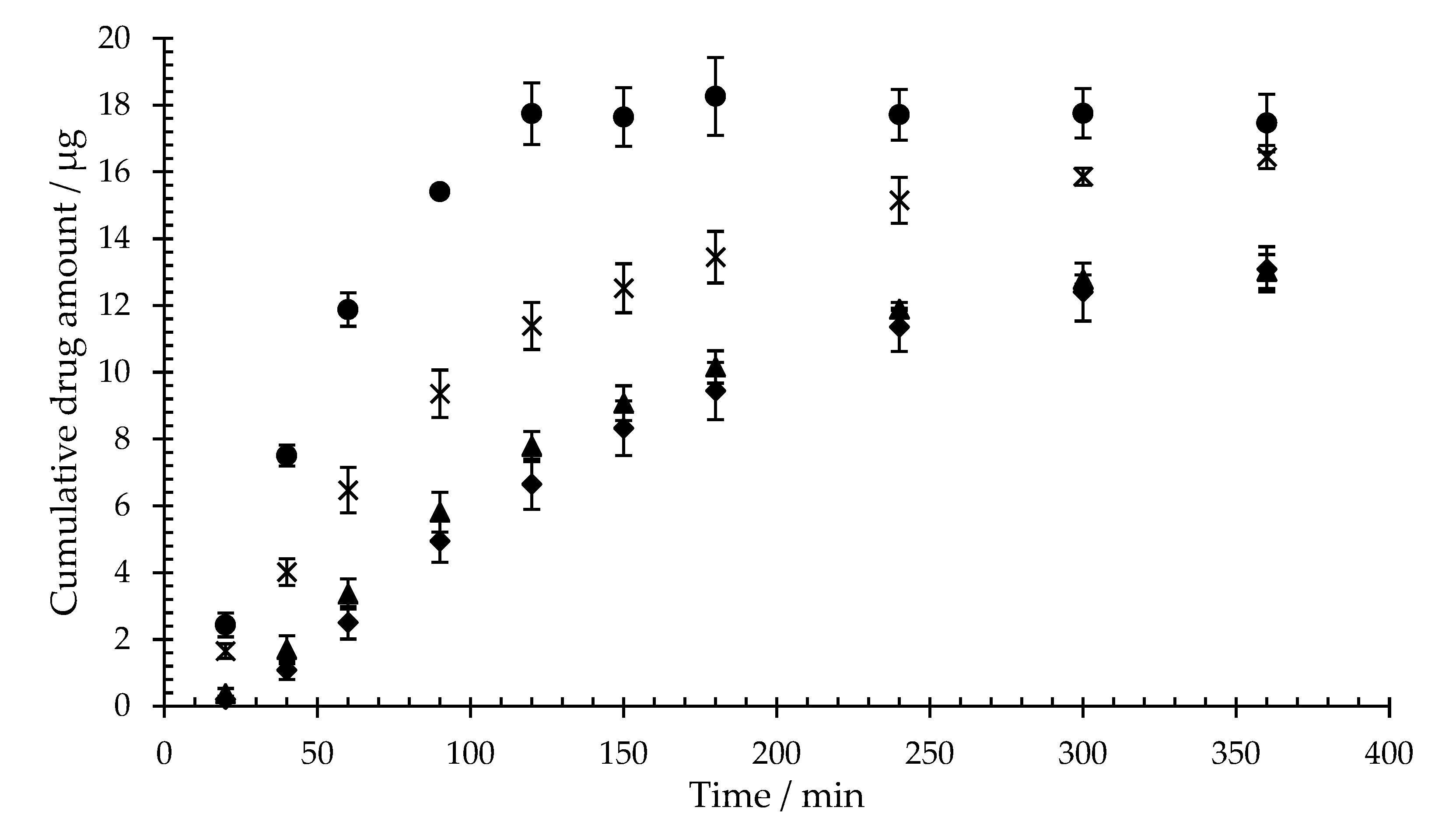
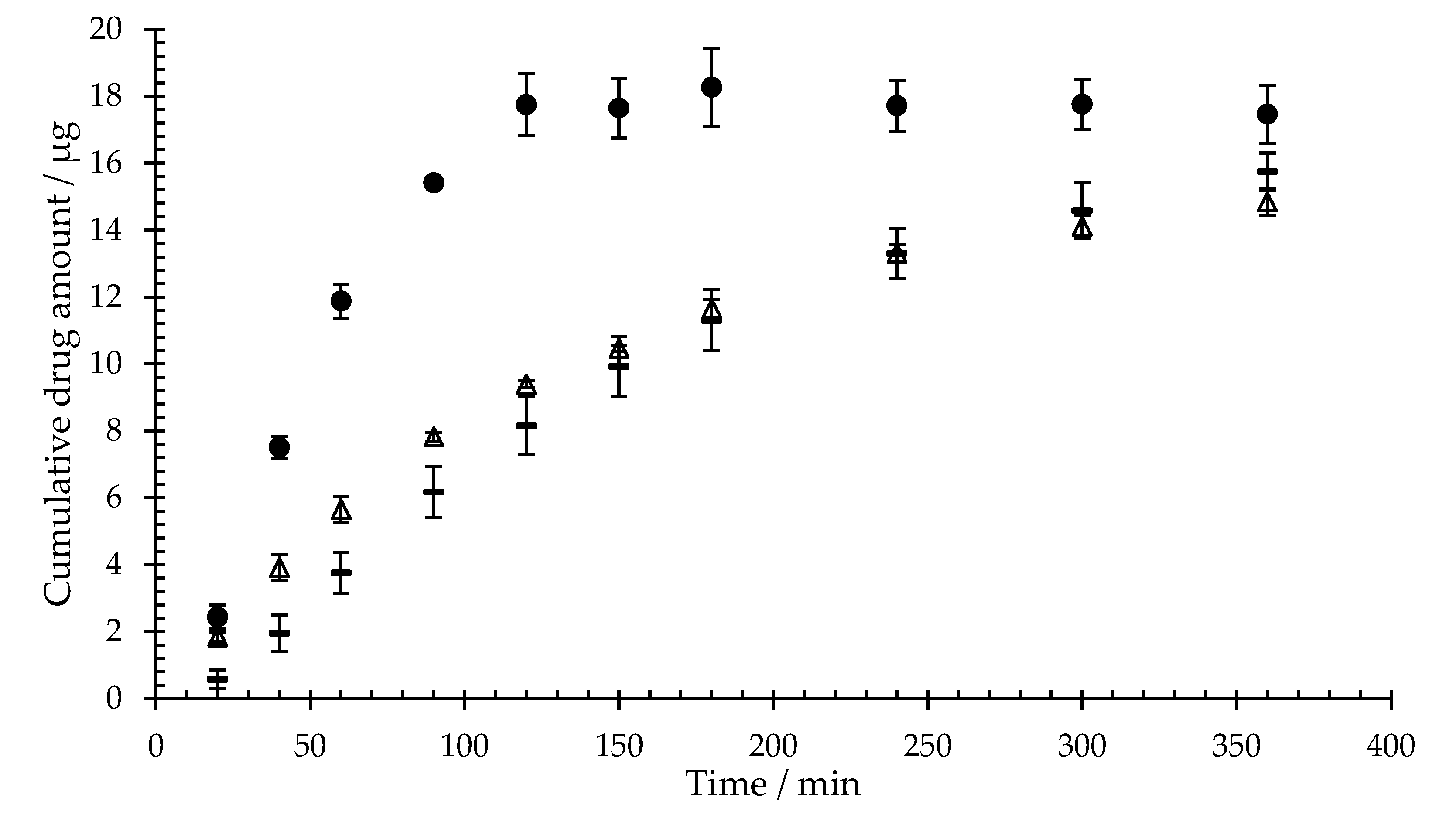
Figure 4 shows the cumulative diffusion of sodium fluorescein across mixed cellulose ester membranes covered with three different hydrogel complex concentrations (isla Conc. 1-3) or artificial saliva. The diffusion was tracked for six hours.
All hydrogel complexes, obtained by dissolving isla
® medic in different volumes of artificial saliva, result in a reduction of sodium fluorescein diffusion. Since the incubation period with the hydrogel complexes and the virus in the preventive plaque assay is 60 minutes, the diffusion of sodium fluorescein at 60 min will be compared in the following. After 60 minutes, 11.9 ± 0.5 µg sodium fluorescein can be detected in the acceptor medium of the diffusion cells loaded with artificial saliva. In contrast, a total of 2.5 ± 0.5 µg of the 20-µg sodium fluorescein applied diffuses through isla Conc. 1 (0.23 g lozenge/mL). The reduction in sodium fluorescein diffusion depends on the concentration of the hydrogel complex of 3.4 ± 0.5 µg (0.115 g lozenge/mL, isla Conc. 2) and 6.5 ± 0.7 µg (0.077 g lozenge/mL, isla Conc. 3). In relation to the untreated control, isla Conc. 1 reduces the sodium fluorescein diffusion at 60 min by 78.9 ± 4.1% (isla Conc. 2 71.8 ± 3.8%, isla Conc. 3 45.5 ± 5.8%).
Figure 5 shows the cumulative diffusion of sodium fluorescein across mixed cellulose ester membranes covered with 0.35% xanthan gum and 0.65% sodium hyaluronate in artificial saliva.
The diffusion of sodium fluorescein is also reduced by these two hydrogels. In 60 minutes, 5.7 ± 0.4 µg of sodium fluorescein diffuses through 0.35% xanthan gum and 3.8 ± 0.6 µg through 0.65% sodium hyaluronate. This results in a reduction of 52.4% ± 3.3% for xanthan gum and 68.3% ± 5.1% for sodium hyaluronate.
Permeation studies were conducted with RV-14 to investigate whether the inhibition of diffusion caused by the hydrogels also works for rhinoviruses. The amount of virus in the acceptor medium was quantified by determining the virus titer as TCID50/0.2 mL. Higher virus titers can be detected in the virus solutions permeated through the hydrogels, which indicates that the virus concentration in the acceptor medium is lower than the control without a hydrogel, 104.59 ± 100.36 TCID50/0.2 mL (control), 10 2.44 ± 100.03 TCID50/0.2 mL (0.23 g lozenge/mL, isla Conc. 1), 103.42 ± 100.06 TCID50/0.2 mL (0.115 g lozenge/mL isla Conc. 2), and 103.44 ± 100.04 TCID50/0.2 mL (0.077 g lozenge/mL, isla Conc. 3). With regard to the untreated control, a titer reduction with isla Conc. 1 (99% ± 0.5%), isla Conc. 2 (92% ± 5%), and isla Conc. 3 (91% ± 5%) was conducted.
3.3. Hydrogels Reduce Rhinovirus Infections In vitro and Have a Curative Effect on Infected H1-HeLa Cells
First, the influence of the hydrogel complexes (isla® medic Conc. 1-3) and the hydrogels based on xanthan gum or sodium hyaluronate on H1-HeLa cell viability was examined over 2 h to determine which concentrations are suitable for the in vitro experiments. The two-hour incubation period of the samples served to test whether a one-hour to two-hour virus adsorption time is feasible in the plaque assay without having any effects on the cells and the monolayer. All tested substances show acceptable viability within the system. After a 2-h incubation with isla Conc. 1 (0.23 g lozenge/mL), the H1-HeLa cells show a viability of 71.0% ± 6.2%. The average viabilities of the H1-HeLa cells incubated with 0.115 g lozenge/mL (isla Conc. 2) and 0.077 g lozenge/mL (isla Conc. 3) were 92.9% ± 1.0% and 96.6% ± 3.3%, respectively. Therefore, the plaque assay to determine the preventive function of the hydrogels on rhinovirus infections was carried out with 0.115 g lozenge/mL and 0.077 g lozenge/mL. The two-hour incubation with two of the main components of the hydrogel complex contained in isla® medic, xanthan gum, and sodium hyaluronate, resulted in relative cell viabilities of 101.5% ± 1.5% (xanthan gum) and 93.7% ± 4.9% (sodium hyaluronate), which indicates that the hydrogels can also be used in the preventive plaque assay.
To determine the effect of the hydrogels in terms of preventing rhinovirus infections in vitro, the physical barrier function was tested in a plaque assay with RV-14. H1-HeLa monolayers were covered with isla Conc. 2 (0.115 g lozenge/mL) or isla Conc. 3 (0.077 g lozenge/mL) and RV-14 was applied onto the hydrogels. As shown in
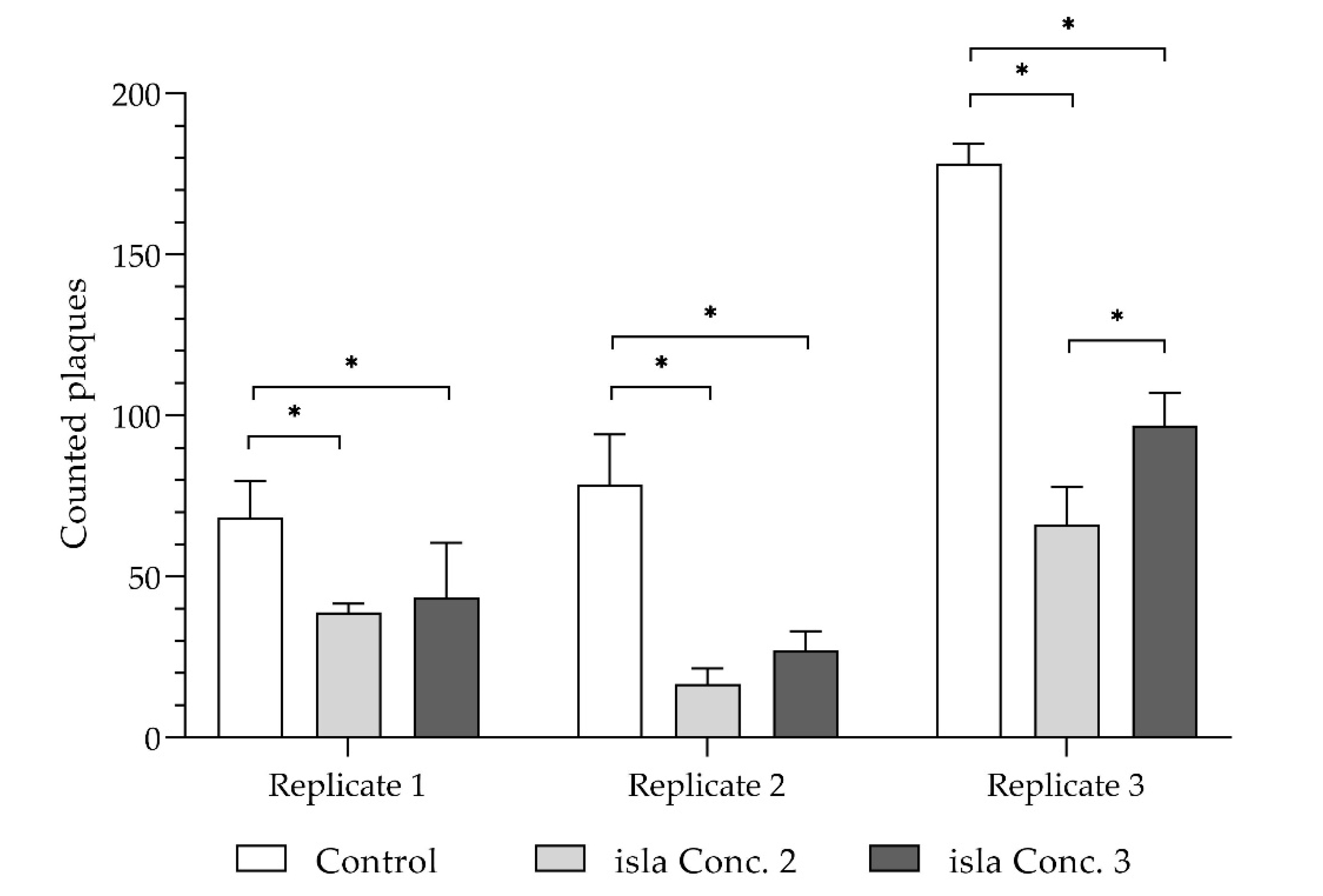
Figure 6, the monolayers covered with the hydrogels have fewer plaques than the untreated control.
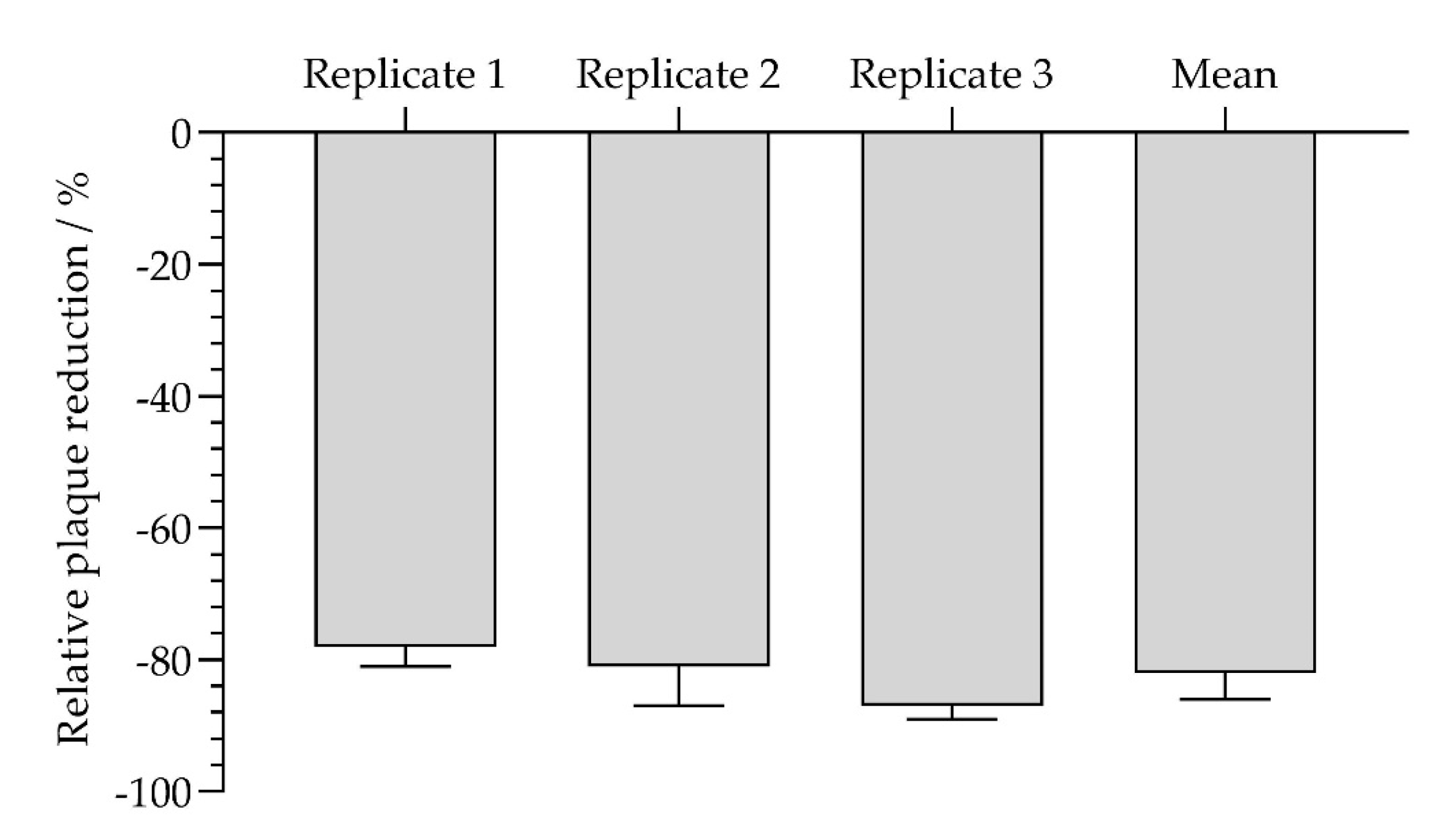
In total, in all three independent experiments, significantly fewer plaques were formed in the cell monolayers covered with isla Conc. 2 and isla Conc. 3. With regard to the untreated control, isla Conc. 2 (0.115 g lozenge/mL) reduces plaque formation by a mean of 61.7% ± 14.7% and isla Conc. 3 by 49.3% ± 12.5% (
Figure 7). Taking into account the standard deviation, no concentration-dependent effect can be determined.
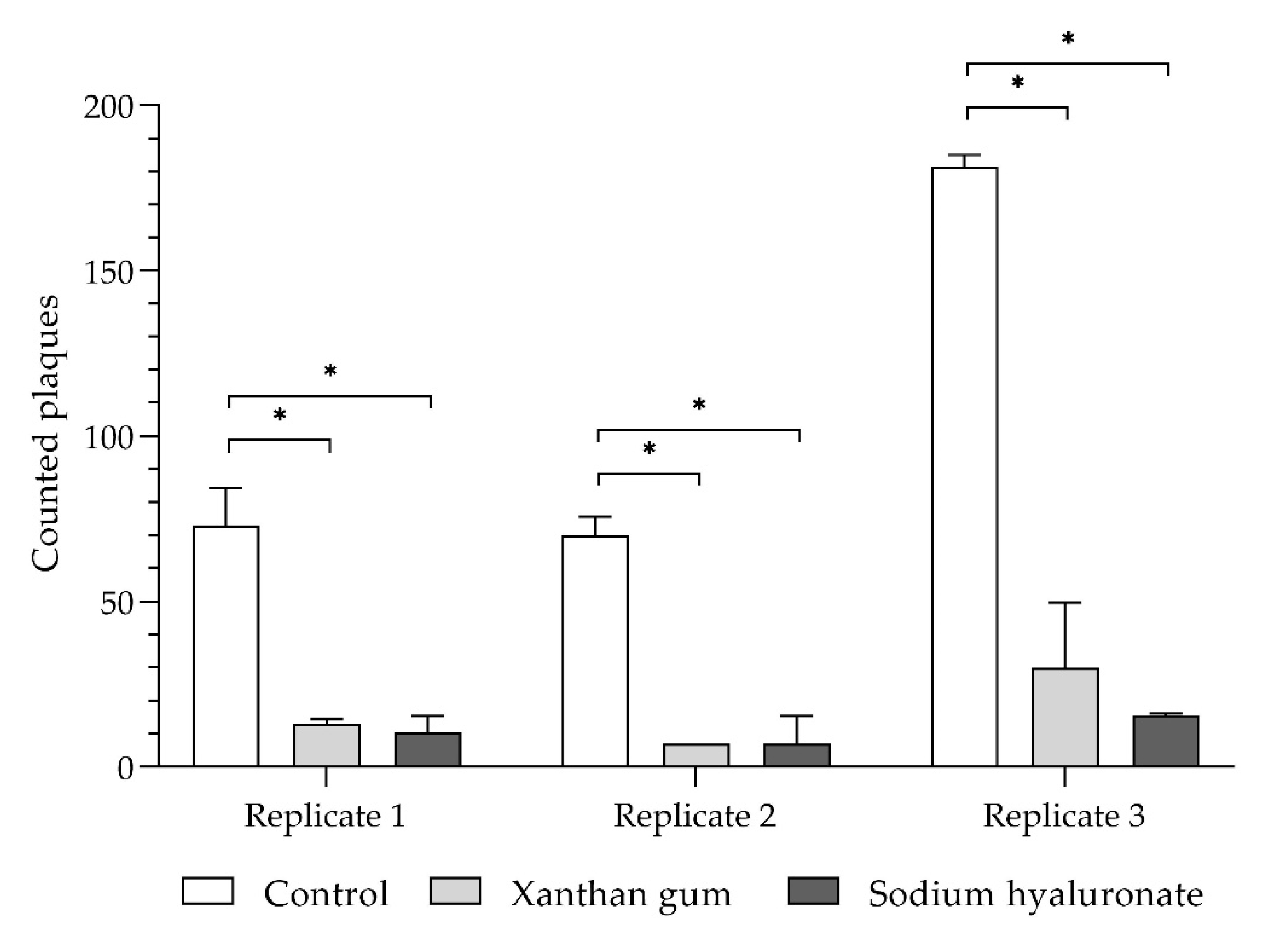
Figure 8 shows the results of the preventive plaque assay carried out with 0.35% xanthan gum or 0.65% sodium hyaluronate.
Even the individual components of the hydrogel complex contained in isla® medic, such as xanthan gum, reduces plaque formation significantly by 85.1% ± 4.3% and sodium hyaluronate by 89.0% ± 3.1%.
A curative plaque assay was performed to investigate whether the hydrogel complex not only has a preventive protective function but also a curative effect on an existing rhinovirus infection. After infection of the cells, an overlay medium was applied for 72 h to which isla Conc. 3 was added in different amounts. To determine the ideal amount of the product for the curative plaque assay, the cell viability was examined after a 72-h incubation time with different proportions of isla Conc. 3 (20%, 30%, 40%, and 50%) in the overlay medium. The average cell viabilities of the H1-HeLa cells incubated with 20%, 30%, 40%, and 50% isla Conc. 3 (0.077 g lozenge/mL) for 72 h were 83.4% ± 2.7%, 76.7% ± 3.3%, 70.3% ± 3.7%, and 56.5% ± 2.7%, respectively. These preliminary plaque assay studies have shown that 40% isla Conc. 3 is the highest concentration where the osmolality does not affect the cell monolayer after 72 h (
Figure 9b) [
27].
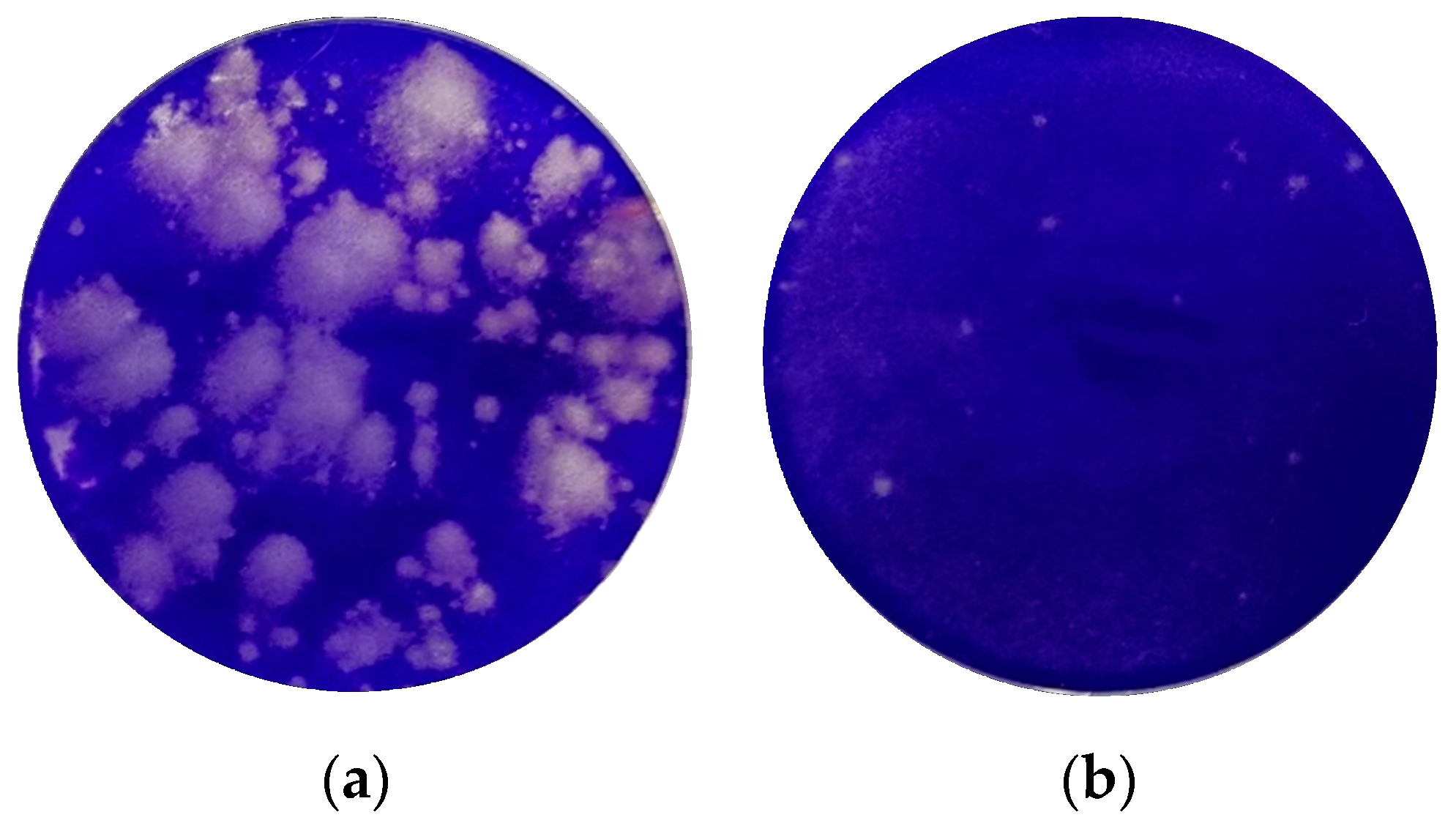
As shown in
Figure 9, fewer and smaller plaques were formed on the monolayers incubated with the hydrogel isla Conc. 3 (0.077 g lozenge/mL) after rhinovirus infection.
In all three independent experiments, fewer and smaller plaques were formed in the monolayers incubated with 40% isla Conc. 3 after rhinovirus infection when compared with the controls. In total, 40% isla Conc. 3 in the overlay medium results in plaque reduction of 87% ± 4% (
Figure 10). Therefore, the hydrogels have an inhibitory effect on the spread of rhinovirus infections.
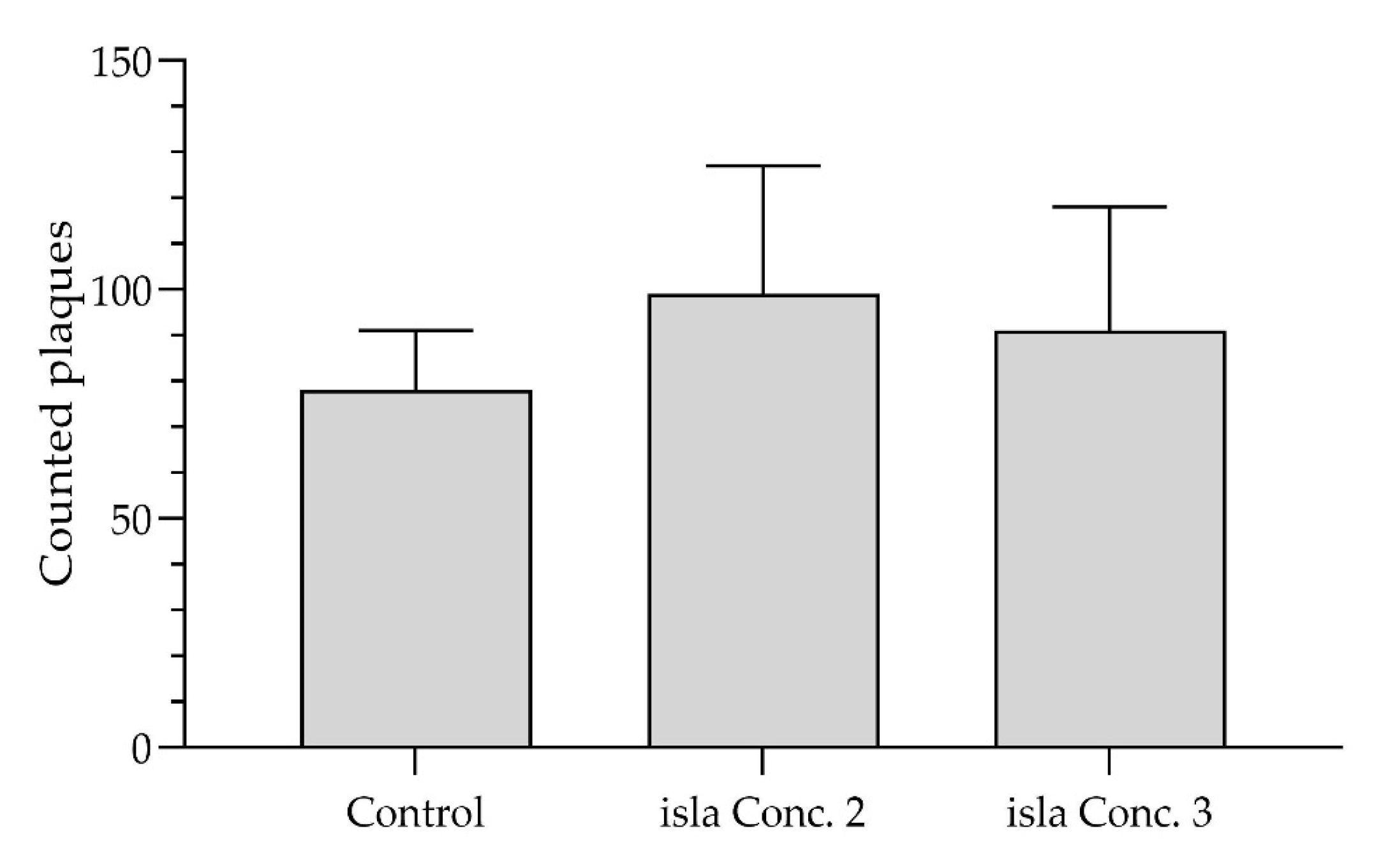
To ensure that the incubation of the cells with the hydrogels does not affect plaque formation in the preventive plaque assay on a metabolic level, an alternative plaque assay, in which the cells were exposed to the hydrogel complex prior to rhinovirus infection, was performed. A similar number of plaques were formed in the control monolayers and the cell monolayers were treated with the hydrogels in all three independent experiments. In total, 78 ± 13 plaques were formed in the control monolayers, 99 ± 28 plaques with isla Conc. 2 (0.115 g lozenge/mL), and 91 ± 27 with isla Conc. 3 (0.077 g lozenge/mL) (
Figure 11). A two-way analysis of variance (ANOVA), followed by a Tukey test, showed no significant difference between the mean values of the control and isla Conc. 2 or isla Conc. 3 (
p ≤ 0.05).
This indicates that plaques could still be formed after the cells were treated with the hydrogel complex.
3.4. Artificial Saliva Thickened by the Polymers Shows Increased Mucoadhesion
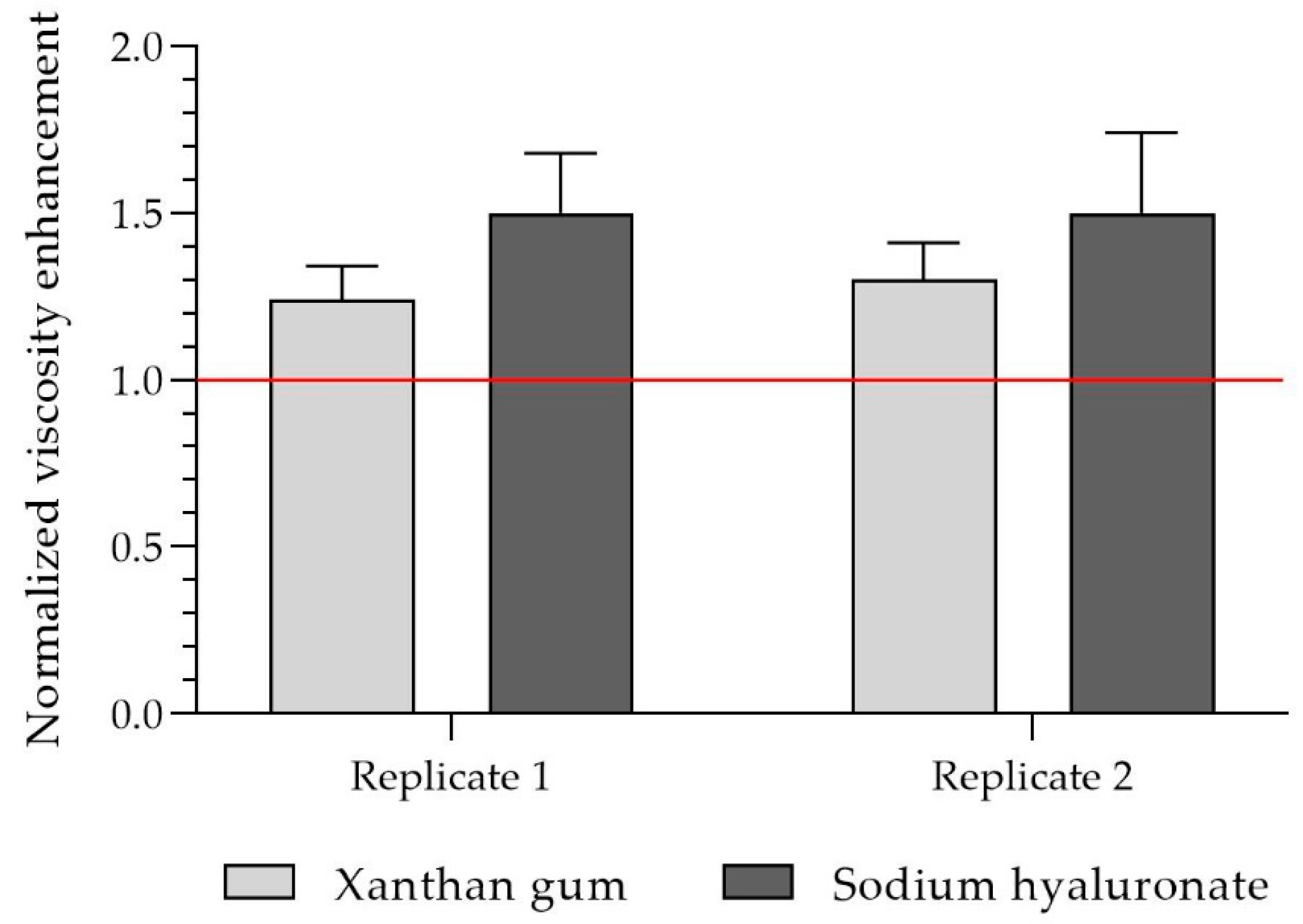
Figure 12 shows the mean values of the relative viscosity increase in xanthan gum and sodium hyaluronate mixed with porcine mucin at a shear rate range of 1–100 s
−1. All normalized values are higher than 1, which implies that mixing with porcine mucin results in a synergistic increase in viscosity. Overall, the values for xanthan gum at the different shear rates are 1.3, and for sodium hyaluronate, they are slightly higher at around 1.5.
Calculated from the viscosity data using the Bingham model, mixing xanthan gum with mucin increases the yield point from 0.98 mPa∙s ± 0.01 mPa∙s to 1.67 mPa∙s ± 0.09 mPa∙s. An increase in the yield point can also be observed with sodium hyaluronate and mucin. In this case, an increase from 0.33 mPa∙s ± 0.06 mPa∙s to 1.14 mPa∙s ± 0.24 mPa∙s is seen.
4. Discussions
In this work, we demonstrate that the hydrogel complex in isla
® medic voice lozenges and hydrogels reduce rhinovirus infections in vitro. Isla
® medic lozenges are a medical device from Engelhard Arzneimittel GmbH & Co. KG, which are recommended to relieve cold and throat complaints [
34]. The viscosity measurements in
Figure 2 show that dissolving the lozenge in artificial saliva causes the saliva to thicken. The thickening can be associated with the polymers contained in the product (carbomer, aqueous extract of Icelandic moss, sodium hyaluronate, and xanthan gum), which are capable of forming a hydrogel complex in combination with the artificial saliva [
35,
36,
37]. This is caused by the polymers absorbing the saliva and forming three-dimensional structures in the solution, which are stabilized by hydrogen or ionic bonds [
38]. In addition,
Figure 2 shows that a higher concentration of the dissolved product is associated with higher viscosity, which is due to the higher number of polymers in the sample. The addition of xanthan gum or sodium hyaluronate also causes artificial saliva to thicken. Therefore, it can be assumed that hydrogels are formed (
Figure 3). Furthermore, the results show that the viscosity decreases as the shear rate increases, which demonstrates a shear-thinning behavior of the hydrogels [
33]. This is due to the orientation of the polymer chains in shear direction, which results in a shear-dependent disentanglement. This, in turn, reduces the flow resistance and, thus, the viscosity [
33]. Sodium fluorescein diffusion assays present a reduction in diffusion of fluorescein in all samples compared to artificial saliva (
Figure 4 and
Figure 5). Thus, it can be proven that the thickening of the saliva with isla
® medic, sodium hyaluronate, and xanthan gum creates a physical barrier for the diffusion of the model drug. In addition, the results show that the viscosity of the hydrogels is not the only factor affecting the diffusion through the gels. This becomes particularly apparent while comparing the different concentrations of isla
® medic in terms of viscosity and diffusion of sodium fluorescein through the samples, which are not linearly correlated. For example, the difference in viscosity in the investigated shear rate range rises in an almost proportionate fashion with the concentration from isla Conc. 3 (0.077 g lozenge/mL) to isla Conc. 1 (0.23 g lozenge/mL). In contrast, the diffusion rates of sodium fluorescein through isla Conc. 1 and isla Conc. 2 differ only slightly at 60 min (isla Conc. 1 78.9% ± 4.1%, isla Conc. 2 71.8% ± 3.8%), while the difference in diffusion rates of isla Conc. 2 and isla Conc. 3 (45.5% ± 5.8%) is much more pronounced. Drug diffusion through a hydrogel matrix is mainly dependent on the mesh structure, the pore number, and their distribution within the network of the gel [
39]. These factors vary depending on the polymers used, which, therefore, influence the diffusion flow of particles [
40]. Consequently, it can be concluded that the structure of the formed hydrogels and the hydrogel complex might be the reason for the differences between the samples in terms of the reduction in sodium fluorescein diffusion. The RV-14 diffusion tests show that the hydrogel complex of isla
® medic also acts as a barrier for the viruses, as there is a reduction in virus titer when compared to the control.
Both hydrogels based on xanthan gum and sodium hyaluronate, as well as the hydrogel complex from isla
® medic, result in significantly reduced plaque formation in the preventive plaque assay (
Figure 7 and
Figure 8). The individual polymers contained in isla
® medic show, in the chosen application concentrations, a remarkable effect on plaque formation as well as on the diffusion of fluorescein. We have chosen the time point of 1 h, as the decisive time for the diffusion studies and the preventive plaque assay, because, in a survey by Staiger et al. [
41], 82% of the patients who used isla
® medic stated that the noticeable effect lasted up to 60 min or longer after sucking. Overall, the results of the preventive plaque assay demonstrate that the physical barrier function is also present in the in vitro experiments. Consequently, the hydrogels represent a barrier to RV-14 and most likely prevent the virus from reaching and infecting the cells. A possible trigger for an RV infection of the lower respiratory tract can be a previous URTI, as increased swallowing of infectious postnasal secretions and, due to extended mouth breathing, dry and irritated mucous membranes in the mouth and throat area contribute to the spread of an RV infection to the lower airways [
42]. Therefore, we are convinced that the oral intake of isla
® medic can contribute to the prevention and treatment of RV infections.
The results of the metabolic plaque assay (
Figure 11) confirm that plaques could still be formed after the cells were treated with the hydrogel complex and support the assumption that the plaque reduction in the preventive plaque assay is due to reduced diffusion of viruses through the hydrogel. In addition, the hydrogel complex has a curative effect on RV-14-infected cell monolayers (
Figure 10). Thus, the hydrogel content in the overlay medium results in much smaller and fewer plaques when compared to the control. The curative effect might be caused by the thickening of the artificial saliva, as the increased viscosity results in less spreading of the viral infection. A similar effect is achieved when performing a plaque assay in the laboratory by applying the viscose overlay medium after infection of the host cells. The overlay medium is intended to prevent the virus infection from spreading indiscriminately during virus replication either by mechanical or convective flow of the liquid medium [
43].
Comparable results with regard to the protective properties against rhinoviruses and other viruses could be shown for the polymer carrageenan [
44,
45]. Furthermore, the work of Grassauer et al. [
44] demonstrates that the polymer prevents the replication of different rhinovirus serotypes to varying degrees. Concerning the applicability of the in vitro results to the situation in vivo, clinical studies by Eccles et al. [
46] and Ludwig et al. [
47] demonstrate that the polymer carrageenan applied as a nasal spray in patients show a cold virus infection leads to a faster resolution of symptoms than in the placebo group. Furthermore, viral titers in nasal fluids showed a significantly higher decrease in carrageenan patients. Therefore, we believe that the polymers investigated in our work also have the potential to lead to positive effects in vivo regarding rhinovirus infections.
Since there are more than 100 serotypes of the rhinovirus, developing suitable extensive prophylaxis and therapy for rhinovirus infections is extremely complex [
3]. However, we are convinced that the principle of a physical barrier, represented by polymer-based hydrogels, can be applied to various rhinovirus serotypes.
In view of the studies on the mucoadhesive properties of the polymers, an increase in viscosity after mixing the sample with porcine mucin indicates synergism between the polymers and the mucin and, thus, mucoadhesive properties [
28]. According to the publication of Thirawong et al. [
28], a relative increase in viscosity higher than 1 demonstrates synergism between polymers and mucin. Consequently, both xanthan gum and sodium hyaluronate have mucoadhesive properties (
Figure 12), which are also indicated by the increase in yield point. Since both polymers are part of the isla
® medic, the mucoadhesive properties can also be transferred to the lozenges. Therefore, the thickened saliva, which is produced when the lozenge is sucked in vivo might have mucoadhesive properties as well. A putative protective function against rhinovirus infections as exemplified in the former assays could be supported by a longer retention time of the physical barrier on the mucous membranes in the mouth and throat area.