Wettability Alteration by Carbonated Brine Injection and Its Impact on Pore-Scale Multiphase Flow for Carbon Capture and Storage and Enhanced Oil Recovery in a Carbonate Reservoir
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Rock Characterization
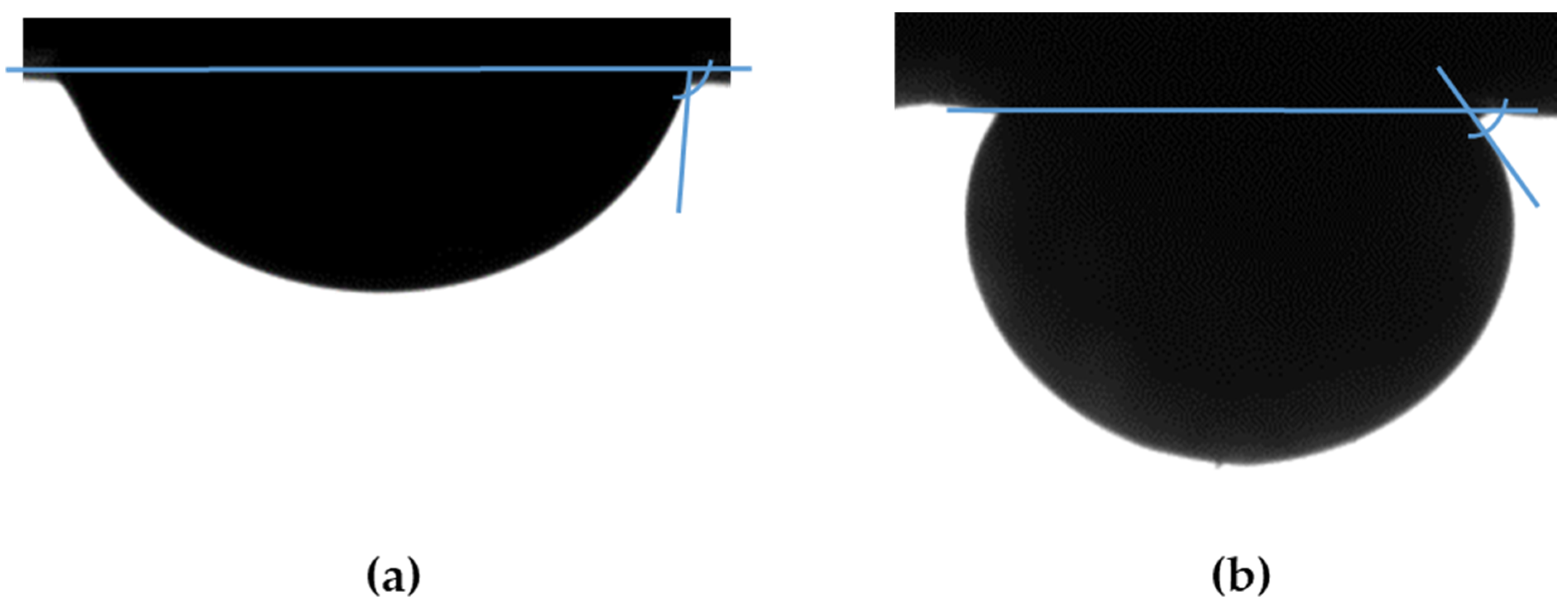
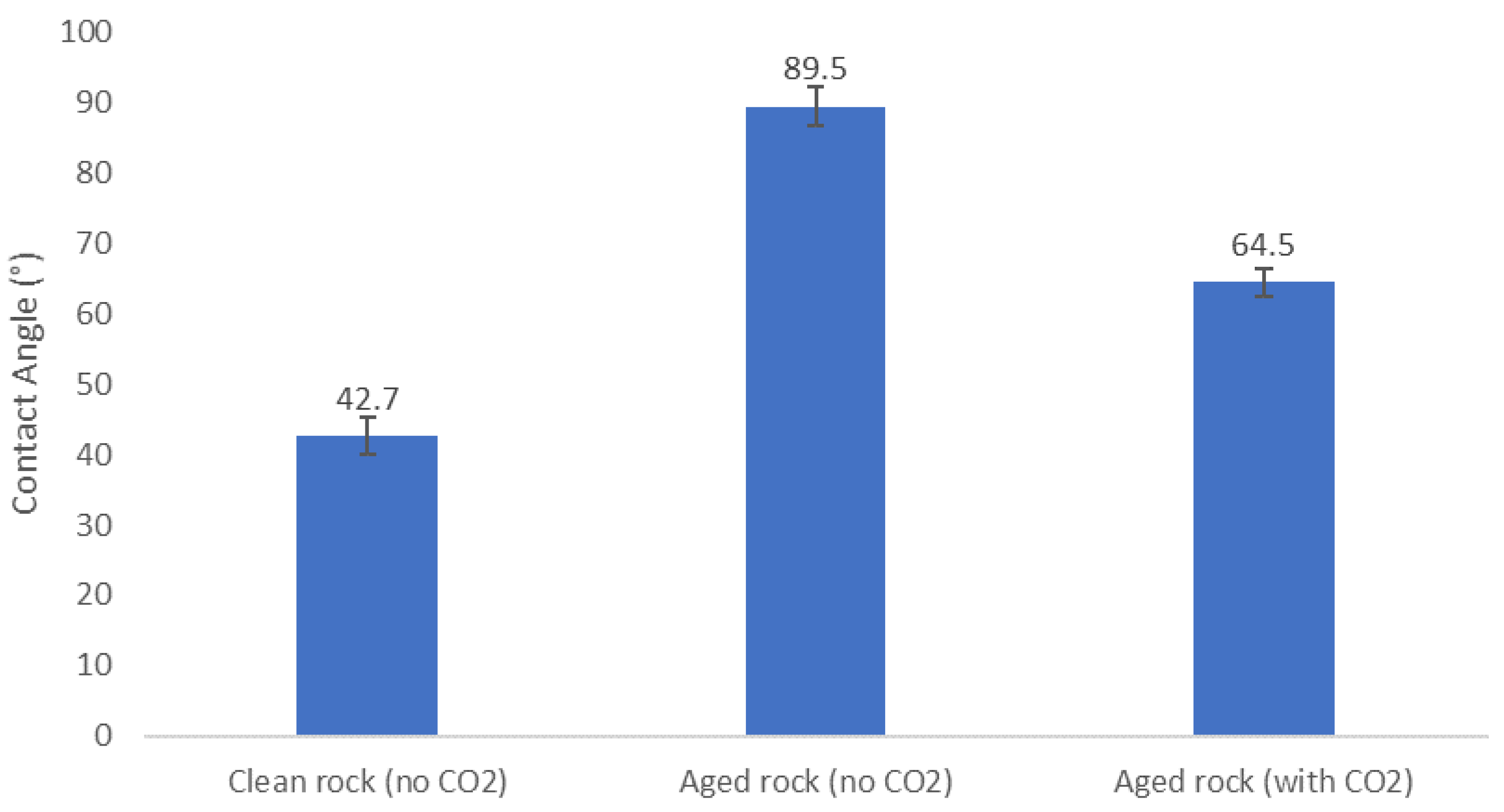
3.2. Contact Angle Measurements
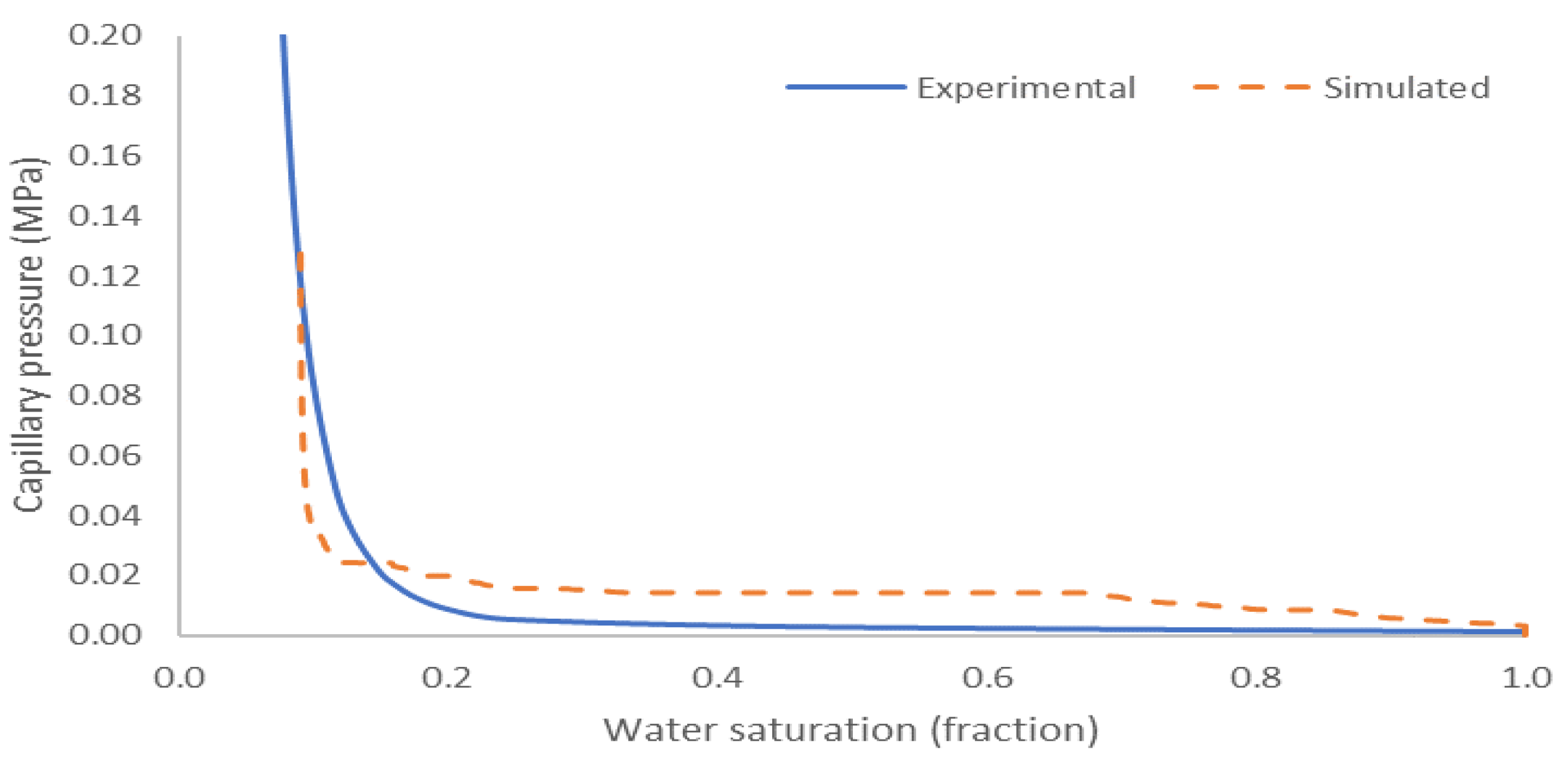
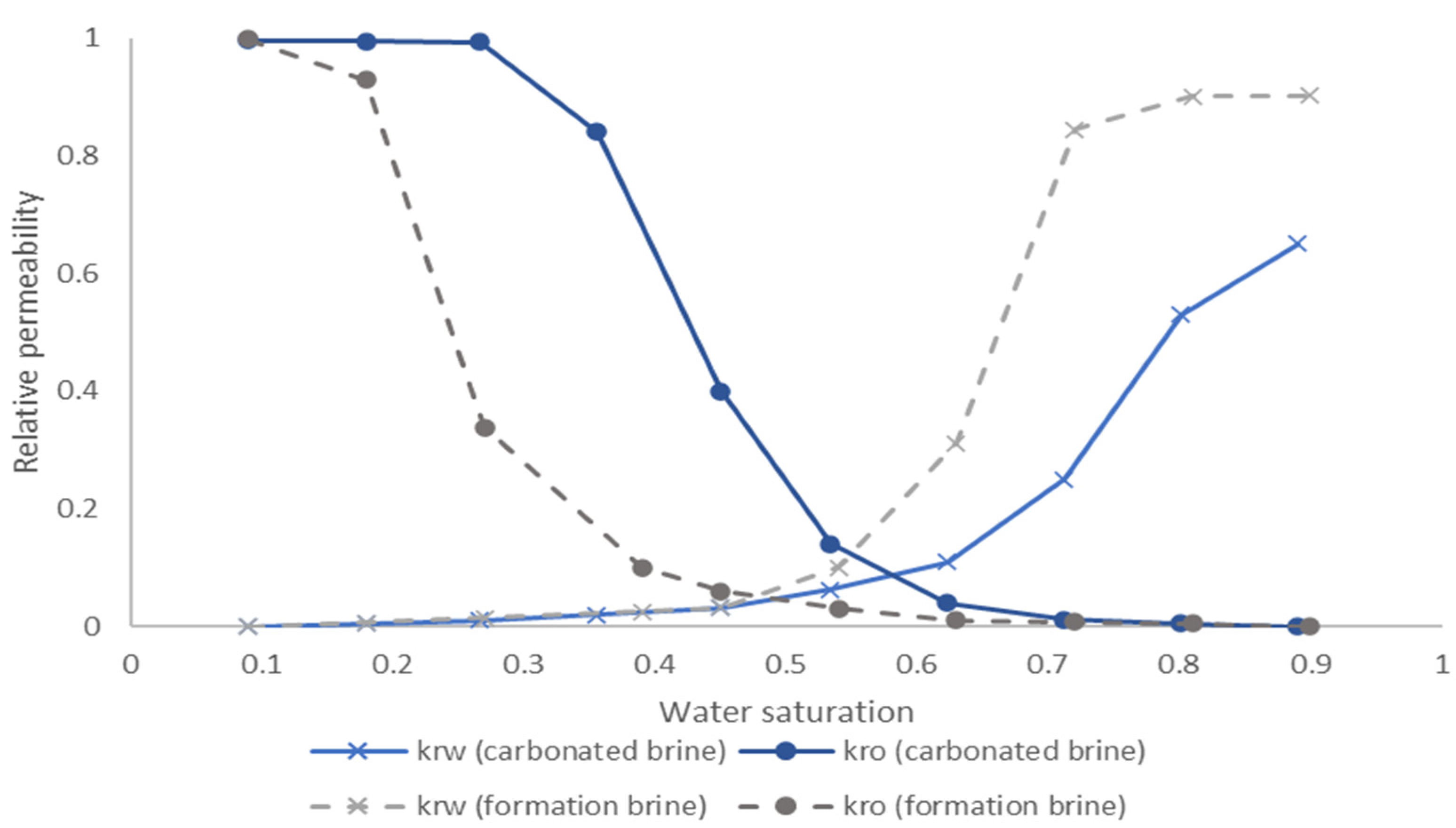
3.3. Capillary Pressure and Relative Permeability Curves
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kriegler, E.; Luderer, G.; Bauer, N.; Baumstark, L.; Fujimori, S.; Popp, A.; Rogelj, J.; Strefler, J.; van Vuuren, D.P. Pathways limiting warming to 1.5 °C: A tale of turning around in no time? Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 2018, 376, 20160457. [Google Scholar] [CrossRef] [PubMed]
- Mac Dowell, N.; Fennell, P.S.; Shah, N.; Maitland, G.C. The role of CO2 capture and utilization in mitigating climate change. Nat. Clim. Chang. 2017, 7, 243–249. [Google Scholar] [CrossRef]
- Guo, J.-X.; Huang, C.; Wang, J.-L.; Meng, X.-Y. Integrated operation for the planning of CO2 capture path in CCS–EOR project. J. Pet. Sci. Eng. 2020, 186, 106720. [Google Scholar] [CrossRef]
- Mohsin, I.; Al-Attas, T.A.; Sumon, K.Z.; Bergerson, J.; McCoy, S.; Kibria, M.G. Economic and Environmental Assessment of Integrated Carbon Capture and Utilization. Cell Rep. Phys. Sci. 2020, 100104. [Google Scholar] [CrossRef]
- Zhang, Z.; Pan, S.-Y.; Li, H.; Cai, J.; Olabi, A.G.; Anthony, E.J.; Manovic, V. Recent advances in carbon dioxide utilization. Renew. Sustain. Energy Rev. 2020, 125, 109799. [Google Scholar] [CrossRef]
- Berstad, D.; Nekså, P.; Gjøvåg, G.A. Low-temperature syngas separation and CO2 capture for enhanced efficiency of IGCC power plants. Energy Proc. 2011, 4, 1260–1267. [Google Scholar] [CrossRef]
- Aliff Radzuan, M.R.; Syarina, N.A.; Wan Rosdi, W.M.; Hussin, A.H.; Adnan, M.F. Sustainable Optimization of Natural Gas Sweetening Using A Process Simulation Approach and Sustainability Evaluator. Mater. Today Proc. 2019, 19, 1628–1637. [Google Scholar] [CrossRef]
- Vega, F.; Baena-Moreno, F.M.; Gallego Fernández, L.M.; Portillo, E.; Navarrete, B.; Zhang, Z. Current status of CO2 chemical absorption research applied to CCS: Towards full deployment at industrial scale. Appl. Energy 2020, 260, 114313. [Google Scholar] [CrossRef]
- Bui, M.; Adjiman, C.S.; Bardow, A.; Anthony, E.J.; Boston, A.; Brown, S.; Fennell, P.S.; Fuss, S.; Galindo, A.; Hackett, L.A.; et al. Carbon capture and storage (CCS): The way forward. Energy Environ. Sci. 2018, 11, 1062–1176. [Google Scholar] [CrossRef]
- Sanz-Pérez, E.S.; Murdock, C.R.; Didas, S.A.; Jones, C.W. Direct Capture of CO(2) from Ambient Air. Chem. Rev. 2016, 116, 11840–11876. [Google Scholar] [CrossRef]
- Webley, P.A. Adsorption technology for CO2 separation and capture: A perspective. Adsorption 2014, 20, 225–231. [Google Scholar] [CrossRef]
- Duan, J.; Jin, W.; Kitagawa, S. Water-resistant porous coordination polymers for gas separation. Coord. Chem. Rev. 2017, 332, 48–74. [Google Scholar] [CrossRef]
- Ahmed, I.; Jhung, S.H. Applications of metal-organic frameworks in adsorption/separation processes via hydrogen bonding interactions. Chem. Eng. J. 2017, 310, 197–215. [Google Scholar] [CrossRef]
- Staciwa, P.; Narkiewicz, U.; Sibera, D.; Moszyński, D.; Wróbel, R.J.; Cormia, R.D. Carbon Spheres as CO2 Sorbents. Appl. Sci. 2019, 9, 3349. [Google Scholar] [CrossRef]
- Deng, X.; Yang, W.; Li, S.; Liang, H.; Shi, Z.; Qiao, Z. Large-Scale Screening and Machine Learning to Predict the Computation-Ready, Experimental Metal-Organic Frameworks for CO2 Capture from Air. Appl. Sci. 2020, 10, 569. [Google Scholar] [CrossRef]
- Brunetti, A.; Scura, F.; Barbieri, G.; Drioli, E. Membrane technologies for CO2 separation. J. Memb. Sci. 2010, 359, 115–125. [Google Scholar] [CrossRef]
- Scholes, C.A.; Smith, K.H.; Kentish, S.E.; Stevens, G.W. CO2 capture from pre-combustion processes—Strategies for membrane gas separation. Int. J. Greenh. Gas Control 2010, 4, 739–755. [Google Scholar] [CrossRef]
- Belaissaoui, B.; Favre, E. Membrane Separation Processes for Post-Combustion Carbon Dioxide Capture: State of the Art and Critical Overview. Oil Gas Sci. Technol. Rev. IFP Energies Nouv. 2014, 69, 1005–1020. [Google Scholar] [CrossRef]
- Chi, C.; Wang, X.; Peng, Y.; Qian, Y.; Hu, Z.; Dong, J.; Zhao, D. Facile Preparation of Graphene Oxide Membranes for Gas Separation. Chem. Mater. 2016, 28, 2921–2927. [Google Scholar] [CrossRef]
- Celia, M.A.; Bachu, S.; Nordbotten, J.M.; Bandilla, K.W. Status of CO2 storage in deep saline aquifers with emphasis on modeling approaches and practical simulations. Water Resour. Res. 2015, 51, 6846–6892. [Google Scholar] [CrossRef]
- Gal, F.; Pokryszka, Z.; Labat, N.; Michel, K.; Lafortune, S.; Marblé, A. Soil-Gas Concentrations and Flux Monitoring at the Lacq-Rousse CO2-Geological Storage Pilot Site (French Pyrenean Foreland): From Pre-Injection to Post-Injection. Appl. Sci. 2019, 9, 645. [Google Scholar] [CrossRef]
- Jeong, S.-M.; Ko, S.; Sean, W.-Y. Numerical Prediction of the Behavior of CO2 Bubbles Leaked from Seafloor and Their Convection and Diffusion near Southeastern Coast of Korea. Appl. Sci. 2020, 10, 4237. [Google Scholar] [CrossRef]
- Pedro, J.; Araújo, A.A.; Moita, P.; Beltrame, M.; Lopes, L.; Chambel, A.; Berrezueta, E.; Carneiro, J. Mineral Carbonation of CO2 in Mafic Plutonic Rocks, I—Screening Criteria and Application to a Case Study in Southwest Portugal. Appl. Sci. 2020, 10, 4879. [Google Scholar] [CrossRef]
- Gou, Y.; Hou, Z.; Liu, H.; Zhou, L.; Were, P. Numerical simulation of carbon dioxide injection for enhanced gas recovery (CO2-EGR) in Altmark natural gas field. Acta Geotech. 2014, 9, 49–58. [Google Scholar] [CrossRef]
- Mazzotti, M.; Pini, R.; Storti, G. Enhanced coalbed methane recovery. J. Supercrit. Fluids 2009, 47, 619–627. [Google Scholar] [CrossRef]
- Iino, A.; Onishi, T.; Datta-Gupta, A. Optimizing CO2—And Field-Gas-Injection EOR in Unconventional Reservoirs Using the Fast-Marching Method. SPE Reserv. Eval. Eng. 2020, 23, 261–281. [Google Scholar] [CrossRef]
- Sheng, J. Enhanced Oil Recovery Field Case Studies; Gulf Professional Publishing: Waltham, MA, USA, 2013; ISBN 9780123865465. [Google Scholar]
- Thorne, R.J.; Sundseth, K.; Bouman, E.; Czarnowska, L.; Mathisen, A.; Skagestad, R.; Stanek, W.; Pacyna, J.M.; Pacyna, E.G. Technical and environmental viability of a European CO2 EOR system. Int. J. Greenh. Gas Control 2020, 92, 102857. [Google Scholar] [CrossRef]
- De Nevers, N. A Calculation Method for Carbonated Water Flooding. Soc. Pet. Eng. J. 1964, 4, 9–20. [Google Scholar] [CrossRef]
- Kechut, N.I.; Riazi, M.; Sohrabi, M.; Jamiolahmady, M. Tertiary Oil Recovery and CO2 Sequestration by Carbonated Water Injection (CWI). In Proceedings of the SPE International Conference on CO2 Capture, Storage, and Utilization, New Orleans, LA, USA, 10–12 November 2010; p. 12. [Google Scholar]
- Fjelde, I.; Asen, S.M. Wettability alteration during water flooding and carbon dioxide flooding of reservoir chalk rocks. In Proceedings of the SPE EUROPEC/EAGE Annual Conference and Exhibition, Barcelona, Spain, 14–17 June 2010. [Google Scholar]
- Al-Mutairi, S.M.; Abu-khamsin, S.A.; Hossain, M.E. A Novel Approach to Handle Continuous Wettability Alteration during Immiscible CO2 Flooding Process. In Proceedings of the Abu Dhabi International Petroleum Conference and Exhibition, Abu Dhabi, UAE, 11–14 November 2012. [Google Scholar]
- Seyyedi, M.; Sohrabi, M.; Farzaneh, A. Investigation of Rock Wettability Alteration by Carbonated Water through Contact Angle Measurements. Energy Fuels 2015, 29, 5544–5553. [Google Scholar] [CrossRef]
- Ruidiaz, E.M.; Winter, A.; Trevisan, O.V. Oil recovery and wettability alteration in carbonates due to carbonate water injection. J. Pet. Explor. Prod. Technol. 2018, 8, 249–258. [Google Scholar] [CrossRef]
- Teklu, T.W.; Alameri, W.; Kazemi, H.; Graves, R.M. Contact Angle Measurements on Conventional and Unconventional Reservoir Cores. In Proceedings of the Unconventional Resources Technology Conference, San Antonio, TX, USA, 20–22 July 2015; p. 17. [Google Scholar]
- Zhu, Z.; Li, M.; Lin, M.; Peng, B.; Sun, L.; Chen, L. Investigation on Variations in Wettability of Reservoir Rock Induced by CO2-Brine-Rock Interactions. In Proceedings of the SPE EUROPEC/EAGE Annual Conference and Exhibition, Vienna, Austria, 12–15 June 2011; p. 11. [Google Scholar]
- Chen, Y.; Sari, A.; Xie, Q.; Saeedi, A. Insights into the wettability alteration of CO2-assisted EOR in carbonate reservoirs. J. Mol. Liq. 2019, 279, 420–426. [Google Scholar] [CrossRef]
- Drexler, S.; Silveira, T.M.G.; De Belli, G.; Couto, P. Experimental study of the effect of carbonated brine on wettability and oil displacement for EOR application in the Brazilian Pre-Salt reservoirs. Energy Sources Part A Recover. Util. Environ. Eff. 2019, 1–15. [Google Scholar] [CrossRef]
- Sohrabi, M.; Emadi, A.; Farzaneh, S.A.; Ireland, S. A Thorough Investigation of Mechanisms of Enhanced Oil Recovery by Carbonated Water Injection. In Proceedings of the SPE Annual Technical Conference and Exhibition, Houston, TX, USA, 28–30 September 2015; p. 33. [Google Scholar]
- De Almeida, A.S.; Lima, S.D.T.C.; Rocha, P.S.; De Andrade, A.M.T.; Branco, C.C.M.; Pinto, A.C.C. CCGS opportunities in the Santos Basin pre-salt development. In Proceedings of the SPE International Conference on Health, Safety and Environment in Oil and Gas Exploration and Production 2010, Rio de Janeiro, Brazil, 12–14 April 2010; Volume 2, pp. 840–849. [Google Scholar]
- Pepin, A.; Bize-Forest, N.; Montoya Padilla, S.; Abad, C.; Schlicht, P.; de Castro Machado, A. Pre-Salt Carbonate Reservoir Analog Selection for Stimulation Optimization. In Proceedings of the International Petroleum Technology Conference, Kuala Lumpur, Malaysia, 10–12 December 2014. [Google Scholar]
- Pizarro, J.O.D.S.; Branco, C.C.M. Challenges in Implementing an EOR Project in the Pre-Salt Province in Deep Offshore Brasil. In Proceedings of the SPE EOR Conference at Oil and Gas West Asia, Muscat, Oman, 16–18 April 2012; p. 13. [Google Scholar]
- Drexler, S.; Correia, E.L.; Jerdy, A.C.; Cavadas, L.A.; Couto, P. Effect of CO2 on the dynamic and equilibrium interfacial tension between crude oil and formation brine for a deepwater Pre-salt field. J. Pet. Sci. Eng. 2020, 190, 107095. [Google Scholar] [CrossRef]
- Campos Neto, O.P.d.A.; Lima, W.S.; Cruz, F.E.G. Bacia de Sergipe-Alagoas (Sergipe-Alagoas Basin). Bol. Geocienc. da Petrobras 2007, 15, 405–415. [Google Scholar]
- Pettijohn, F.J. Sedimentary Rocks, 2nd ed.; Harper Brothers: New York, NY, USA, 1957; ISBN 978-1114189805. [Google Scholar]
- Schäfer, W. Ecology and Palaeoecology of Marine Environments; The University of Chicago Press: Chicago, IL, USA, 1972. [Google Scholar]
- Azambuja, N.C.; Arienti, L.M.; da Cruz, F.E. Guidebook to the Rift-Drift Sergipe-Alagoas Passive Margin Basin, Brazil, Proceedings of the The 1998 AAPG International Conference and Exhibition, Rio de Janeiro, Brazil, 8–11 November 1998; American Association of Petroleum Geologists: Tulsa, OK, USA, 1998; p. 113. [Google Scholar]
- Tavares, A.C.; Borghi, L.; Corbett, P.; Nobre-Lopes, J.; Câmara, R. Facies and depositional environments for the coquinas of the Morro do Chaves Formation, Sergipe-Alagoas Basin, defined by taphonomic and compositional criteria. Braz. J. Geol. 2015, 45, 415–429. [Google Scholar] [CrossRef]
- Corbett, P.W.M.; Estrella, R.; Morales Rodriguez, A.; Shoeir, A.; Borghi, L.; Tavares, A.C. Integration of Cretaceous Morro do Chaves rock properties (NE Brazil) with the Holocene Hamelin Coquina architecture (Shark Bay, Western Australia) to model effective permeability. Pet. Geosci. 2016, 22, 105–122. [Google Scholar] [CrossRef]
- Chinelatto, G.F.; Vidal, A.C.; Kuroda, M.C.; Basilici, G. A taphofacies model for coquina sedimentation in lakes (Lower Cretaceous, Morro do Chaves Formation, NE Brazil). Cretac. Res. 2018, 85, 1–19. [Google Scholar] [CrossRef]
- Hoerlle, F.O.; Rios, E.H.; de Azevedo Lopes da Silva, W.G.; Pontedeiro, E.M.B.D.; da Costa de Oliveira Lima, M.; Corbett, P.W.M.; Alves, J.L.D.; Couto, P. Nuclear Magnetic Resonance to Characterize the Pore System of Coquinas from Morro do Chaves Formation, Sergipe-Alagoas Basin, Brazil. Braz. J. Geophys. 2018, 36, 317–324. [Google Scholar] [CrossRef]
- da Costa de Oliveira Lima, M.; Martins, L.P.; Rios, E.H.; Boyd, A.; Pontedeiro, E.M.B.D.; Hoerlle, F.O.; Lipovetski, T.; Neto, A.O.; Mendes, M.; Borghi, L.; et al. Rock Typing of Coquinas from the Morro do Chaves Formation, Proceedings of the Sixteenth International Congress of the Brazilian Geophysical Society, Rio de Janeiro, Brazil, 17–22 August 2019; Brazilian Geophysical Society: Rio de Janeiro, Brazil, 2019; pp. 1–6. [Google Scholar]
- Coates, G.R.; Xiao, L.; Prammer, M.G. NMR Logging: Principles and Applications; Halliburton Energy Services: Houston, TX, USA, 1999; p. 234. [Google Scholar]
- Hassler, G.L.; Brunner, E. Measurement of Capillary Pressures in Small Core Samples. Trans. AIME 1945, 160, 114–123. [Google Scholar] [CrossRef]
- Albuquerque, M.R.; Eler, F.M.; Camargo, H.V.R.; Compan, A.L.M.; Cruz, D.A.; Pedreira, C.E. Estimation of Capillary Pressure Curves from Centrifuge Measurements Using Inverse Methods, Proceedings of the Rio Oil & Gas; IBP: Rio de Janeiro, Brazil, 24–27 September 2018. [Google Scholar]
- Silin, D.; Patzek, T. Pore space morphology analysis using maximal inscribed spheres. Phys. A Stat. Mech. Its Appl. 2006, 371, 336–360. [Google Scholar] [CrossRef]
- Dong, H. Micro CT Imaging and Pore Network Extraction; Imperial College London: London, UK, 2007. [Google Scholar]
- Zhou, D.; Blunt, M.; Orr, F.M. Hydrocarbon Drainage along Corners of Noncircular Capillaries. J. Colloid Interface Sci. 1997, 187, 11–21. [Google Scholar] [CrossRef] [PubMed]
- Raoof, A.; Hassanizadeh, S.M. A New Method for Generating Pore-Network Models of Porous Media. Transp. Porous Media 2010, 81, 391–407. [Google Scholar] [CrossRef]
- Joekar-Niasar, V.; Majid Hassanizadeh, S. Effect of fluids properties on non-equilibrium capillarity effects: Dynamic pore-network modeling. Int. J. Multiph. Flow 2011, 37, 198–214. [Google Scholar] [CrossRef]
- Blunt, M.J. Flow in porous media—Pore-network models and multiphase flow. Curr. Opin. Colloid Interface Sci. 2001, 6, 197–207. [Google Scholar] [CrossRef]
- Buades, A.; Coll, B.; Morel, J. A non-local algorithm for image denoising. In Proceedings of the 2005 IEEE Computer Society Conference on Computer Vision and Pattern Recognition (CVPR’05), San Diego, CA, USA, 20–25 June 2005; Volume 2, pp. 60–65. [Google Scholar]
- Kittler, J.; Illingworth, J. Minimum error thresholding. Pattern Recognit. 1986, 19, 41–47. [Google Scholar] [CrossRef]
- Blunt, M.J. Multiphase Flow in Permeable Media: A Pore-Scale Perspective; Cambridge University Press: Cambridge, UK, 2017; ISBN 9781107093461. [Google Scholar]
- Werner, J.; Persson, I.; Björneholm, O.; Kawecki, D.; Saak, C.-M.; Walz, M.-M.; Ekholm, V.; Unger, I.; Valtl, C.; Caleman, C.; et al. Shifted equilibria of organic acids and bases in the aqueous surface region. Phys. Chem. Chem. Phys. 2018, 20, 23281–23293. [Google Scholar] [CrossRef] [PubMed]
- Danielli, J.F. The Relations between Surface pH, Ion Concentrations and Interfacial Tension. Proc. R. Soc. Lond. Ser. B Biol. Sci. 1937, 122, 155–174. [Google Scholar]
- Lashkarbolooki, M.; Riazi, M.; Ayatollahi, S. Effect of CO2 and natural surfactant of crude oil on the dynamic interfacial tensions during carbonated water flooding: Experimental and modeling investigation. J. Pet. Sci. Eng. 2017, 159, 58–67. [Google Scholar] [CrossRef]
- Lashkarbolooki, M.; Ayatollahi, S. The effects of pH, acidity, asphaltene and resin fraction on crude oil/water interfacial tension. J. Pet. Sci. Eng. 2018, 162, 341–347. [Google Scholar] [CrossRef]
- Buckley, J.S.; Takamura, K.; Morrow, N.R. Influence of Electrical Surface Charges on the Wetting Properties of Crude Oils. SPE Reserv. Eng. 1989, 4, 332–340. [Google Scholar] [CrossRef]
- Drexler, S.; Hoerlle, F.O.; Silveira, T.M.G.; Cavadas, L.A.; Couto, P. Impact of Rock Aging Time on the Initial Wettability of Minerals and Analogue Rocks Using Pre-Salt Fluids Under Reservoir Conditions. In Proceedings of the Offshore Technology Conference Brasil, Rio de Janeiro, Brazil, 29–31 October 2019; p. 8. [Google Scholar]
- Strand, S.; Høgnesen, E.J.; Austad, T. Wettability alteration of carbonates—Effects of potential determining ions (Ca2+ and SO42−) and temperature. Colloids Surf. A Physicochem. Eng. Asp. 2006, 275, 1–10. [Google Scholar] [CrossRef]
- Austad, T.; Strand, S.; Madland, M.V.; Puntervold, T.; Korsnes, R.I. Seawater in Chalk: An EOR and Compaction Fluid. SPE Reserv. Eval. Eng. 2008, 11, 648–654. [Google Scholar] [CrossRef]
- Tölke, J.; Baldwin, C.; Mu, Y.; Derzhi, N.; Fang, Q.; Grader, A.; Dvorkin, J. Computer simulations of fluid flow in sediment: From images to permeability. Lead. Edge 2010, 29, 68–74. [Google Scholar] [CrossRef]
- Dernaika, M.R.; Sahib, M.R.; Gonzalez, D.; Mansour, B.; Al Jallad, O.; Koronfol, S.; Sinclair, G.; Kayali, A. Core Characterization and Numerical Flow Simulation in Representative Rock Types of the Raudhatain Field in Kuwait. In Proceedings of the SPE Kuwait Oil & Gas Show and Conference, Kuwait City, Kuwait, 15–18 October 2017; p. 24. [Google Scholar]
- Zambrano, M.; Tondi, E.; Mancini, L.; Lanzafame, G.; Trias, F.X.; Arzilli, F.; Materazzi, M.; Torrieri, S. Fluid flow simulation and permeability computation in deformed porous carbonate grainstones. Adv. Water Resour. 2018, 115, 95–111. [Google Scholar] [CrossRef]
- AlJallad, O.; Dernaika, M.; Koronfol, S.; Naseer Uddin, Y.; Mishra, P. Evaluation of Complex Carbonates from Pore-Scale to Core-Scale. In Proceedings of the International Petroleum Technology Conference, Dhahran, Kingdom of Saudi Arabia, 13–15 January 2020; p. 21. [Google Scholar]
- Craig, F.F. The Reservoir Engineering Aspects of Waterflooding; Henry, L., Doherty, H.L., Eds.; Memorial Fund of AIME: Richardson, TX, USA, 1971; ISBN 9780895202024. [Google Scholar]
- Anderson, W.G. Wettability Literature Survey Part 5: The Effects of Wettability on Relative Permeability. J. Pet. Technol. 1987, 39, 1453–1468. [Google Scholar] [CrossRef]
- Lake, L.W. Enhanced Oil Recovery; Prentice Hall: Upper Saddle River, NJ, USA, 1989; ISBN 9780132816014. [Google Scholar]
- Picchi, D.; Battiato, I. Relative Permeability Scaling From Pore-Scale Flow Regimes. Water Resour. Res. 2019, 55, 3215–3233. [Google Scholar] [CrossRef]
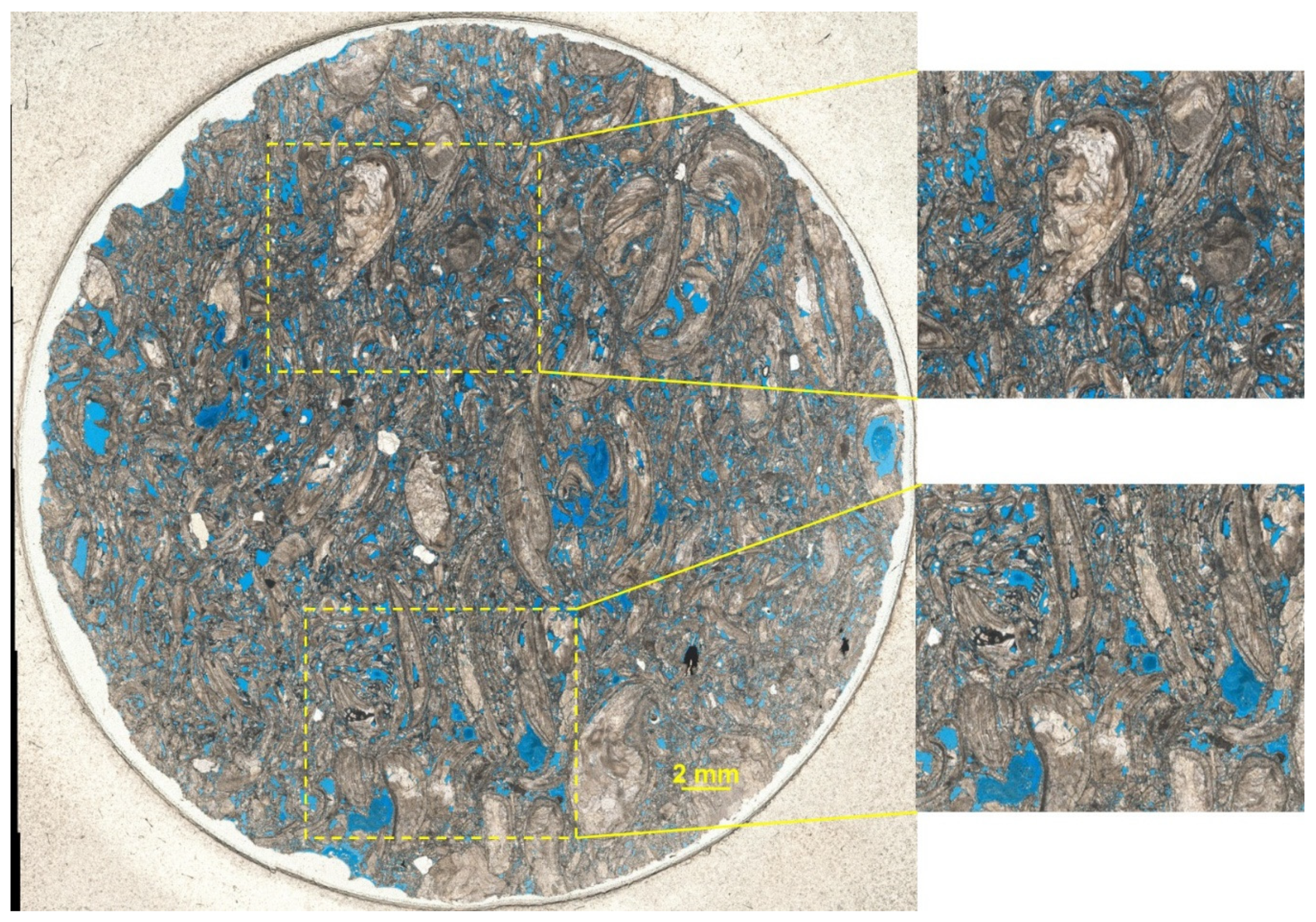
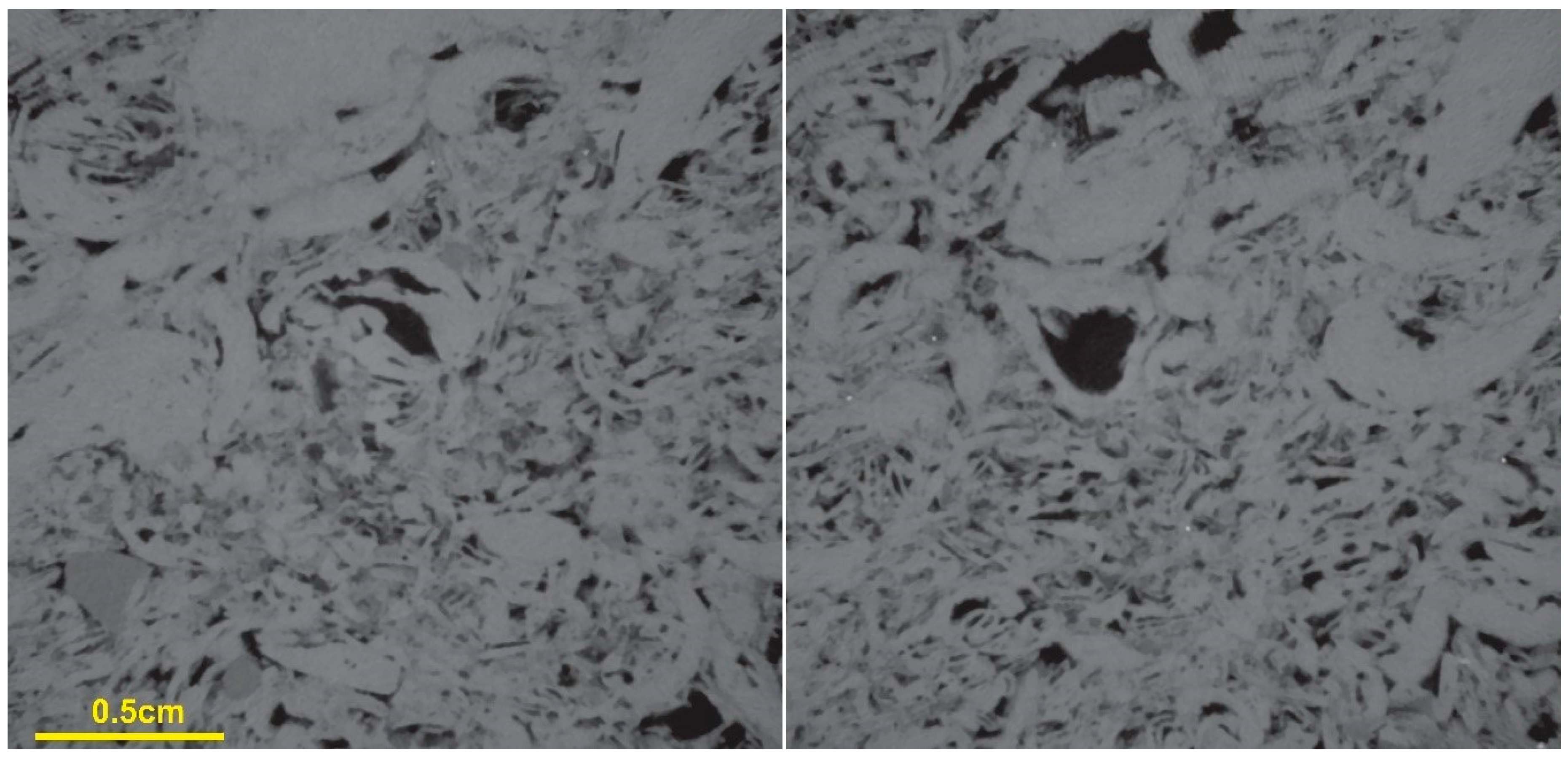
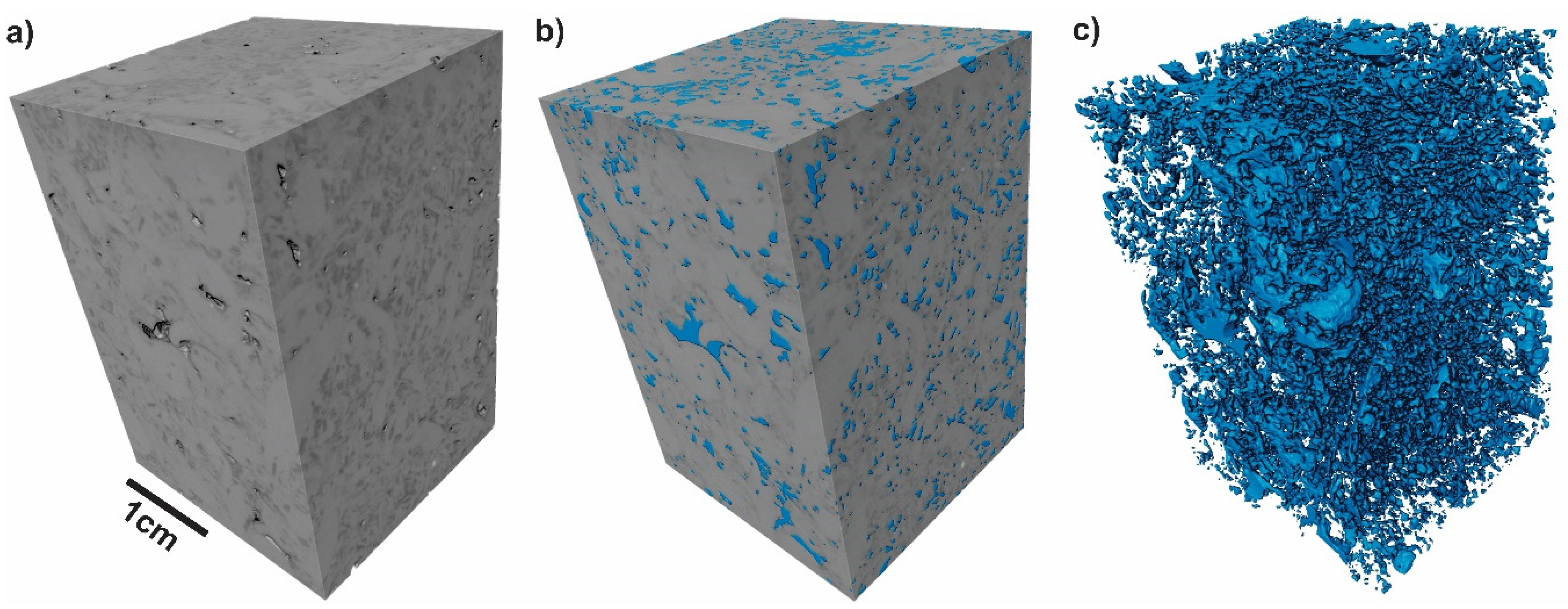
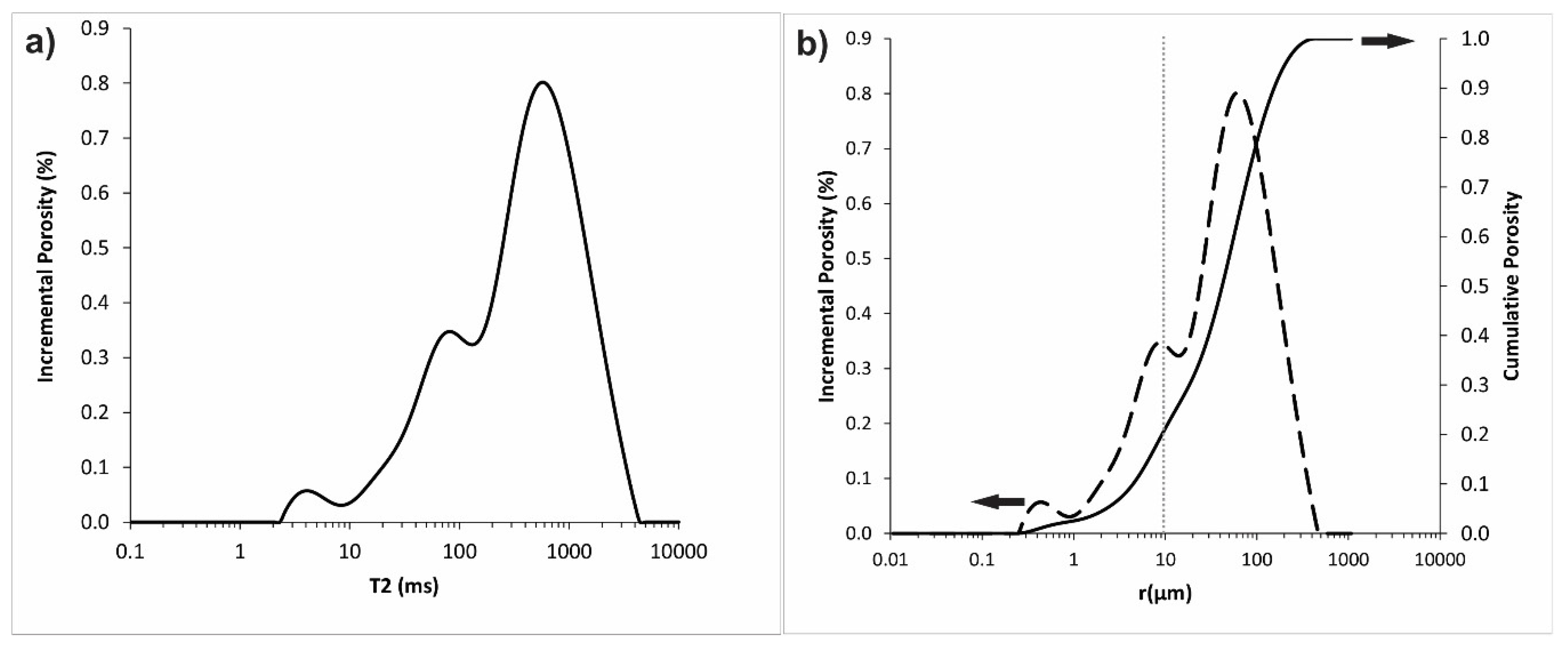
| Ions | Concentration [mg/L] |
|---|---|
| Na+ | 57,580 |
| Ca2+ | 24,250 |
| Mg2+ | 2120 |
| K+ | 1200 |
| Ba2+ | 24 |
| Sr2+ | 1260 |
| SO42− | 54 |
| Cl− | 139,900 |
| TDS | 226,388 |
| Crude Oil Properties | |
|---|---|
| Saturates (wt.%) | 64.06 |
| Aromatics (wt.%) | 25.98 |
| Resins (wt.%) | 8.46 |
| Asphaltenes (wt.%) | 1.50 |
| Total Acid Number (mg KOH/g) | 0.37 |
| Total Base Number (mg KOH/g) | 4.00 |
| Sample | Pore Volume (cc) | Porosity (%) | Permeability (mD) | Grain Density (g/cc) | Diameter (cm) | Length (cm) | Weight (g) |
|---|---|---|---|---|---|---|---|
| Coquina | 6.8 | 19.8 | 765.1 | 2.69 | 3.5 | 3.6 | 74.3 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Drexler, S.; Hoerlle, F.; Godoy, W.; Boyd, A.; Couto, P. Wettability Alteration by Carbonated Brine Injection and Its Impact on Pore-Scale Multiphase Flow for Carbon Capture and Storage and Enhanced Oil Recovery in a Carbonate Reservoir. Appl. Sci. 2020, 10, 6496. https://doi.org/10.3390/app10186496
Drexler S, Hoerlle F, Godoy W, Boyd A, Couto P. Wettability Alteration by Carbonated Brine Injection and Its Impact on Pore-Scale Multiphase Flow for Carbon Capture and Storage and Enhanced Oil Recovery in a Carbonate Reservoir. Applied Sciences. 2020; 10(18):6496. https://doi.org/10.3390/app10186496
Chicago/Turabian StyleDrexler, Santiago, Fernanda Hoerlle, William Godoy, Austin Boyd, and Paulo Couto. 2020. "Wettability Alteration by Carbonated Brine Injection and Its Impact on Pore-Scale Multiphase Flow for Carbon Capture and Storage and Enhanced Oil Recovery in a Carbonate Reservoir" Applied Sciences 10, no. 18: 6496. https://doi.org/10.3390/app10186496
APA StyleDrexler, S., Hoerlle, F., Godoy, W., Boyd, A., & Couto, P. (2020). Wettability Alteration by Carbonated Brine Injection and Its Impact on Pore-Scale Multiphase Flow for Carbon Capture and Storage and Enhanced Oil Recovery in a Carbonate Reservoir. Applied Sciences, 10(18), 6496. https://doi.org/10.3390/app10186496





