Folic Acid/Methotrexate Functionalized Mesoporous Silica Nanoflakes from Different Supports: Comparative Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of Mesoporous Silica Flakes
2.3. Folic Acid Functionalization
2.4. Drug Loading
2.5. Release Study
2.6. Degradation of Silica Flakes
2.7. Characterization Techniques
3. Results
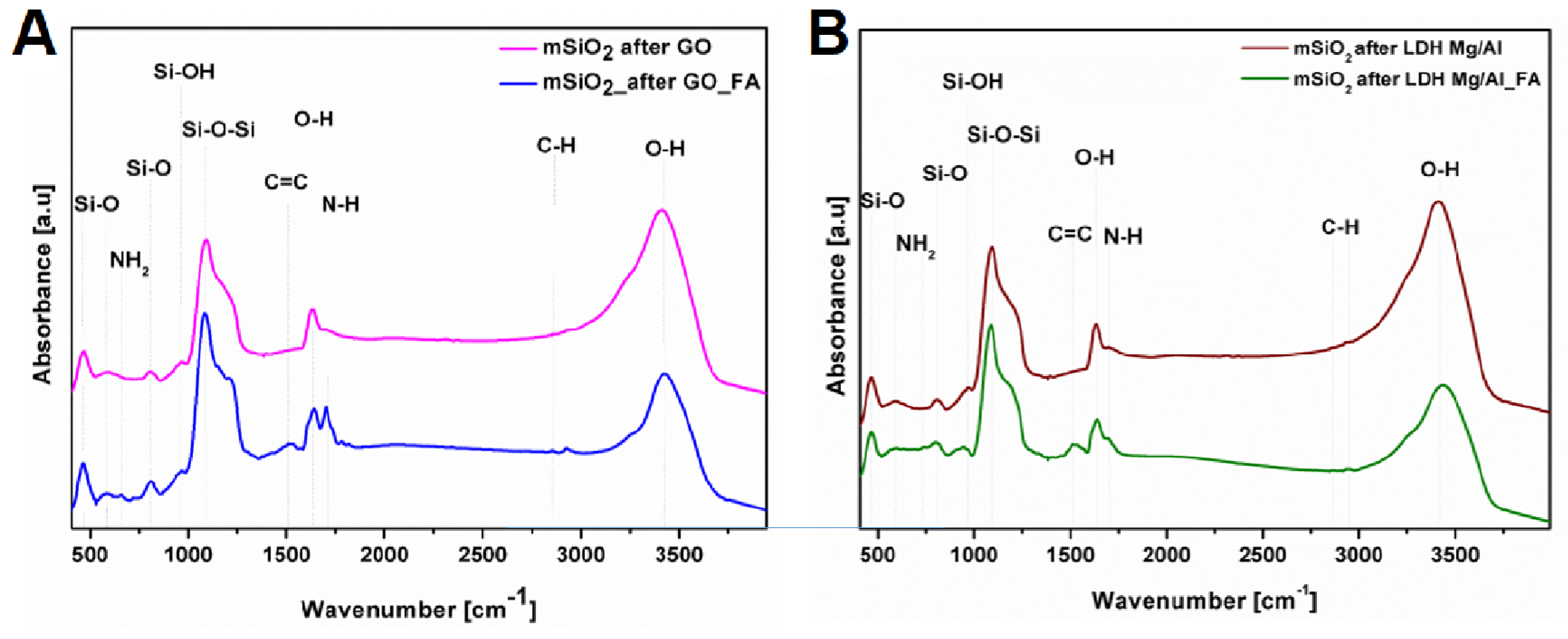
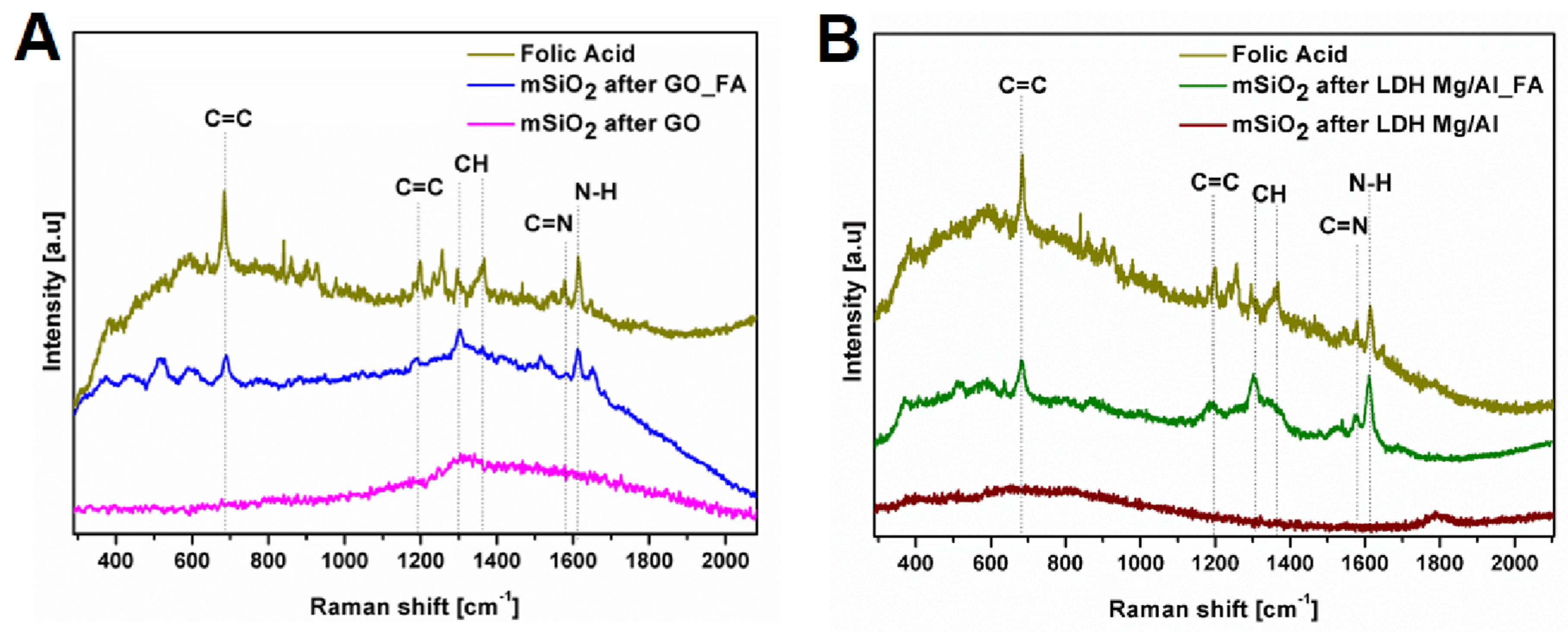
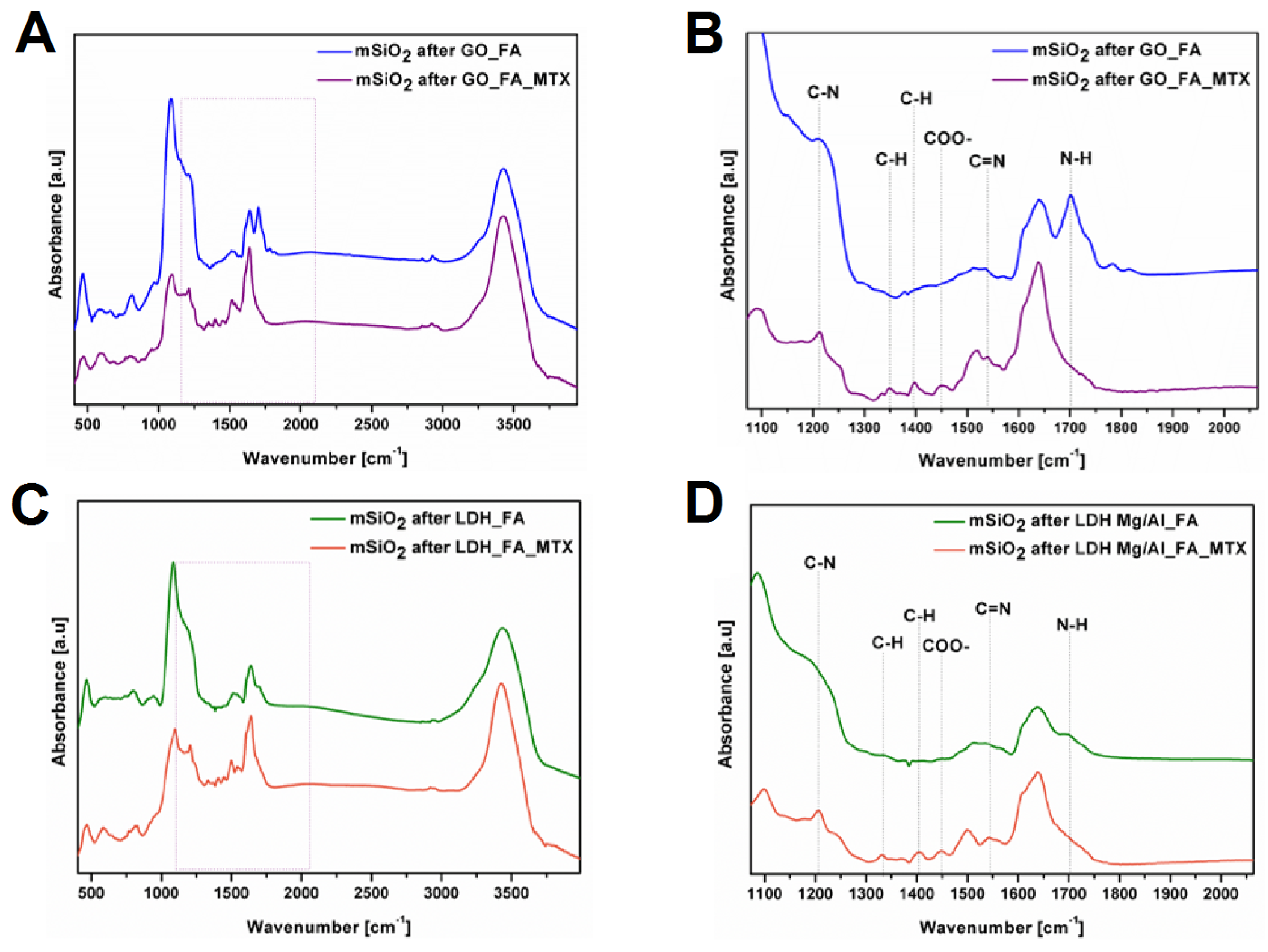
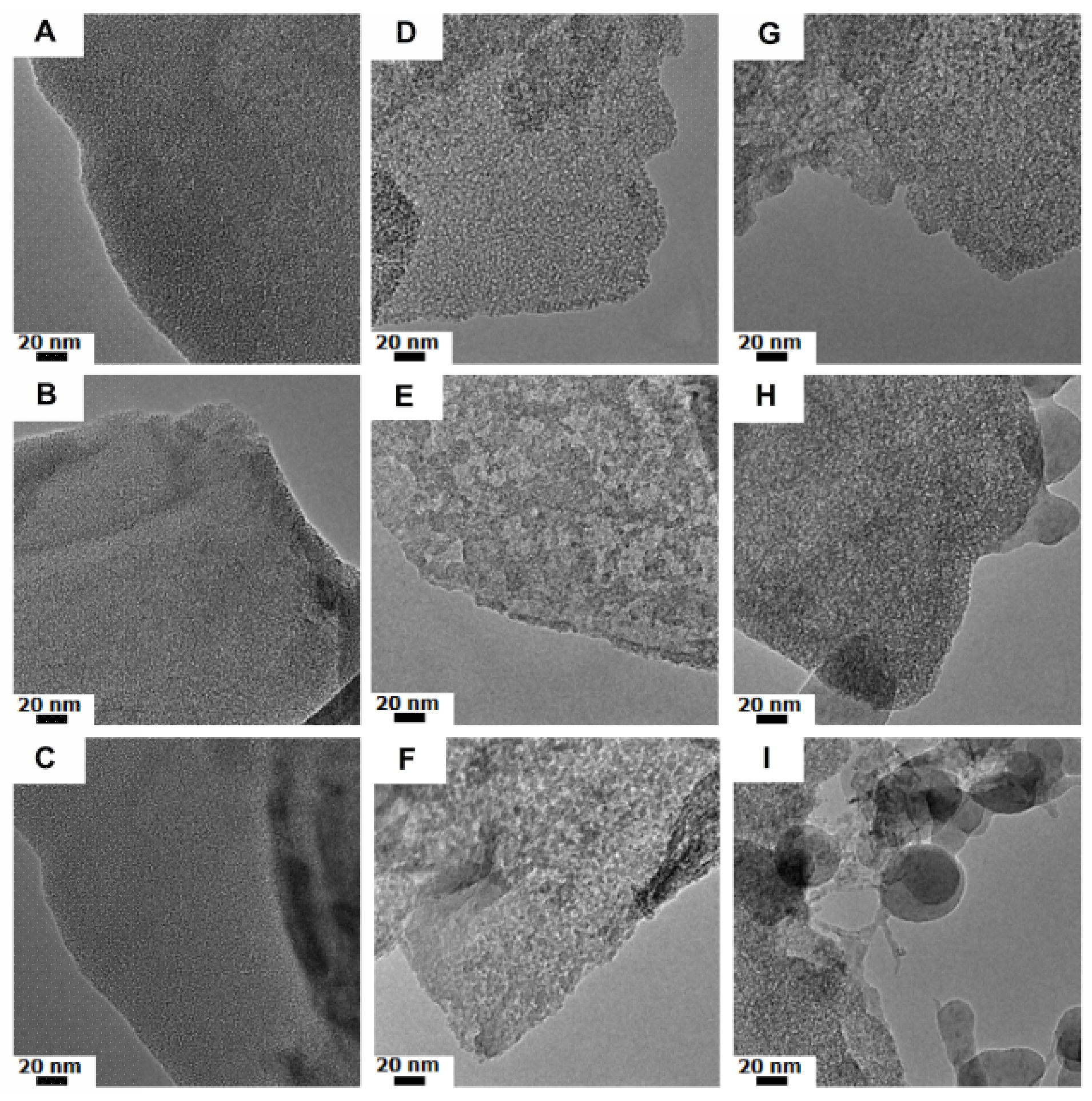
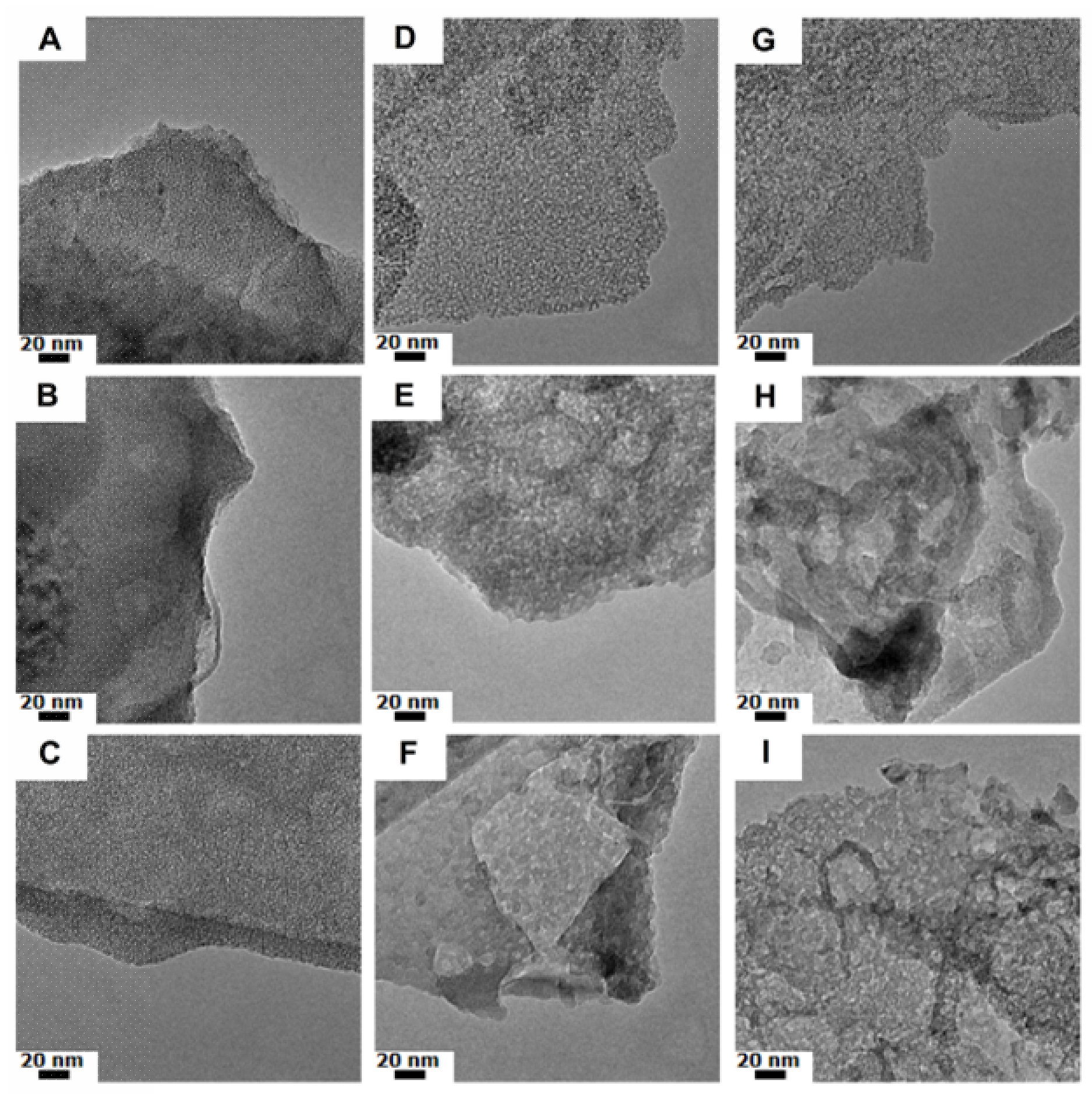
3.1. Silica Nanoflakes Characterization
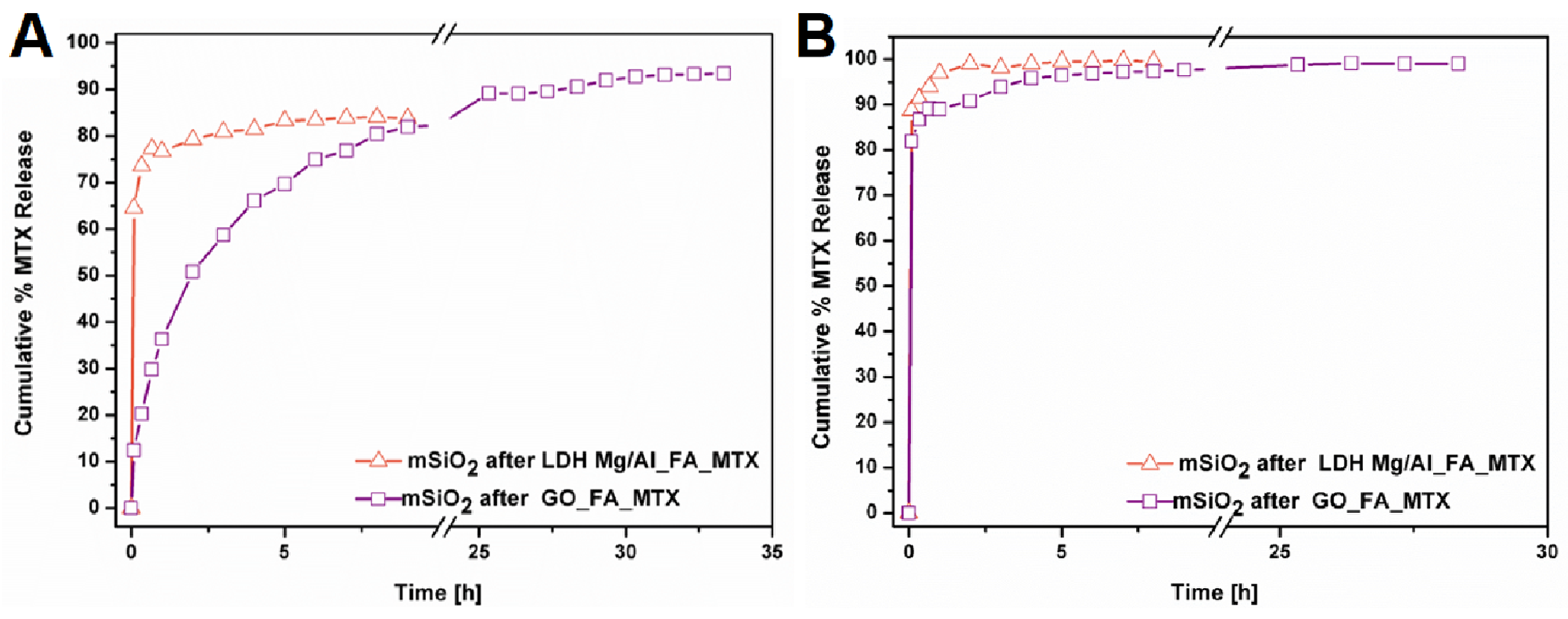
3.2. Drug Release
3.3. Degradation of Silica Flakes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Fiori, G.; Bonaccorso, F.; Iannaccone, G.; Palacios, T.; Neumaier, D.; Seabaugh, A.; Banerjee, S.K.; Colombo, L. Electronics based on two-dimensional materials. Nat. Nanotechnol. 2014, 9, 768–779. [Google Scholar] [CrossRef] [PubMed]
- Deng, D.; Novoselov, K.; Fu, Q.; Zheng, N.; Tian, Z.; Bao, X. Catalysis with two-dimensional materials and their heterostructures. Nat. Nanotechnol. 2016, 11, 218–230. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Feng, X.; Wang, L.; Tang, K.; Maier, J.; Müllen, K. Graphene-based nanosheets with a sandwich structure. Angew. Chem. Int. Ed. 2010, 49, 4795–4799. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.M.; Wang, W.; Coombs, N.; Soheilnia, N.; Ozin, G.A. Graphene oxide-periodic mesoporous silica sandwich nanocomposites with vertically oriented channels. ACS Nano 2010, 4, 7437–7450. [Google Scholar] [CrossRef] [PubMed]
- Guardia, L.; Suárez-García, F.; Paredes, J.I.; Solís-Fernández, P.; Rozada, R.; Fernández-Merino, M.J.; Martínez-Alonso, A.; Tascón, M.D. Synthesis and characterization of graphene-mesoporous silica nanoparticle hybrids. Microporous Mesoporous Mater. 2012, 160, 18–24. [Google Scholar] [CrossRef]
- Liu, Y.; Li, W.; Shen, D.; Wang, C.; Li, X.; Pal, M.; Zhang, R.; Chen, L.; Yao, C.; Wei, Y.; et al. Synthesis of Mesoporous Silica/Reduced Graphene Oxide Sandwich-Like Sheets with Enlarged and “Funneling” Mesochannels. Chem. Mater. 2015, 27, 5577–5586. [Google Scholar] [CrossRef]
- Harrison, R.; Li, L.; Gu, Z.; Xu, Z.P. Controlling mesoporous silica-coating of layered double hydroxide nanoparticles for drug control release. Micropor. Mesoporous Mater. 2017, 238, 97–106. [Google Scholar] [CrossRef]
- Trewyn, B.G.; Slowing, I.I.; Giri, S.; Chen, H.T.; Lin, V.Y. Synthesis and functionalization of a mesoporous silica nanoparticle based on the sol–gel process and applications in controlled release. Acc. Chem. Res. 2007, 40, 846–853. [Google Scholar] [CrossRef]
- Vialpando, M.; Aerts, A.; Persoons, J.; Martens, J.; Van Den Mooter, G. Evaluation of ordered mesoporous silica as a carrier for poorly soluble drugs: Influence of pressure on the structure and drug release. J. Pharm. Sci. 2011, 100, 3411–3420. [Google Scholar] [CrossRef]
- Van Speybroeck, M.; Barillaro, V.; Thi, T.D.; Mellaerts, R.; Martens, J.; Van Humbeeck, J.; Vermant, J.; Annaert, P.; Van Den Mooter, G.; Augustijns, P. Ordered mesoporous silica material SBA-15: A broad-spectrum formulation platform for poorly soluble drugs. J. Pharm. Sci. 2009, 98, 2648–2658. [Google Scholar] [CrossRef]
- Tarn, D.; Ashley, C.E.; Xue, M.; Carnes, E.C.; Zink, J.I.; Brinker, C.J. Mesoporous Silica Nanoparticle Nanocarriers: Biofunctionality and Biocompatibility. Acc. Chem. Res. 2013, 46, 3792–3801. [Google Scholar] [CrossRef]
- Takahashi, R.; Sato, S.; Sodesawa, T.; Kawakita, M.; Ogura, K. High Surface-Area Silica with Controlled Pore Size Prepared from Nanocomposite of Silica and Citric Acid. J. Phys. Chem. B 2000, 104, 12184–12191. [Google Scholar] [CrossRef]
- Zhu, Y.; Shi, J.; Li, Y.; Chen, H.; Shen, W.; Dong, X. Storage and release of ibuprofen drug molecules in hollow mesoporous silica spheres with modified pore surface. Microporous Mesoporous Mater. 2005, 85, 75–81. [Google Scholar] [CrossRef]
- Tang, F.; Li, L.; Chen, D. Mesoporous Silica Nanoparticles: Synthesis, Biocompatibility and Drug Delivery. Adv. Mater. 2012, 24, 1504–1534. [Google Scholar] [CrossRef]
- Li, J.; Li, X.; Huang, Y.; Zhong, Y.; Lan, Q.; Wu, X.; Hu, R.; Zhang, G.; Hu, X.; Yang, Z. Biofunctionalized mesoporous silica nanospheres for the ultrasensitive chemiluminescence immunoassay of tumor markers. New J. Chem. 2018, 42, 11264–11267. [Google Scholar] [CrossRef]
- Cha, B.G.; Kim, J. Functional mesoporous silica nanoparticles for bio-imaging applications. WIREs Nanomed. Nanobiotechnol. 2018, 11, 11–e1515. [Google Scholar] [CrossRef]
- Martín-Saavedra, F.M.; Ruíz-Hernández, E.; Boré, A.; Arcos, D.; Vallet-Regí, M.; Vilaboa, N. Magnetic mesoporous silica spheres for hyperthermia therapy. Acta Biomater. 2010, 6, 4522–4531. [Google Scholar] [CrossRef]
- Fang, W.; Tang, S.; Liu, P.; Fang, X.; Gong, J.; Zheng, N. Pd nanosheet-covered hollow mesoporous silica nanoparticles as a platform for the chemo-photothermal treatment of cancer cells. Small 2012, 8, 3816–3822. [Google Scholar] [CrossRef]
- Tsai, C.P.; Chen, C.Y.; Hung, Y.; Chang, F.H.; Mou, C.Y. Monoclonal antibody-functionalized mesoporous silica nanoparticles (MSN) for selective targeting breast cancer cells. J. Mater. Chem. 2009, 19, 5737–5743. [Google Scholar] [CrossRef]
- Tan, J.; Yang, N.; Hu, Z.; Su, J.; Zhong, J.; Yang, Y.; Yu, Y.; Zhu, J.; Xue, D.; Huang, Y.; et al. Aptamer-Functionalized Fluorescent Silica Nanoparticles for Highly Sensitive Detection of Leukemia Cells. Nanoscale Res. Lett. 2016, 11, 298–306. [Google Scholar] [CrossRef] [PubMed]
- Rosenholm, J.M.; Meinander, A.; Peuhu, E.; Niemi, R.; Eriksson, J.E.; Sahlgren, C.; Lindén, M. Targeting of Porous Hybrid Silica Nanoparticles to Cancer Cells. ACS Nano 2009, 3, 197–206. [Google Scholar] [CrossRef]
- Shan, W.; Wang, B.; Zhanga, Y.; Tang, Y. Fabrication of lotus-leaf-like nanoporous silica flakes with controlled thickness. Chem. Commun. 2005, 1877–1879. [Google Scholar]
- Xiao, Z.; Zhao, Y.; Huang, Z.; Li, Y.; Li, B. Preparation of silica nanoflakes with spherical mesopores using a dual-templating approach. Mater. Lett. 2014, 135, 233–236. [Google Scholar] [CrossRef]
- Mijowska, E.; Cendrowski, K.; Barylak, M.; Konicki, W. Sandwich-like mesoporous silica flakes for anticancer drug transport—Synthesis, characterization and kinetics release study. Colloids Surf. B 2015, 136, 119–125. [Google Scholar] [CrossRef] [PubMed]
- Peruzynska, M.; Szelag, S.; Trzeciak, K.; Kurzawski, M.; Cendrowski, K.; Barylak, M.; Roginska, D.; Piotrowska, K.; Mijowska, E.; Drozdzik, M. In vitro and in vivo evaluation of sandwich-like mesoporous silica nanoflakes as promising anticancer drug delivery system. Int. J. Pharm. 2016, 506, 458–468. [Google Scholar] [CrossRef]
- Low, P.S.; Kularatne, S.A. Folate-targeted therapeutic and imaging agents for cancer. Curr. Opin. Chem. Biol. 2009, 13, 256–262. [Google Scholar] [CrossRef] [PubMed]
- Hagner, N.; Joerger, M. Cancer chemotherapy: Targeting folic acid synthesis. Cancer Manag. Res. 2010, 2, 293–301. [Google Scholar] [PubMed]
- Rezaei, B.; Heidarbeigy, M.; Ensafi, A.A.; Dinari, M. Electrochemical Determination of Papaverine on Mg-Al Layered Double Hydroxide/Graphene Oxide and CNT Modified Carbon Paste Electrode. IEEE Sens. J. 2016, 16, 3496–3503. [Google Scholar] [CrossRef]
- Anovitz, L.M.; Cole, D.R. Characterization and Analysis of Porosity and Pore Structures. Rev. Mineral. Geochem. 2015, 80, 61–164. [Google Scholar] [CrossRef]
- Kruk, M.; Jaroniec, M.; Sayari, A. Application of large pore mcm-41 molecular sieves to improve pore size analysis using nitrogen adsorption measurements. Langmuir 1997, 13, 6267–6273. [Google Scholar] [CrossRef]
- Nandiyanto, A.B.D.; Kim, S.G.; Iskandar, F.; Okuyama, K. Synthesis of spherical mesoporous silica nanoparticles with nanometer-size controllable pores and outer diameter. Microporous Mesoporous Mater. 2009, 120, 447–453. [Google Scholar] [CrossRef]
- Yao, Y.; Lu, H.; Li, J.; Peng, Q.; Wang, Q.; Li, Y.; He, X.; Leng, J. Electrospun silica-based inorganic/organic hybrid membranes with tunable performance in appropriate solvent systems. RSC Adv. 2015, 5, 89113–89120. [Google Scholar] [CrossRef]
- Stevanović, M.; Kovačević, B.; Petković, J.; Filipič, M.; Uskoković, D. Effect of poly-α, γ, L-glutamic acid as a capping agent on morphology and oxidative stress-dependent toxicity of silver nanoparticles. Int. J. Nanomed. 2011, 6, 2837–2847. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Sumayya, A.; Panicker, C.Y.; Varghese, H.T.; Harikumar, B. Vibrational Spectroscopic Studies and Ab Initio Calculations of L-Glutamic Acid 5-Amide. Rasayan J. Chem. 2008, 1, 548–555. [Google Scholar]
- Castillo, J.J.; Rindzevicius, T.; Rozo, C.E.; Boisen, A. Adsorption and Vibrational Study of Folic Acid on Gold Nanopillar Structures Using Surface-enhanced Raman Scattering Spectroscopy. Nanomater. Nanotechnol. 2015, 5, 29–37. [Google Scholar] [CrossRef]
- Castillo, J.J.; Rindzevicius, T.; Wu, K.; Rozo, C.E.; Schmidt, M.S.; Boisen, A. Silver-capped silicon nanopillar platforms for adsorption studies of folic acid using surface enhanced Raman spectroscopy and density functional theory. J. Raman Spectrosc. 2015, 46, 1087–1094. [Google Scholar] [CrossRef]
- Kokaislová, A.; Helešicová, T.; Ončák, M.; Matějka, P. Spectroscopic studies of folic acid adsorbed on various metal substrates: Does the type of substrate play an essential role in temperature dependence of spectral features? J. Raman Spectrosc. 2014, 45, 750–757. [Google Scholar] [CrossRef]
- Wang, W.Y.; Zhao, X.F.; Ju, X.H.; Wang, Y.; Wang, L.; Li, S.P.; Li, X.D. Novel morphology change of Au-Methotrexate conjugates: From nanochains to discrete nanoparticles. Int. J. Pharm. 2016, 515, 221–232. [Google Scholar] [CrossRef]
- Xu, Y.; Li, D.; Liu, M.; Niu, F.; Liu, J.; Wang, E. Enhanced-quantum yield sulfur/nitrogen co-doped fluorescent carbon nanodots produced from biomass Enteromorpha prolifera: Synthesis, posttreatment, applications and mechanism study. Sci. Rep. 2017, 7, 4499. [Google Scholar] [CrossRef]
- Huo, X.; Dai, C.; Li, S.; Li, X. Synthesis of Au yolk/LDH shell nanoparticles as anticancer vehicles. RSC Adv. 2015, 5, 8689–8692. [Google Scholar] [CrossRef]
- Wang, C.F.; Mäkilä, E.M.; Kaasalainen, M.H.; Hagström, M.V.; Salonen, J.J.; Hirvonen, J.T.; Santos, H.A. Dual-drug delivery by porous silicon nanoparticles for improved cellular uptake, sustained release, and combination therapy. Acta Biomater. 2015, 16, 206–214. [Google Scholar] [CrossRef] [PubMed]
- DRUGBANK. Available online: https://www.drugbank.ca/drugs/DB00563 (accessed on 13 May 2020).
- Yamada, H.; Urata, C.; Aoyama, Y.; Osada, S.; Yamauchi, Y.; Kuroda, K. Preparation of colloidal mesoporous silica nanoparticles with different diameters and their unique degradation behavior in static aqueous systems. Chem. Mater. 2012, 24, 1462–1471. [Google Scholar] [CrossRef]
- Hao, N.; Liu, H.; Li, L.; Chen, D.; Li, L.; Tang, F. In vitro degradation behavior of silica nanoparticles under physiological conditions. J. Nanosci. Nanotechnol. 2012, 12, 6346–6354. [Google Scholar] [CrossRef] [PubMed]
- Choi, E.; Kim, S. How can doxorubicin loading orchestrate in vitro degradation behaviors of mesoporous silica nanoparticles under a physiological condition? Langmuir 2017, 33, 4974–4980. [Google Scholar] [CrossRef]
- Gouze, B.; Cambedouzou, J.; Parrès-Maynadié, S.; Rébiscoula, D. How hexagonal mesoporous silica evolves in water on short and long term: Role of pore size and silica wall porosity. Microporous Mesoporous Mater. 2014, 183, 168–176169. [Google Scholar] [CrossRef]
- Trewyn, B.G.; Nieweg, J.A.; Zhao, Y.; Lin, V.S.Y. Biocompatible mesoporous silica nanoparticles with different morphologies for animal cell membrane penetration. Chem. Eng. J. 2008, 137, 23–29. [Google Scholar] [CrossRef]
- Lin, Y.S.; Tsai, C.P.; Huang, H.Y.; Kuo, C.T.; Hung, Y.; Huang, D.M.; Chen, Y.C.; Mou, C.Y. Well-ordered mesoporous silica nanoparticles as cell markers. Chem. Mater. 2005, 17, 4570–4573. [Google Scholar] [CrossRef]
- Huang, X.; Teng, X.; Chen, D.; Tang, F.; He, J. The effect of the shape of mesoporous silica nanoparticles on cellular uptake and cell function. Biomaterials 2010, 31, 438–448. [Google Scholar] [CrossRef] [PubMed]
- Hao, N.; Linlin, L.; Zhang, Q.; Huang, X.; Meng, X.; Zhang, Y.; Chen, D. The shape effect of PEGylated mesoporous silica nanoparticles on cellular uptake pathway in Hela cells. Microporous Mesoporous Mater. 2012, 162, 14–23. [Google Scholar] [CrossRef]
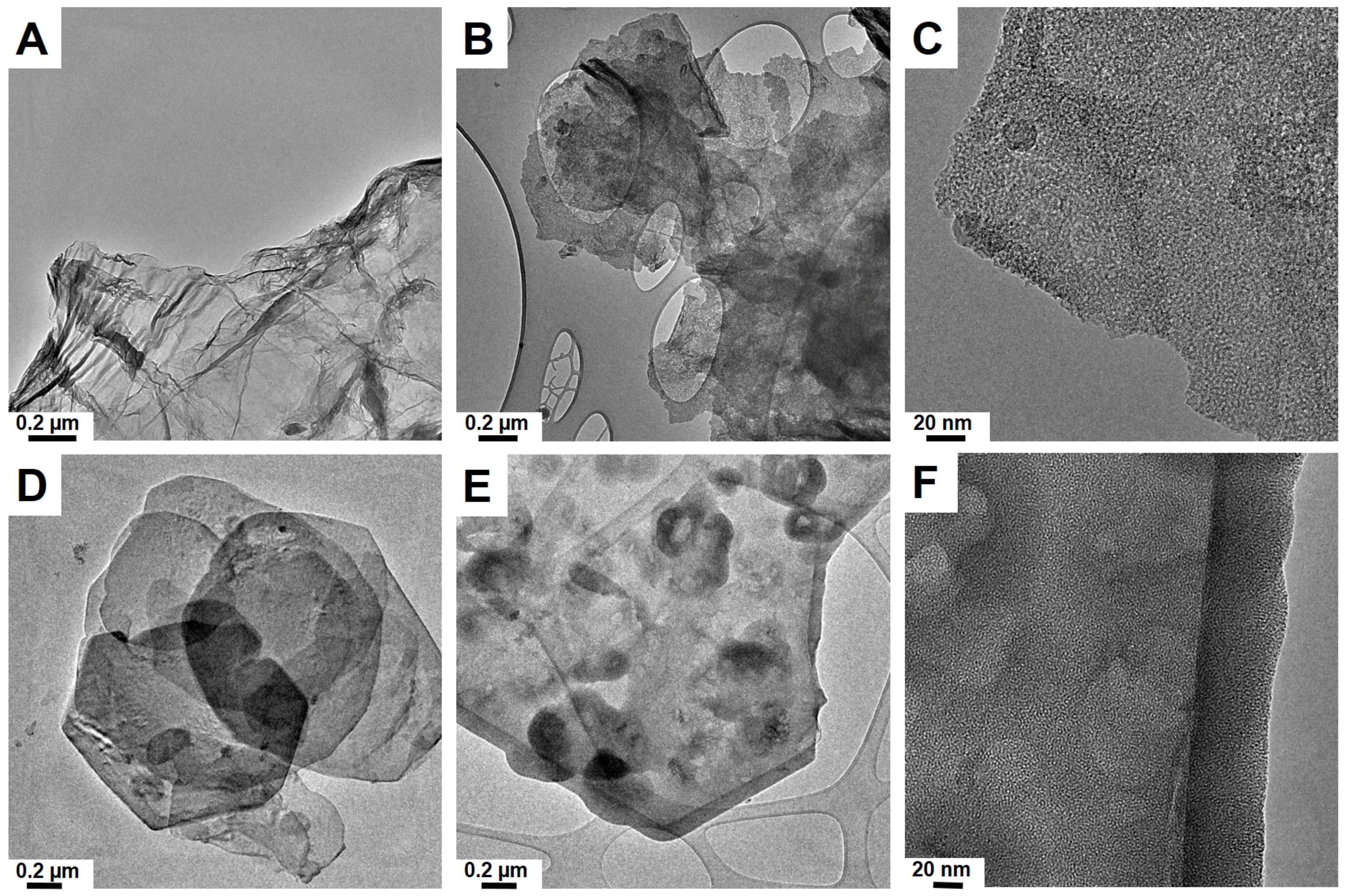
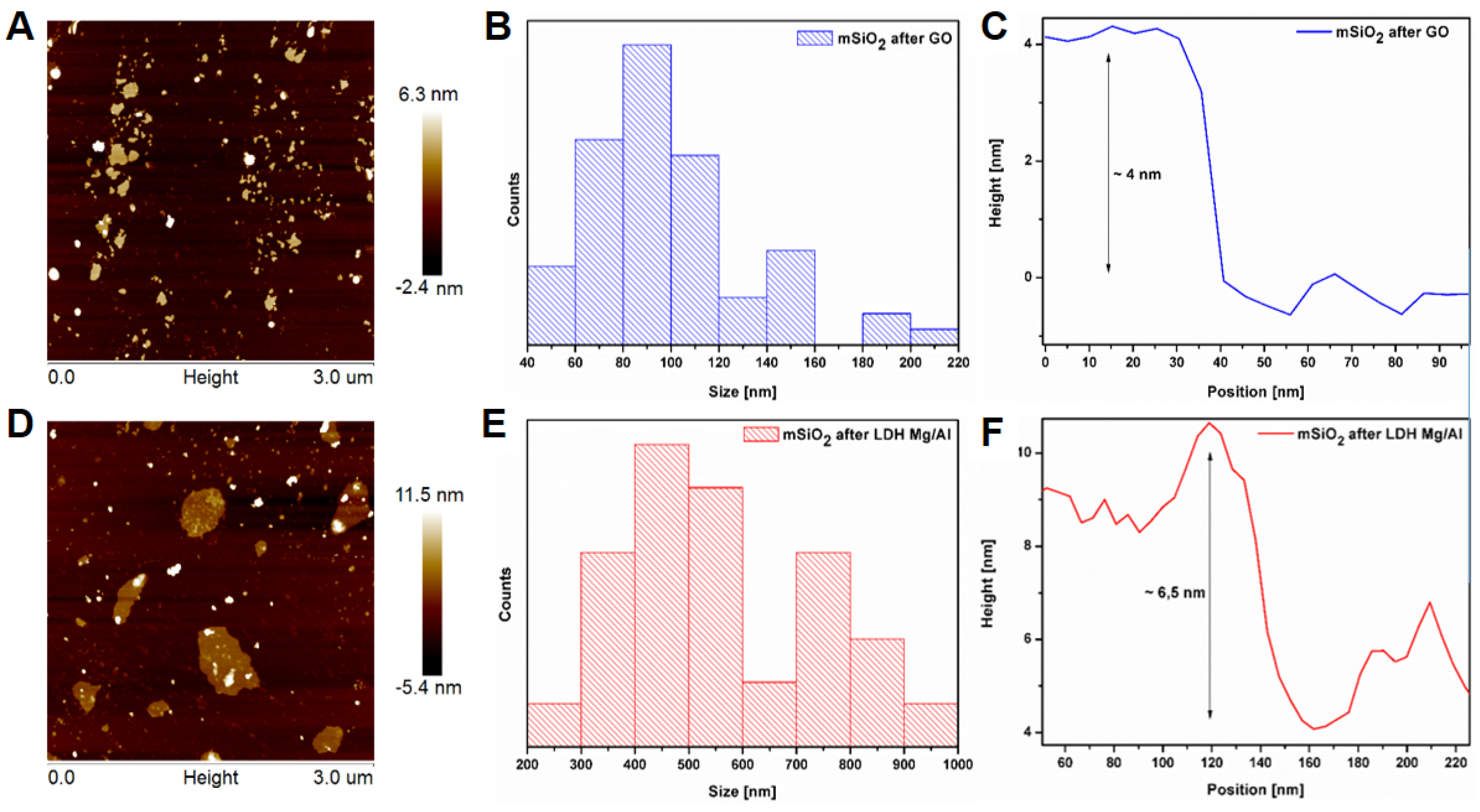
| Sample | SBET (m2/g) | VTOTAL (cm3/g) | Pore Size (nm) | Flake Size (nm) | Flake Thickness |
|---|---|---|---|---|---|
| mSiO2 after GO | 738 | 0.1501 | 1.95–6 (1.95–3) | 40–220 (80–100) | ~4 |
| mSiO2 after LDH Mg/Al | 839 | 0.052 | 2.2–4.85 (2.2–3.4) | 200–1000 (400–500) | ~6.5 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Trukawka, M.; Cendrowski, K.; Konicki, W.; Mijowska, E. Folic Acid/Methotrexate Functionalized Mesoporous Silica Nanoflakes from Different Supports: Comparative Study. Appl. Sci. 2020, 10, 6465. https://doi.org/10.3390/app10186465
Trukawka M, Cendrowski K, Konicki W, Mijowska E. Folic Acid/Methotrexate Functionalized Mesoporous Silica Nanoflakes from Different Supports: Comparative Study. Applied Sciences. 2020; 10(18):6465. https://doi.org/10.3390/app10186465
Chicago/Turabian StyleTrukawka, Martyna, Krzysztof Cendrowski, Wojciech Konicki, and Ewa Mijowska. 2020. "Folic Acid/Methotrexate Functionalized Mesoporous Silica Nanoflakes from Different Supports: Comparative Study" Applied Sciences 10, no. 18: 6465. https://doi.org/10.3390/app10186465
APA StyleTrukawka, M., Cendrowski, K., Konicki, W., & Mijowska, E. (2020). Folic Acid/Methotrexate Functionalized Mesoporous Silica Nanoflakes from Different Supports: Comparative Study. Applied Sciences, 10(18), 6465. https://doi.org/10.3390/app10186465





