The Oral Microbiome of Healthy Japanese People at the Age of 90
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design, Setting, and Participants
2.2. Sampling and Microbial DNA Extraction
2.3. Microbial-Community Analysis
2.4. Bioinformatics Analysis
2.5. Ethics Approval
3. Results
3.1. Study Participants
3.2. Sequence Data
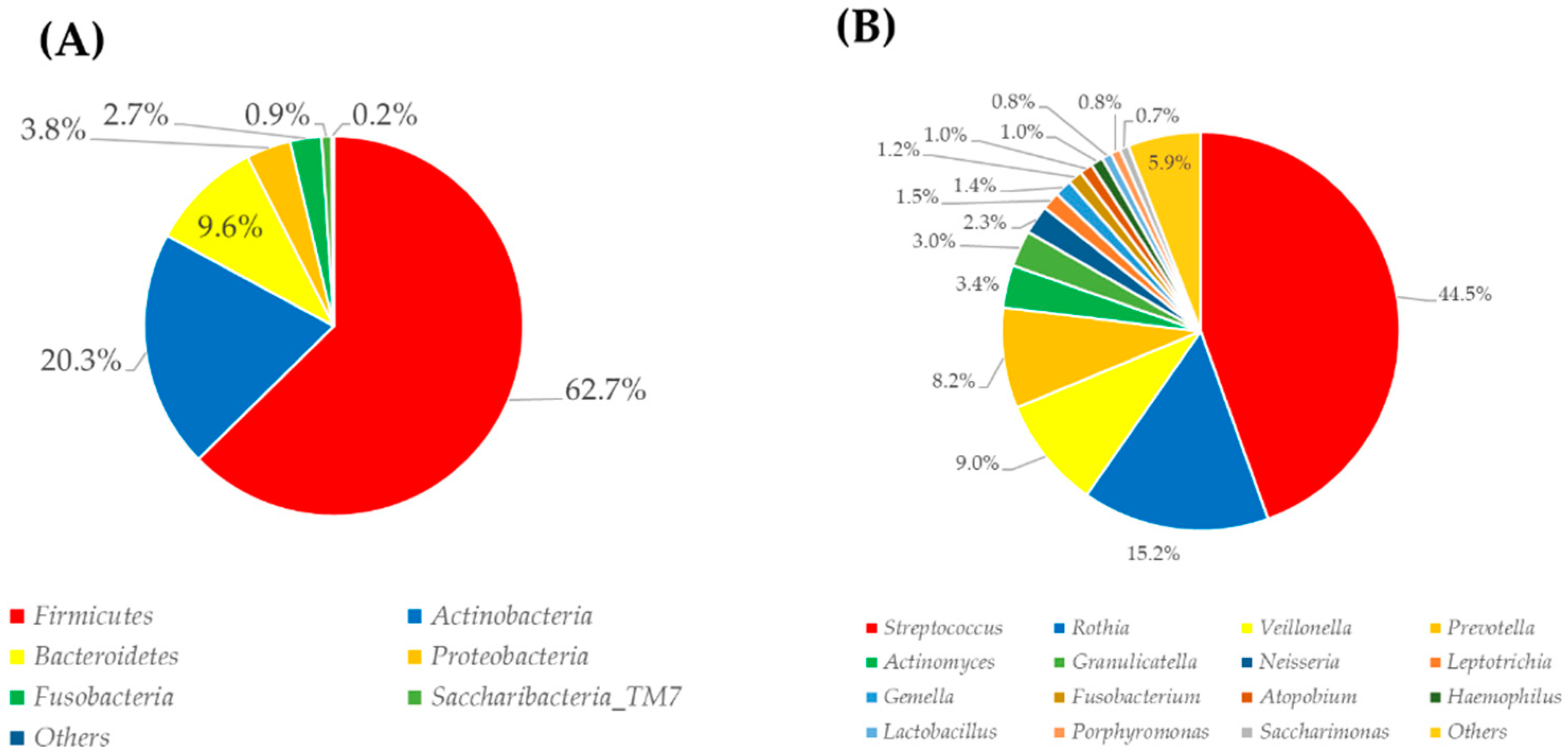
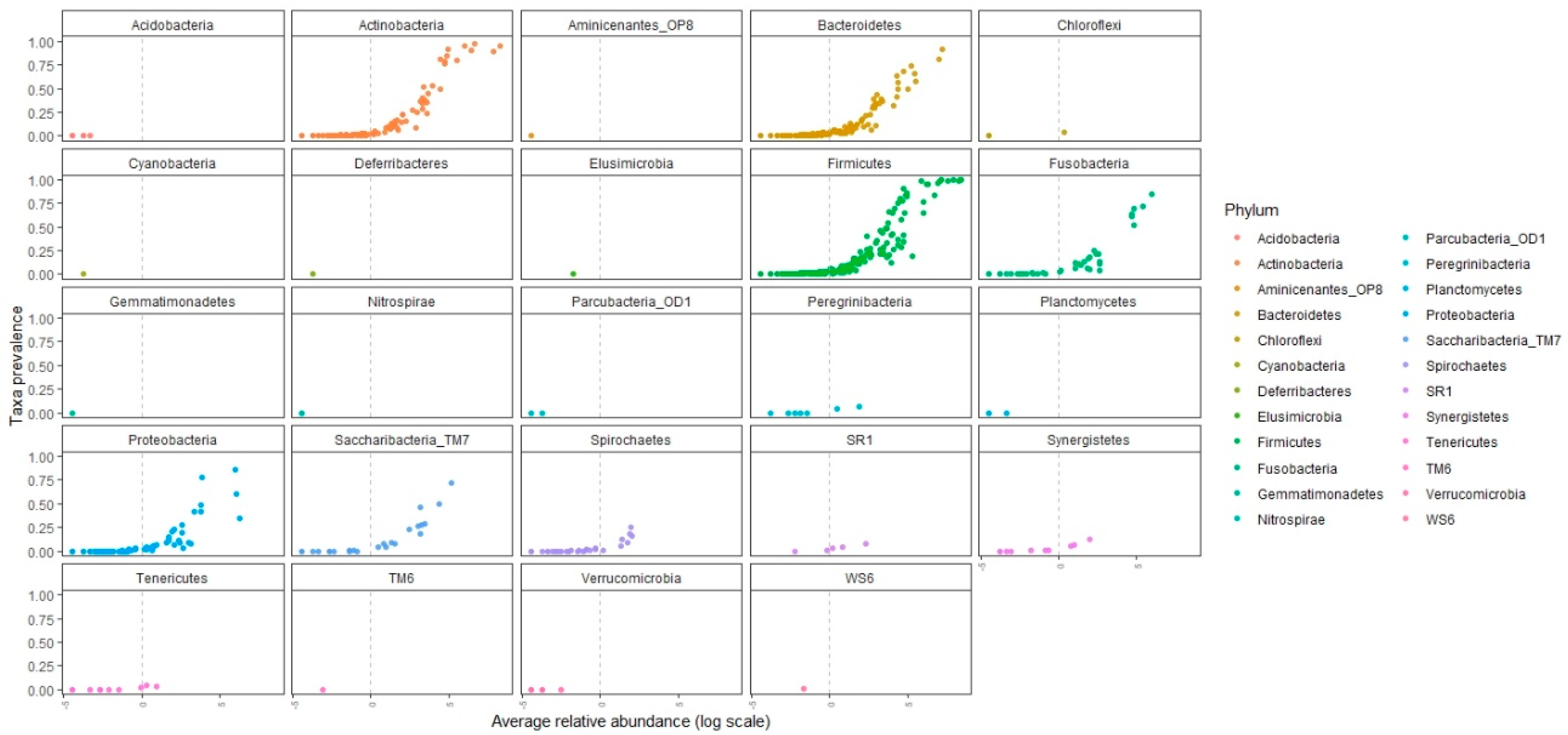
3.3. Oral-Microbiome Structure
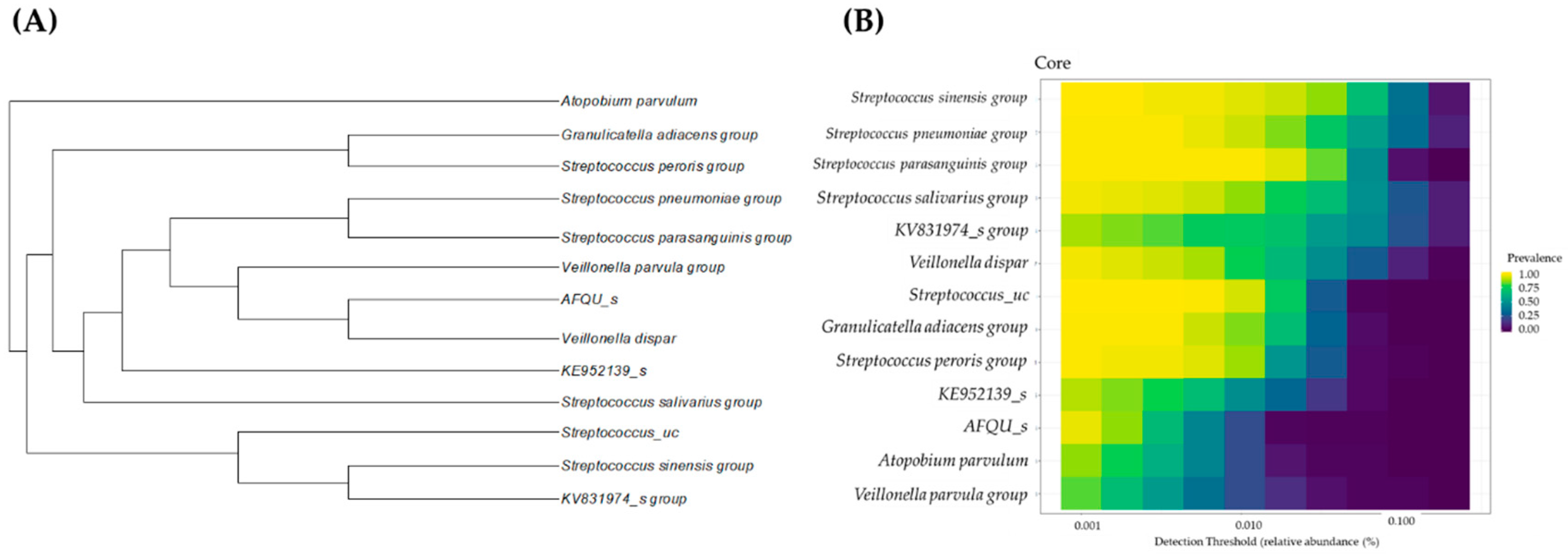
3.4. Candidates for Core Microbiome and Core-Microbiome Analysis
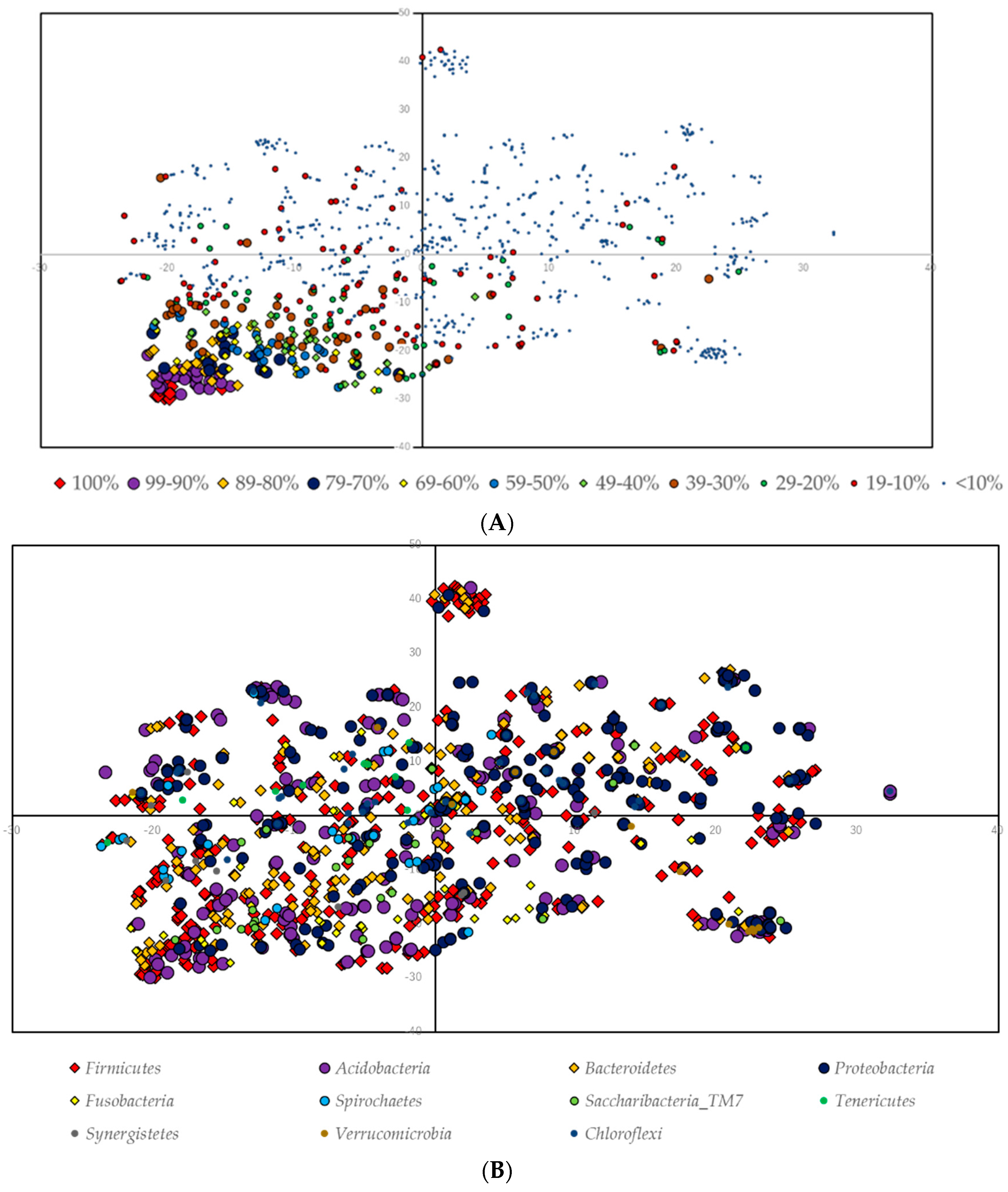
3.5. Ordination Analysis
4. Discussion
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Lynch, S.V.; Pedersen, O. The Human Intestinal Microbiome in Health and Disease. N. Engl. J. Med. 2016, 375, 2369–2379. [Google Scholar] [CrossRef] [PubMed]
- Silbergeld, E.K. The Microbiome. Toxicol. Pathol. 2017, 45, 190–194. [Google Scholar] [CrossRef] [PubMed]
- Tuddenham, S.; Sears, C.L. The intestinal microbiome and health. Curr. Opin. Infect. Dis. 2015, 28, 464–470. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.K.; Chang, H.W.; Yan, D.; Lee, K.M.; Ucmak, D.; Wong, K.; Abrouk, M.; Farahnik, B.; Nakamura, M.; Zhu, T.H.; et al. Influence of diet on the gut microbiome and implications for human health. J. Transl. Med. 2017, 15, 73. [Google Scholar] [CrossRef]
- Rinninella, E.; Raoul, P.; Cintoni, M.; Franceschi, F.; Miggiano, G.A.D.; Gasbarrini, A.; Mele, M.C. What is the Healthy Gut Microbiota Composition? A Changing Ecosystem across Age, Environment, Diet, and Diseases. Microorganisms 2019, 7, 14. [Google Scholar] [CrossRef]
- Schwartz, M.H.; Wang, H.; Pan, J.N.; Clark, W.C.; Cui, S.; Eckwahl, M.J.; Pan, D.W.; Parisien, M.; Owens, S.M.; Cheng, B.L.; et al. Microbiome characterization by high-throughput transfer RNA sequencing and modification analysis. Nat. Commun. 2018, 9, 5353. [Google Scholar] [CrossRef]
- Papapanou, P.N.; Park, H.; Cheng, B.; Kokaras, A.; Paster, B.; Burkett, S.; Watson, C.W.; Annavajhala, M.K.; Uhlemann, A.C.; Noble, J.M. Subgingival microbiome and clinical periodontal status in an elderly cohort: The WHICAP ancillary study of oral health. J. Periodontol. 2020. [Google Scholar] [CrossRef]
- Farrell, J.J.; Zhang, L.; Zhou, H.; Chia, D.; Elashoff, D.; Akin, D.; Paster, B.J.; Joshipura, K.; Wong, D.T. Variations of oral microbiota are associated with pancreatic diseases including pancreatic cancer. Gut 2012, 61, 582–588. [Google Scholar] [CrossRef]
- Demmer, R.T.; Jacobs, D.R., Jr.; Singh, R.; Zuk, A.; Rosenbaum, M.; Papapanou, P.N.; Desvarieux, M. Periodontal Bacteria and Prediabetes Prevalence in ORIGINS: The Oral Infections, Glucose Intolerance, and Insulin Resistance Study. J. Dent. Res. 2015, 94, 201S–211S. [Google Scholar] [CrossRef]
- Docktor, M.J.; Paster, B.J.; Abramowicz, S.; Ingram, J.; Wang, Y.E.; Correll, M.; Jiang, H.; Cotton, S.L.; Kokaras, A.S.; Bousvaros, A. Alterations in diversity of the oral microbiome in pediatric inflammatory bowel disease. Inflamm. Bowel Dis. 2012, 18, 935–942. [Google Scholar] [CrossRef]
- Liu, X.R.; Xu, Q.; Xiao, J.; Deng, Y.M.; Tang, Z.H.; Tang, Y.L.; Liu, L.S. Role of oral microbiota in atherosclerosis. Clin. Chim. Acta 2020, 506, 191–195. [Google Scholar] [CrossRef] [PubMed]
- Cobb, C.M.; Kelly, P.J.; Williams, K.B.; Babbar, S.; Angolkar, M.; Derman, R.J. The oral microbiome and adverse pregnancy outcomes. Int. J. Womens Health 2017, 9, 551–559. [Google Scholar] [CrossRef] [PubMed]
- Teles, F.R.F.; Alawi, F.; Castilho, R.M.; Wang, Y. Association or Causation? Exploring the Oral Microbiome and Cancer Links. J. Dent. Res. 2020. [Google Scholar] [CrossRef] [PubMed]
- Kleinstein, S.E.; Nelson, K.E.; Freire, M. Inflammatory Networks Linking Oral Microbiome with Systemic Health and Disease. J. Dent. Res. 2020, 99, 1131–1139. [Google Scholar] [CrossRef] [PubMed]
- Integrative HMP (iHMP) Research Network Consortium. The Integrative Human Microbiome Project. Nature 2019, 569, 641–648. [Google Scholar] [CrossRef] [PubMed]
- Severance, E.G.; Yolken, R.H.; Eaton, W.W. Autoimmune diseases, gastrointestinal disorders and the microbiome in schizophrenia: More than a gut feeling. Schizophr. Res. 2016, 176, 23–35. [Google Scholar] [CrossRef] [PubMed]
- Barengolts, E.; Green, S.J.; Chlipala, G.E.; Layden, B.T.; Eisenberg, Y.; Priyadarshini, M.; Dugas, L.R. Predictors of Obesity among Gut Microbiota Biomarkers in African American Men with and without Diabetes. Microorganisms 2019, 7, 320. [Google Scholar] [CrossRef]
- Zhang, F.; Wang, M.; Yang, J.; Xu, Q.; Liang, C.; Chen, B.; Zhang, J.; Yang, Y.; Wang, H.; Shang, Y.; et al. Response of gut microbiota in type 2 diabetes to hypoglycemic agents. Endocrine 2019, 66, 485–493. [Google Scholar] [CrossRef]
- Ulloa, C.P.; van der Veen, M.H.; Krom, B.P. Review: Modulation of the oral microbiome by the host to promote ecological balance. Odontology 2019, 107, 437–448. [Google Scholar] [CrossRef]
- Marsh, P.D. In Sickness and in Health-What Does the Oral Microbiome Mean to Us? An Ecological Perspective. Adv. Dent. Res. 2018, 29, 60–65. [Google Scholar] [CrossRef]
- Rosier, B.T.; Marsh, P.D.; Mira, A. Resilience of the Oral Microbiota in Health: Mechanisms That Prevent Dysbiosis. J. Dent. Res. 2018, 97, 371–380. [Google Scholar] [CrossRef] [PubMed]
- Tam, J.; Hoffmann, T.; Fischer, S.; Bornstein, S.; Gräßler, J.; Noack, B. Obesity alters composition and diversity of the oral microbiota in patients with type 2 diabetes mellitus independently of glycemic control. PLoS ONE 2018, 13, e0204724. [Google Scholar] [CrossRef]
- Abe, K.; Takahashi, A.; Fujita, M.; Imaizumi, H.; Hayashi, M.; Okai, K.; Ohira, H. Dysbiosis of oral microbiota and its association with salivary immunological biomarkers in autoimmune liver disease. PLoS ONE 2018, 13, e0198757. [Google Scholar] [CrossRef] [PubMed]
- Gaetti-Jardim, E., Jr.; Jardim, E.C.; Schweitzer, C.M.; da Silva, J.C.; Oliveira, M.M.; Masocatto, D.C.; dos Santos, C.M. Supragingival and subgingival microbiota from patients with poor oral hygiene submitted to radiotherapy for head and neck cancer treatment. Arch. Oral Biol. 2018, 90, 45–52. [Google Scholar] [CrossRef] [PubMed]
- Si, J.; Lee, C.; Ko, G. Oral Microbiota: Microbial Biomarkers of Metabolic Syndrome Independent of Host Genetic Factors. Front. Cell. Infect. Microbiol. 2017, 7, 516. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, T.; Ogawa, H.M.; Ikebe, K.; Notomi, Y.; Iwamoto, Y.; Shirobayashi, I.; Hata, S.; Kibi, M.; Masayasu, S.; Sasaki, S.; et al. Characterizations of oral microbiota in elderly nursing home residents with diabetes. J. Oral Sci. 2017, 59, 549–555. [Google Scholar] [CrossRef]
- Flemer, B.; Warren, R.D.; Barrett, M.P.; Cisek, K.; Das, A.; Jeffery, I.B.; Hurley, E.; O’Riordain, M.; Shanahan, F.; O’Toole, P.W. The oral microbiota in colorectal cancer is distinctive and predictive. Gut 2018, 67, 1454–1463. [Google Scholar] [CrossRef]
- Olson, S.H.; Satagopan, J.; Xu, Y.; Ling, L.; Leong, S.; Orlow, I.; Saldia, A.; Li, P.; Nunes, P.; Madonia, V.; et al. The oral microbiota in patients with pancreatic cancer, patients with IPMNs, and controls: A pilot study. Cancer Causes Control 2017, 28, 959–969. [Google Scholar] [CrossRef]
- Zhu, X.X.; Yang, X.J.; Chao, Y.L.; Zheng, H.M.; Sheng, H.F.; Liu, H.Y.; He, Y.; Zhou, H.W. The Potential Effect of Oral Microbiota in the Prediction of Mucositis during Radiotherapy for Nasopharyngeal Carcinoma. EBioMedicine 2017, 18, 23–31. [Google Scholar] [CrossRef]
- Chen, X.; Winckler, B.; Lu, M.; Cheng, H.; Yuan, Z.; Yang, Y.; Jin, L.; Ye, W. Oral Microbiota and Risk for Esophageal Squamous Cell Carcinoma in a High-Risk Area of China. PLoS ONE 2015, 10, e0143603. [Google Scholar] [CrossRef]
- Gaiser, R.A.; Halimi, A.; Alkharaan, H.; Lu, L.; Davanian, H.; Healy, K.; Hugerth, L.W.; Ateeb, Z.; Valente, R.; Fernández Moro, F.C.; et al. Enrichment of oral microbiota in early cystic precursors to invasive pancreatic cancer. Gut 2019, 68, 2186–2194. [Google Scholar] [CrossRef] [PubMed]
- Nomura, Y.; Otsuka, R.; Hasegawa, R.; Hanada, N. Oral Microbiome of Children Living in an Isolated Area in Myanmar. Int. J. Environ. Res. Public Health 2020, 17, 4033. [Google Scholar] [CrossRef] [PubMed]
- Iwauchi, M.; Horigome, A.; Ishikawa, K.; Mikuni, A.; Nakano, M.; Xiao, J.Z.; Odamaki, T.; Hironaka, S. Relationship between oral and gut microbiota in elderly people. Immun. Inflamm. Dis. 2019, 7, 229–236. [Google Scholar] [CrossRef]
- Asakawa, M.; Takeshita, T.; Furuta, M.; Kageyama, S.; Takeuchi, K.; Hata, J.; Ninomiya, T.; Yamashita, Y. Tongue Microbiota and Oral Health Status in Community-Dwelling Elderly Adults. mSphere 2018, 3, e00332-18. [Google Scholar] [CrossRef]
- Hirotomi, T.; Yoshihara, A.; Ogawa, H.; Ito, K.; Igarashi, A.; Miyazaki, H. A preliminary study on the relationship between stimulated saliva and periodontal conditions in community-dwelling elderly people. J. Dent. 2006, 34, 692–698. [Google Scholar] [CrossRef] [PubMed]
- Yamaga, T.; Ogawa, H.; Miyazaki, H. Influence of occlusal deterioration considering prosthetics on subsequent all-cause mortality in a Japanese elderly independent population. Gerodontology 2019, 36, 163–170. [Google Scholar] [CrossRef] [PubMed]
- Nomura, Y.; Kakuta, E.; Okada, A.; Otsuka, R.; Shimada, M.; Tomizawa, Y.; Taguchi, C.; Arikawa, K.; Daikoku, H.; Sato, T.; et al. Oral microbiome in four female centenarians. Appl. Sci. 2020, 10, 5312. [Google Scholar] [CrossRef]
- Kim, O.S.; Cho, Y.J.; Lee, K.; Yoon, S.H.; Kim, M.; Na, H.; Park, S.C.; Jeon, Y.S.; Lee, J.H.; Yi, H.; et al. Introducing EzTaxon-e: A prokaryotic 16S rRNA gene sequence database with phylotypes that represent uncultured species. Int. J. Syst. Evol. Microbiol. 2012, 62, 716–721. [Google Scholar] [CrossRef]
- Yoon, S.H.; Ha, S.M.; Kwon, S.; Lim, J.; Kim, Y.; Seo, H.; Chun, J. Introducing EzBioCloud: A taxonomically united database of 16S rRNA and whole genome assemblies. Int. J. Syst. Evol. Microbiol. 2017, 67, 1613–1617. [Google Scholar] [CrossRef]
- Lahti, L.; Shetty, S. Introduction to the Microbiome R Package. 2020. Available online: https://microbiome.github.io/tutorials/ (accessed on 31 August 2020).
- Shetty, S.A.; Hugenholtz, F.; Lahti, L.; Smidt, H.; de Vos, W.M. Intestinal microbiome landscaping: Insight in community assemblage and implications for microbial modulation strategies. FEMS Microbiol. Rev. 2017, 41, 182–199. [Google Scholar] [CrossRef]
- Bagwell, C.B. High-Dimensional Modeling for Cytometry: Building Rock Solid Models Using GemStone™ and Verity Cen-se’™ High-Definition t-SNE Mapping. Methods Mol. Biol. 2018, 1678, 11–36. [Google Scholar] [CrossRef]
- Jiang, Q.; Liu, J.; Chen, L.; Gan, N.; Yang, D. The Oral Microbiome in the Elderly with Dental Caries and Health. Front. Cell. Infect. Microbiol. 2019, 8, 442. [Google Scholar] [CrossRef] [PubMed]
- Balle, C.; Esra, R.; Havyarimana, E.; Jaumdally, S.Z.; Lennard, K.; Konstantinus, I.N.; Barnabas, S.L.; Happel, A.U.; Gill, K.; Pidwell, T.; et al. Relationship between the Oral and Vaginal Microbiota of South African Adolescents with High Prevalence of Bacterial Vaginosis. Microorganisms 2020, 8, 1004. [Google Scholar] [CrossRef]
- Matsha, T.E.; Prince, Y.; Davids, S.; Chikte, U.; Erasmus, R.T.; Kengne, A.P.; Davison, G.M. Oral Microbiome Signatures in Diabetes Mellitus and Periodontal Disease. J. Dent. Res. 2020, 99, 658–665. [Google Scholar] [CrossRef]
- Burcham, Z.M.; Garneau, N.L.; Comstock, S.S.; Tucke, R.M.; Knight, R.; Metcalf, J.L. Patterns of Oral Microbiota Diversity in Adults and Children: A Crowdsourced Population Study. Sci. Rep. 2020, 10, 2133. [Google Scholar] [CrossRef] [PubMed]
- Nakano, M.; Wakabayashi, H.; Sugahara, H.; Odamaki, T.; Yamauchi, K.; Abe, F.; Xiao, J.Z.; Murakami, K.; Ishikawa, K.; Hironaka, S. Effects of lactoferrin and lactoperoxidase-containing food on the oral microbiota of older individuals. Microbiol. Immunol. 2017, 61, 416–426. [Google Scholar] [CrossRef]
- Anderson, A.C.; Rothballer, M.; Altenburger, M.J.; Woelber, J.P.; Karygianni, L.; Vach, K.; Hellwig, E.; Al-Ahmad, A. Long-term fluctuation of oral biofilm microbiota following different dietary phases. Appl. Environ. Microbiol. 2020. [Google Scholar] [CrossRef]
- Sampaio-Maia, B.; Monteiro-Silva, F. Acquisition and maturation of oral microbiome throughout childhood: An update. Dent. Res. J. 2014, 11, 291–301. [Google Scholar]
- Anderson, A.C.; Rothballer, M.; Altenburger, M.J.; Woelber, J.P.; Karygianni, L.; Lagkouvardos, I.; Hellwig, E.; Al-Ahmad, A. In-vivo shift of the microbiota in oral biofilm in response to frequent sucrose consumption. Sci. Rep. 2018, 8, 14202. [Google Scholar] [CrossRef]
- Yang, L.; Dunlap, D.G.; Qin, S.; Fitch, A.; Li, K.; Koch, C.D.; Nouraie, M.; DeSensi, R.; Ho, K.S.; Martinson, J.J.; et al. Alterations in Oral Microbiota in HIV Are Related to Decreased Pulmonary Function. Am. J. Respir. Crit. Care Med. 2020, 201, 445–457. [Google Scholar] [CrossRef]
- Starr, J.R.; Huang, Y.; Lee, K.H.; Murphy, C.M.; Moscicki, A.B.; Shiboski, C.H.; Ryder, M.I.; Yao, T.J.; Faller, L.L.; Dyke, V.R.B.; et al. Oral microbiota in youth with perinatally acquired HIV infection. Microbiome 2018, 6, 100. [Google Scholar] [CrossRef] [PubMed]
- Anbalagan, R.; Srikanth, P.; Mani, M.; Barani, R.; Seshadri, K.G.; Janarthanan, R. Next generation sequencing of oral microbiota in Type 2 diabetes mellitus prior to and after neem stick usage and correlation with serum monocyte chemoattractant-1. Diabetes Res. Clin. Pract. 2017, 130, 204–210. [Google Scholar] [CrossRef] [PubMed]
- Querido, N.B.; de Araujo, W.C. Selective isolation of Neisseria sicca from the human oral cavity on eosin methylene blue agar. Appl. Environ. Microbiol. 1976, 4, 612–614. [Google Scholar] [CrossRef] [PubMed]
- Cephas, K.D.; Kim, J.; Mathai, R.A.; Barry, K.A.; Dowd, S.E.; Meline, B.S.; Swanson, K.S. Comparative analysis of salivary bacterial microbiome diversity in edentulous infants and their mothers or primary care givers using pyrosequencing. PLoS ONE 2011, 6, e23503. [Google Scholar] [CrossRef]
- Shi, W.; Qin, M.; Chen, F.; Xia, B. Supragingival Microbial Profiles of Permanent and Deciduous Teeth in Children with Mixed Dentition. PLoS ONE 2016, 11, e0146938. [Google Scholar] [CrossRef]
- Lassalle, F.; Spagnoletti, M.; Fumagalli, M.; Shaw, L.; Dyble, M.; Walker, C.; Thomas, M.G.; Migliano, A.B.; Balloux, F. Oral microbiomes from hunter-gatherers and traditional farmers reveal shifts in commensal balance and pathogen load linked to diet. Mol. Ecol. 2018, 27, 182–195. [Google Scholar] [CrossRef]
- Ruiz, L.; Bacigalupe, R.; García-Carral, C.; Boix-Amoros, A.; Argüello, H.; Silva, C.B.; de los Angeles Checa, M.; Mira, A.; Rodríguez, J. Microbiota of human precolostrum and its potential role as a source of bacteria to the infant mouth. Sci. Rep. 2019, 9, 8435. [Google Scholar] [CrossRef]
- Biagi, E.; Aceti, A.; Quercia, S.; Beghetti, I.; Rampelli, S.; Turroni, S.; Soverini, M.; Zambrini, A.V.; Faldella, G.; Candela, M.; et al. Microbial Community Dynamics in Mother’s Milk and Infant’s Mouth and Gut in Moderately Preterm Infants. Front. Microbiol. 2018, 22, 2512. [Google Scholar] [CrossRef]
- Bidossi, A.; de Grandi, R.; Toscano, M.; Bottagisio, M.; de Vecchi, E.; Gelardi, M.; Drago, L. Probiotics Streptococcus salivarius 24SMB and Streptococcus oralis 89a interfere with biofilm formation of pathogens of the upper respiratory tract. BMC Infect. Dis. 2018, 18, 653. [Google Scholar] [CrossRef]
- Humphreys, G.J.; McBain, A.J. Antagonistic effects of Streptococcus and Lactobacillus probiotics in pharyngeal biofilms. Lett. Appl. Microbiol. 2019, 68, 303–312. [Google Scholar] [CrossRef]
- Woo, P.C.; Teng, J.L.; Leung, K.W.; Lau, S.K.; Tse, H.; Wong, B.H.; Yuen, K.Y. Streptococcus sinensis may react with Lancefield group F antiserum. J. Med. Microbiol. 2004, 53, 1083–1088. [Google Scholar] [CrossRef] [PubMed]
- Faibis, F.; Mihaila, L.; Perna, S.; Lefort, J.F.; Demachy, M.C.; le Flèche-Matéos, A.; Bouvet, A. Streptococcus sinensis: An emerging agent of infective endocarditis. J. Med. Microbiol. 2008, 57, 528–531. [Google Scholar] [CrossRef] [PubMed]
- Leonard, A.; Lalk, M. Infection and metabolism—Streptococcus pneumoniae metabolism facing the host environment. Cytokine 2018, 112, 75–86. [Google Scholar] [CrossRef] [PubMed]
- Valm, A.M.; Welch, J.L.; Rieken, C.W.; Hasegawa, Y.; Sogin, M.L.; Oldenbourg, R.; Dewhirst, F.E.; Borisy, G.G. Systems-level analysis of microbial community organization through combinatorial labeling and spectral imaging. Proc. Natl. Acad. Sci. USA 2011, 108, 4152–4157. [Google Scholar] [CrossRef] [PubMed]
- Zaura, E.; Keijser, B.J.; Huse, S.M.; Crielaard, W. Defining the healthy “core microbiome” of oral microbial communities. BMC Microbiol. 2009, 9, 259. [Google Scholar] [CrossRef]
- Kanasi, E.; Dewhirst, F.E.; Chalmers, N.I.; Kent, R., Jr.; Moore, A.; Hughes, C.V.; Pradhan, N.; Loo, C.Y.; Tanner, A.C.R. Clonal analysis of the microbiota of severe early childhood caries. Caries Res. 2010, 44, 485–497. [Google Scholar] [CrossRef]
- Khemaleelaku, S.; Baumgartner, J.C.; Pruksakorn, S. Identification of bacteria in acute endodontic infections and their antimicrobial susceptibility. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2002, 94, 746–755. [Google Scholar] [CrossRef]
- Mashima, I.; Kamaguchi, A.; Nakazawa, F. The distribution and frequency of oral veillonella spp. in the tongue biofilm of healthy young adults. Curr. Microbiol. 2011, 63, 403–407. [Google Scholar] [CrossRef]
- Kolenbrander, P.E. Oral microbial communities: Biofilms, interactions, and genetic systems. Annu. Rev. Microbiol. 2000, 54, 413–437. [Google Scholar] [CrossRef]
- Aas, J.A.; Paster, B.J.; Stokes, L.N.; Olsen, I.; Dewhirst, F.E. Defining the normal bacterial flora of the oral cavity. J. Clin. Microbiol. 2005, 43, 5721–5732. [Google Scholar] [CrossRef]
- Jensen, E.; Allen, G.; Bednarz, J.; Couper, J.; Peña, A. Periodontal risk markers in children and adolescents with type 1 diabetes: A systematic review and meta-analysis. Diabetes Metab. Res. Rev. 2020, e3368. [Google Scholar] [CrossRef]
- Wang, J.; Yang, X.; Zou, X.; Zhang, Y.; Wang, J.; Wang, Y. Relationship between periodontal disease and lung cancer: A systematic review and meta-analysis. J. Periodontal. Res. 2020. [Google Scholar] [CrossRef] [PubMed]
- Nadim, R.; Tang, J.; Dilmohamed, A.; Yuan, S.; Wu, C.; Bakre, A.T.; Partridge, M.; Ni, J.; Copeland, J.R.; Anstey, K.J.; et al. Influence of periodontal disease on risk of dementia: A systematic literature review and a meta-analysis. Eur. J. Epidemiol. 2020. [Google Scholar] [CrossRef]
- Priyamvara, A.; Dey, A.K.; Bandyopadhyay, D.; Katikineni, V.; Zaghlol, R.; Basyal, B.; Barssoum, K.; Amarin, R.; Bhatt, D.L.; Lavie, C.J. Periodontal Inflammation and the Risk of Cardiovascular Disease. Curr. Atheroscler. Rep. 2020, 22, 28. [Google Scholar] [CrossRef]
- Orlandi, M.; Graziani, F.; D’Aiuto, F. Periodontal therapy and cardiovascular risk. Periodontol. 2000 2020, 83, 107–124. [Google Scholar] [CrossRef]
- Jepsen, S.; Suvan, J.; Deschner, J. The association of periodontal diseases with metabolic syndrome and obesity. Periodontol. 2000 2020, 83, 125–153. [Google Scholar] [CrossRef]
- Isola, G.; Polizzi, A.; Iorio-Siciliano, V.; Alibrandi, A.; Ramaglia, L.; Leonardi, R. Effectiveness of a nutraceutical agent in the non-surgical periodontal therapy: A randomized, controlled clinical trial. Clin. Oral Investig. 2020, 1–11. [Google Scholar] [CrossRef]
- Isola, G.; Polizzi, A.; Santonocito, S.; Alibrandi, A.; Ferlito, S. Expression of Salivary and Serum Malondialdehyde and Lipid Profile of Patients with Periodontitis and Coronary Heart Disease. Int. J. Mol. Sci. 2019, 20, 6061. [Google Scholar] [CrossRef]
- Isola, G.; Giudice, A.L.; Polizzi, A.; Alibrandi, A.; Patini, R.; Ferlito, S. Periodontitis and Tooth Loss Have Negative Systemic Impact on Circulating Progenitor Cell Levels: A Clinical Study. Genes 2019, 10, 1022. [Google Scholar] [CrossRef]
| Taxonomy | Prevalence (n%) | Abundance (Average) | ||||
|---|---|---|---|---|---|---|
| Phylum | Genus | Species | ||||
| 100% | Firmicutes | Streptococcus | Streptococcus sinensis | 85/85 | 100% | 10.14% |
| Firmicutes | Streptococcus | Streptococcus pneumoniae | 85/85 | 100% | 9.61% | |
| Firmicutes | Streptococcus | Streptococcus salivarius | 85/85 | 100% | 8.75% | |
| Actinobacteria | Rothia | KV831974_s | 85/85 | 100% | 8.30% | |
| Firmicutes | Streptococcus | Streptococcus parasanguinis | 85/85 | 100% | 6.13% | |
| Firmicutes | Veillonella | Veillonella dispar | 85/85 | 100% | 4.31% | |
| Firmicutes | Granulicatella | Granulicatella adiacens | 85/85 | 100% | 2.90% | |
| Firmicutes | Streptococcus | Streptococcus_uc | 85/85 | 100% | 2.80% | |
| Firmicutes | Streptococcus | Streptococcus peroris | 85/85 | 100% | 2.67% | |
| Actinobacteria | Actinomyces | KE952139_s | 85/85 | 100% | 1.72% | |
| Firmicutes | Veillonella | Veillonella parvula | 85/85 | 100% | 1.15% | |
| Actinobacteria | Atopobium | Atopobium parvulum | 85/85 | 100% | 0.89% | |
| Firmicutes | Streptococcus | AFQU_s | 85/85 | 100% | 0.77% | |
| 99%–95% | Firmicutes | Veillonella | Veillonella atypica | 84/85 | 98.8% | 2.22% |
| Actinobacteria | Actinomyces | Actinomyces_uc | 84/85 | 98.8% | 0.30% | |
| Firmicutes | Veillonella | Veillonella_uc | 84/85 | 98.8% | 0.25% | |
| Actinobacteria | Rothia | Rothia mucilaginosa | 83/85 | 97.6% | 5.43% | |
| Firmicutes | Gemella | Gemella haemolysans | 83/85 | 97.6% | 1.23% | |
| Actinobacteria | Rothia | Rothia_uc | 83/85 | 97.6% | 0.28% | |
| Proteobacteria | Campylobacter | Campylobacter concisus | 83/85 | 97.6% | 0.10% | |
| Bacteroides | Prevotella | Prevotella melaninogenica | 82/85 | 96.5% | 2.92% | |
| Firmicutes | Streptococcus | Streptococcus gordonii | 82/85 | 96.5% | 1.78% | |
| Firmicutes | Moryella | Stomatobaculum longum | 82/85 | 96.5% | 0.31% | |
| Actinobacteria | Actinomyces | JVLH_s | 82/85 | 96.5% | 0.18% | |
| Actinobacteria | Rothia | Rothia dentocariosa | 81/85 | 95.3% | 1.36% | |
| Firmicutes | Bulleidia | Solobacterium moorei | 81/85 | 95.3% | 0.20% | |
| >90% | Fusobacteria | Fusobacterium | Fusobacterium nucleatum | 80/85 | 94.1% | 0.87% |
| Firmicutes | Megasphaera | Megasphaera micronuciformis | 80/85 | 94.1% | 0.30% | |
| Actinobacteria | Actinomyces | Actinomyces odontolyticus | 80/85 | 94.1% | 0.24% | |
| Firmicutes | Lachnoanaerobaculum | Lachnoanaerobaculum saburreum | 80/85 | 94.1% | 0.17% | |
| Bacteroides | Prevotella | Prevotella histicola | 79/85 | 92.9% | 2.39% | |
| Proteobacteria | Haemophilus | Haemophilus parainfluenzae | 79/85 | 92.9% | 0.88% | |
| Bacteroides | Prevotella | Prevotella_uc | 79/85 | 92.9% | 0.42% | |
| Firmicutes | Streptococcus | Streptococcus sanguinis | 78/85 | 91.8% | 0.92% | |
| Actinobacteria | Actinomyces | Actinomyces graevenitzii | 78/85 | 91.8% | 0.52% | |
| Bacteroides | Prevotella | Prevotella salivae | 77/85 | 90.6% | 0.25% | |
| Firmicutes | Oribacterium | Oribacterium asaccharolyticum | 77/85 | 90.6% | 0.24% | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nomura, Y.; Kakuta, E.; Kaneko, N.; Nohno, K.; Yoshihara, A.; Hanada, N. The Oral Microbiome of Healthy Japanese People at the Age of 90. Appl. Sci. 2020, 10, 6450. https://doi.org/10.3390/app10186450
Nomura Y, Kakuta E, Kaneko N, Nohno K, Yoshihara A, Hanada N. The Oral Microbiome of Healthy Japanese People at the Age of 90. Applied Sciences. 2020; 10(18):6450. https://doi.org/10.3390/app10186450
Chicago/Turabian StyleNomura, Yoshiaki, Erika Kakuta, Noboru Kaneko, Kaname Nohno, Akihiro Yoshihara, and Nobuhiro Hanada. 2020. "The Oral Microbiome of Healthy Japanese People at the Age of 90" Applied Sciences 10, no. 18: 6450. https://doi.org/10.3390/app10186450
APA StyleNomura, Y., Kakuta, E., Kaneko, N., Nohno, K., Yoshihara, A., & Hanada, N. (2020). The Oral Microbiome of Healthy Japanese People at the Age of 90. Applied Sciences, 10(18), 6450. https://doi.org/10.3390/app10186450






