Rubber Dam Isolation and High-Volume Suction Reduce Ultrafine Dental Aerosol Particles: An Experiment in a Simulated Patient
Abstract
Featured Application
Abstract
1. Introduction
2. Materials and Methods
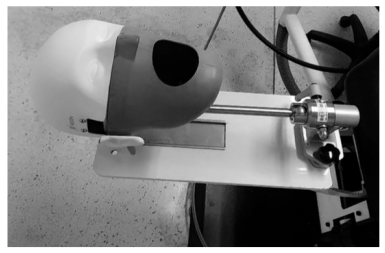
2.1. Dental Simulated Patient
2.2. Particulate Matter Detection and Quantitation
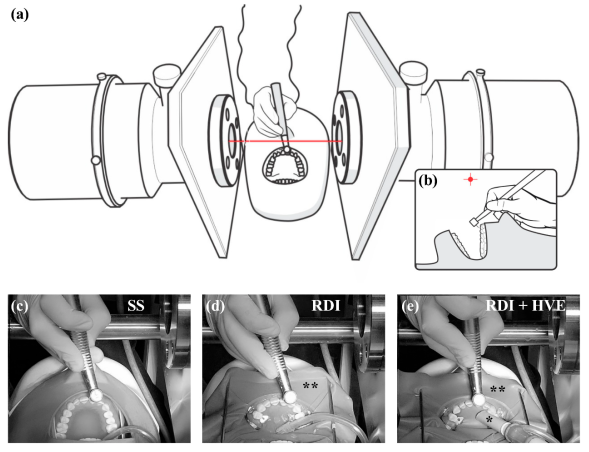
2.3. Dental Isolation and Suction Techniques
2.4. Dental Restorative Procedures
2.5. Statistical Analysis
3. Results
3.1. Ultrafine Dental Aerosol Particles Are Generated during Full-Crown Teeth Preparation
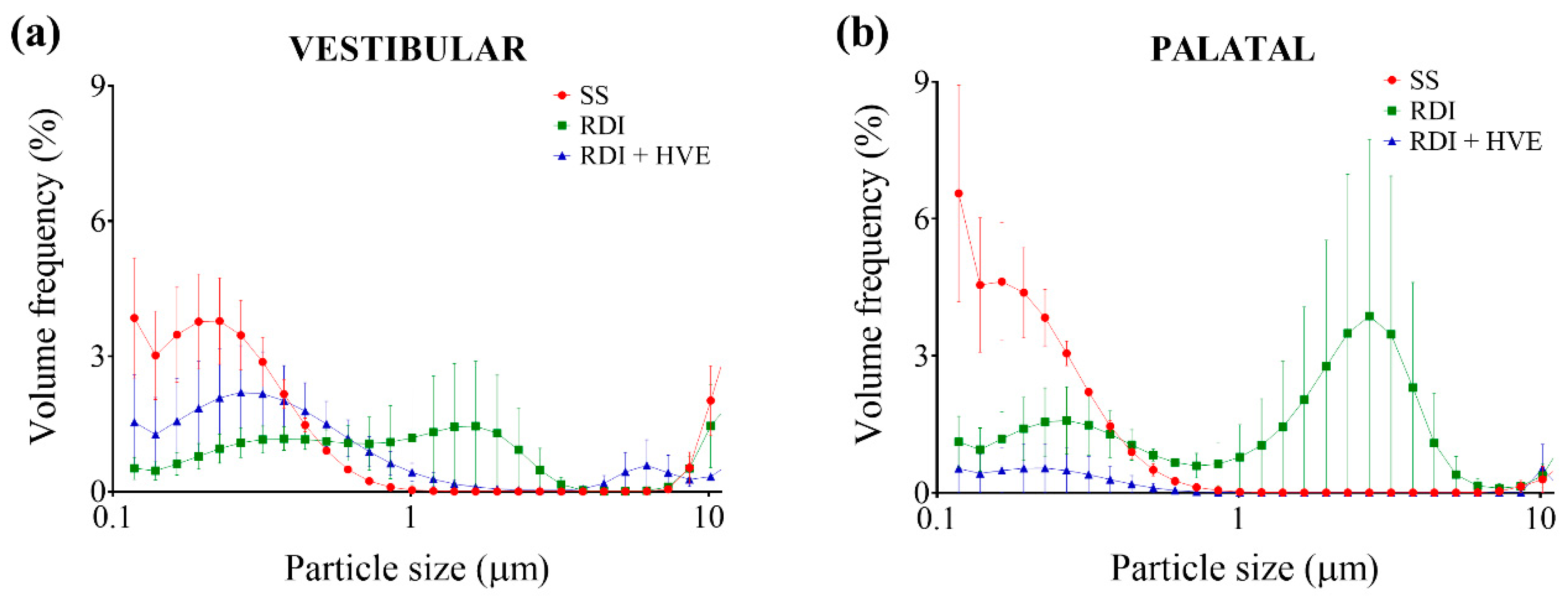
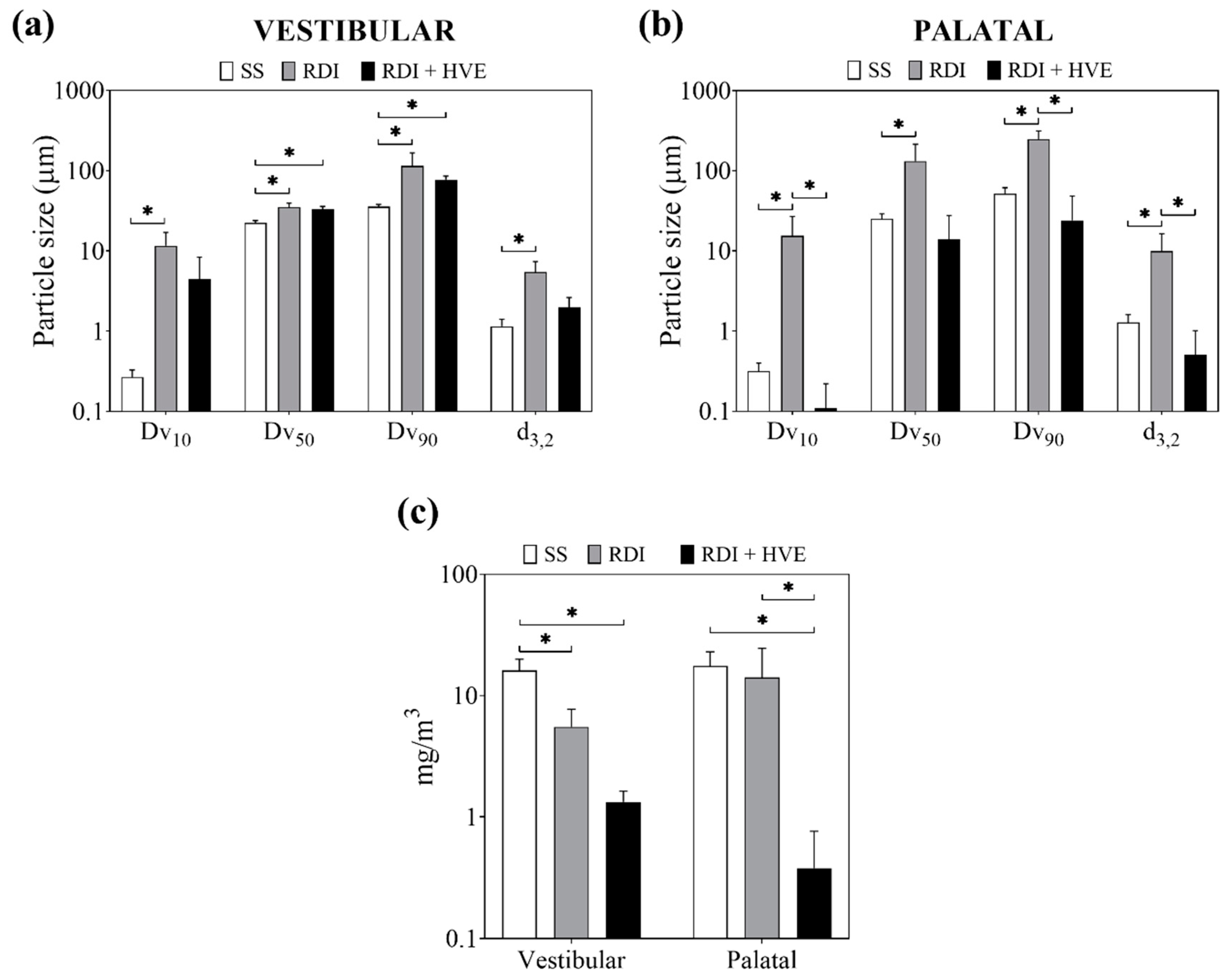
3.2. Rubber Dam Isolation and High-Volume Suction Reduce the Volume Fraction of Ultrafine Dental Aerosol Particles
3.3. Rubber Dam Isolation and High-Volume Suction Reduce the Concentration of Dental Aerosol Particles
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A

References
- Coulthard, P. Dentistry and coronavirus (COVID-19)—moral decision-making. Br. Dent. J. 2020, 228, 503–505. [Google Scholar] [CrossRef] [PubMed]
- Zemouri, C.; Volgenant, C.M.C.; Buijs, M.J.; Crielaard, W.; Rosema, N.A.M.; Brandt, B.W.; Laheij, A.; De Soet, J.J. Dental aerosols: Microbial composition and spatial distribution. J. Oral Microbiol. 2020, 12, 1762040. [Google Scholar] [CrossRef] [PubMed]
- Ather, A.; Patel, B.; Ruparel, N.B.; Diogenes, A.; Hargreaves, K.M. Coronavirus Disease 19 (COVID-19): Implications for Clinical Dental Care. J. Endod. 2020, 46, 584–595. [Google Scholar] [CrossRef] [PubMed]
- Clarkson, J.R.C.; Richards, D.; Robertson, C.; Aceves-Martins, M.; On behalf of the CoDER Working Group. Aerosol Generating Procedures and their Mitigation in International Dental Guidance Documents—A Rapid Review. 2020. Available online: https://oralhealth.cochrane.org/news/aerosol-generating-procedures-and-their-mitigation-international-guidance-documents (accessed on 30 July 2020).
- To, K.K.; Tsang, O.T.; Leung, W.S.; Tam, A.R.; Wu, T.C.; Lung, D.C.; Yip, C.C.; Cai, J.P.; Chan, J.M.; Chik, T.S.; et al. Temporal profiles of viral load in posterior oropharyngeal saliva samples and serum antibody responses during infection by SARS-CoV-2: An observational cohort study. Lancet Infect. Dis. 2020, 20, 565–574. [Google Scholar] [CrossRef]
- Chen, J.H.; Yip, C.C.; Poon, R.W.; Chan, K.H.; Cheng, V.C.; Hung, I.F.; Chan, J.F.; Yuen, K.Y.; To, K.K. Evaluating the use of posterior oropharyngeal saliva in a point-of-care assay for the detection of SARS-CoV-2. Emerg. Microbes Infect 2020, 9, 1356–1359. [Google Scholar] [CrossRef]
- Peng, X.; Xu, X.; Li, Y.; Cheng, L.; Zhou, X.; Ren, B. Transmission routes of 2019-nCoV and controls in dental practice. Int. J. Oral Sci. 2020, 12, 9. [Google Scholar] [CrossRef]
- Patini, R. How to Face the Post-SARS-CoV-2 Outbreak Era in Private Dental Practice: Current Evidence for Avoiding Cross-infections. J. Int. Soc. Prev. Community Dent. 2020, 10, 237–239. [Google Scholar] [CrossRef]
- WHO Global. Considerations for the Provision of Essential Oral Health Services in the Context Of COVID-19. Available online: https://www.who.int/publications/i/item/who-2019-nCoV-oral-health-2020.1 (accessed on 11 August 2020).
- Meng, L.; Hua, F.; Bian, Z. Coronavirus Disease 2019 (COVID-19): Emerging and Future Challenges for Dental and Oral Medicine. J. Dent. Res. 2020, 99, 481–487. [Google Scholar] [CrossRef]
- Izzetti, R.; Nisi, M.; Gabriele, M.; Graziani, F. COVID-19 Transmission in Dental Practice: Brief Review of Preventive Measures in Italy. J. Dent. Res. 2020, 99, 1030–1038. [Google Scholar] [CrossRef]
- Ge, Z.Y.; Yang, L.M.; Xia, J.J.; Fu, X.H.; Zhang, Y.Z. Possible aerosol transmission of COVID-19 and special precautions in dentistry. J. Zhejiang Univ. Sci. B 2020, 21, 361–368. [Google Scholar] [CrossRef]
- Koletsi, D.; Belibasakis, G.N.; Eliades, T. Interventions to Reduce Aerosolized Microbes in Dental Practice: A Systematic Review with Network Meta-analysis of Randomized Controlled Trials. J. Dent. Res. 2020. [Google Scholar] [CrossRef] [PubMed]
- Al-Amad, S.H.; Awad, M.A.; Edher, F.M.; Shahramian, K.; Omran, T.A. The effect of rubber dam on atmospheric bacterial aerosols during restorative dentistry. J. Infect. Public Health 2017, 10, 195–200. [Google Scholar] [CrossRef] [PubMed]
- Holloman, J.L.; Mauriello, S.M.; Pimenta, L.; Arnold, R.R. Comparison of suction device with saliva ejector for aerosol and spatter reduction during ultrasonic scaling. J. Am. Dent. Assoc. 2015, 146, 27–33. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.H.; Chen, C.T.; Chuang, L.C.; Lin, W.M.; Wan, G.H. Removal efficiency of central vacuum system and protective masks to suspended particles from dental treatment. PLoS ONE 2019, 14, e0225644. [Google Scholar] [CrossRef] [PubMed]
- Sotiriou, M.; Ferguson, S.F.; Davey, M.; Wolfson, J.M.; Demokritou, P.; Lawrence, J.; Sax, S.N.; Koutrakis, P. Measurement of particle concentrations in a dental office. Environ. Monit. Assess. 2008, 137, 351–361. [Google Scholar] [CrossRef]
- Wilson, W.E.; Suh, H.H. Fine particles and coarse particles: Concentration relationships relevant to epidemiologic studies. J. Air. Waste Manag. Assoc. 1997, 47, 1238–1249. [Google Scholar] [CrossRef]
- Pope, C.A., 3rd; Bhatnagar, A.; McCracken, J.P.; Abplanalp, W.; Conklin, D.J.; O’Toole, T. Exposure to Fine Particulate Air Pollution Is Associated with Endothelial Injury and Systemic Inflammation. Circ. Res. 2016, 119, 1204–1214. [Google Scholar] [CrossRef]
- Chang, X.; Zhou, L.; Tang, M.; Wang, B. Association of fine particles with respiratory disease mortality: A meta-analysis. Arch. Environ. Occup. Health 2015, 70, 98–101. [Google Scholar] [CrossRef]
- Yang, Y.; Ruan, Z.; Wang, X.; Yang, Y.; Mason, T.G.; Lin, H.; Tian, L. Short-term and long-term exposures to fine particulate matter constituents and health: A systematic review and meta-analysis. Environ. Pollut. 2019, 247, 874–882. [Google Scholar] [CrossRef]
- Zoran, M.A.; Savastru, R.S.; Savastru, D.M.; Tautan, M.N. Assessing the relationship between surface levels of PM2.5 and PM10 particulate matter impact on COVID-19 in Milan, Italy. Sci. Total Environ. 2020, 738, 139825. [Google Scholar] [CrossRef]
- Zhu, Y.; Xie, J.; Huang, F.; Cao, L. Association between short-term exposure to air pollution and COVID-19 infection: Evidence from China. Sci. Total Environ. 2020, 727, 138704. [Google Scholar] [CrossRef]
- Lindsley, W.G.; Blachere, F.M.; Thewlis, R.E.; Vishnu, A.; Davis, K.A.; Cao, G.; Palmer, J.E.; Clark, K.E.; Fisher, M.A.; Khakoo, R.; et al. Measurements of airborne influenza virus in aerosol particles from human coughs. PLoS ONE 2010, 5, e15100. [Google Scholar] [CrossRef]
- Liu, Y.; Ning, Z.; Chen, Y.; Guo, M.; Liu, Y.; Gali, N.K.; Sun, L.; Duan, Y.; Cai, J.; Westerdahl, D.; et al. Aerodynamic analysis of SARS-CoV-2 in two Wuhan hospitals. Nature 2020, 582, 557–560. [Google Scholar] [CrossRef] [PubMed]
- Schraufnagel, D.E. The health effects of ultrafine particles. Exp. Mol. Med. 2020, 52, 311–317. [Google Scholar] [CrossRef] [PubMed]
- Harrel, S.K.; Molinari, J. Aerosols and splatter in dentistry: A brief review of the literature and infection control implications. J. Am. Dent. Assoc. 2004, 135, 429–437. [Google Scholar] [CrossRef] [PubMed]
- Fennelly, K.P. Particle sizes of infectious aerosols: Implications for infection control. Lancet. Respir. Med. 2020, 8, 914–924. [Google Scholar] [CrossRef]
- Grillet, G.; Marjanovic, N.; Diverrez, J.M.; Tattevin, P.; Tadie, J.M.; L’Her, E. Intensive care medical procedures are more complicated, more stressful, and less comfortable with Ebola personal protective equipment: A simulation study. J. Infect. 2015, 71, 703–706. [Google Scholar] [CrossRef]
- Scheuch, G. Breathing Is Enough: For the Spread of Influenza Virus and SARS-CoV-2 by Breathing Only. J. Aerosol. Med. Pulm. Drug Deliv. 2020, 33, 230–234. [Google Scholar] [CrossRef]
- Wolff, D.; Frese, C.; Schoilew, K.; Dalpke, A.; Wolff, B.; Boutin, S. Amplicon-based microbiome study highlights the loss of diversity and the establishment of a set of species in patients with dentin caries. PLoS ONE 2019, 14, e0219714. [Google Scholar] [CrossRef]
- Aas, J.A.; Griffen, A.L.; Dardis, S.R.; Lee, A.M.; Olsen, I.; Dewhirst, F.E.; Leys, E.J.; Paster, B.J. Bacteria of dental caries in primary and permanent teeth in children and young adults. J. Clin. Microbiol. 2008, 46, 1407–1417. [Google Scholar] [CrossRef]
- Polednik, B. Aerosol and bioaerosol particles in a dental office. Environ. Res. 2014, 134, 405–409. [Google Scholar] [CrossRef] [PubMed]
- Tag El Din, A.M.; Ghoname, N.A.H. Efficacy of rubber dam isolation as an infection control procedure in paediatric dentistry. East. Mediterr. Health J. 1997, 3, 530–539. [Google Scholar]
- Samaranayake, L.P.; Reid, J.; Evans, D. The efficacy of rubber dam isolation in reducing atmospheric bacterial contamination. ASDC J. Dent. Child 1989, 56, 442–444. [Google Scholar] [PubMed]
- Chenthamara, D.; Subramaniam, S.; Ramakrishnan, S.G.; Krishnaswamy, S.; Essa, M.M.; Lin, F.H.; Qoronfleh, M.W. Therapeutic efficacy of nanoparticles and routes of administration. Biomater. Res. 2019, 23, 20. [Google Scholar] [CrossRef]
| Side | Particulate Matter Size | Volume Fraction (%) by Group | ||
|---|---|---|---|---|
| SS | RDI | RDI + HVE | ||
| Vestibular | PM0.1 | 3.85 ± 1.33 | [0.52 ± 0.25] * | 1.54 ± 1.06 |
| PM2.5 | 0.00 ± 0.00 | 0.93 ± 0.93 | 0.03 ± 0.01 | |
| PM10 | 2.02 ± 0.77 | 1.45 ± 0.92 | [0.33 ± 0.04] * | |
| Palatal | PM0.1 | 6.56 ± 2.37 | [1.11 ± 0.56] * | [0.53 ± 0.53] * |
| PM2.5 | 0.00 ± 0.00 | 3.49 ± 3.49 | 0.00 ± 0.00 | |
| PM10 | 0.29 ± 0.29 | 0.37 ± 0.25 | 0.53 ± 0.53 | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Balanta-Melo, J.; Gutiérrez, A.; Sinisterra, G.; Díaz-Posso, M.d.M.; Gallego, D.; Villavicencio, J.; Contreras, A. Rubber Dam Isolation and High-Volume Suction Reduce Ultrafine Dental Aerosol Particles: An Experiment in a Simulated Patient. Appl. Sci. 2020, 10, 6345. https://doi.org/10.3390/app10186345
Balanta-Melo J, Gutiérrez A, Sinisterra G, Díaz-Posso MdM, Gallego D, Villavicencio J, Contreras A. Rubber Dam Isolation and High-Volume Suction Reduce Ultrafine Dental Aerosol Particles: An Experiment in a Simulated Patient. Applied Sciences. 2020; 10(18):6345. https://doi.org/10.3390/app10186345
Chicago/Turabian StyleBalanta-Melo, Julián, Albio Gutiérrez, Gustavo Sinisterra, María del Mar Díaz-Posso, David Gallego, Judy Villavicencio, and Adolfo Contreras. 2020. "Rubber Dam Isolation and High-Volume Suction Reduce Ultrafine Dental Aerosol Particles: An Experiment in a Simulated Patient" Applied Sciences 10, no. 18: 6345. https://doi.org/10.3390/app10186345
APA StyleBalanta-Melo, J., Gutiérrez, A., Sinisterra, G., Díaz-Posso, M. d. M., Gallego, D., Villavicencio, J., & Contreras, A. (2020). Rubber Dam Isolation and High-Volume Suction Reduce Ultrafine Dental Aerosol Particles: An Experiment in a Simulated Patient. Applied Sciences, 10(18), 6345. https://doi.org/10.3390/app10186345







