Optimization of Chinese Chive Juice as a Functional Feed Additive
Abstract
Featured Application
Abstract
1. Introduction
2. Materials and Methods
2.1. CC Juice Preparation
2.2. Antibacterial Activity of CC Juice
2.3. Antioxidant Activity and Antioxidant Compound Contents of CC Juice
2.3.1. DPPH Free Radical Scavenging Assay
2.3.2. Determination of Total Phenolic Content
2.3.3. Determination of Total Flavonoid Content
2.4. Fractional Factorial Design (FFD)
2.5. UHPLC-LTQ-Orbitrap-MS/MS Analysis
2.6. Statistical Analysis
3. Results
3.1. Antibacterial Stability of CC Juice under Heat Treatment
3.2. Antioxidant Stability of CC Juice under Heat Treatment
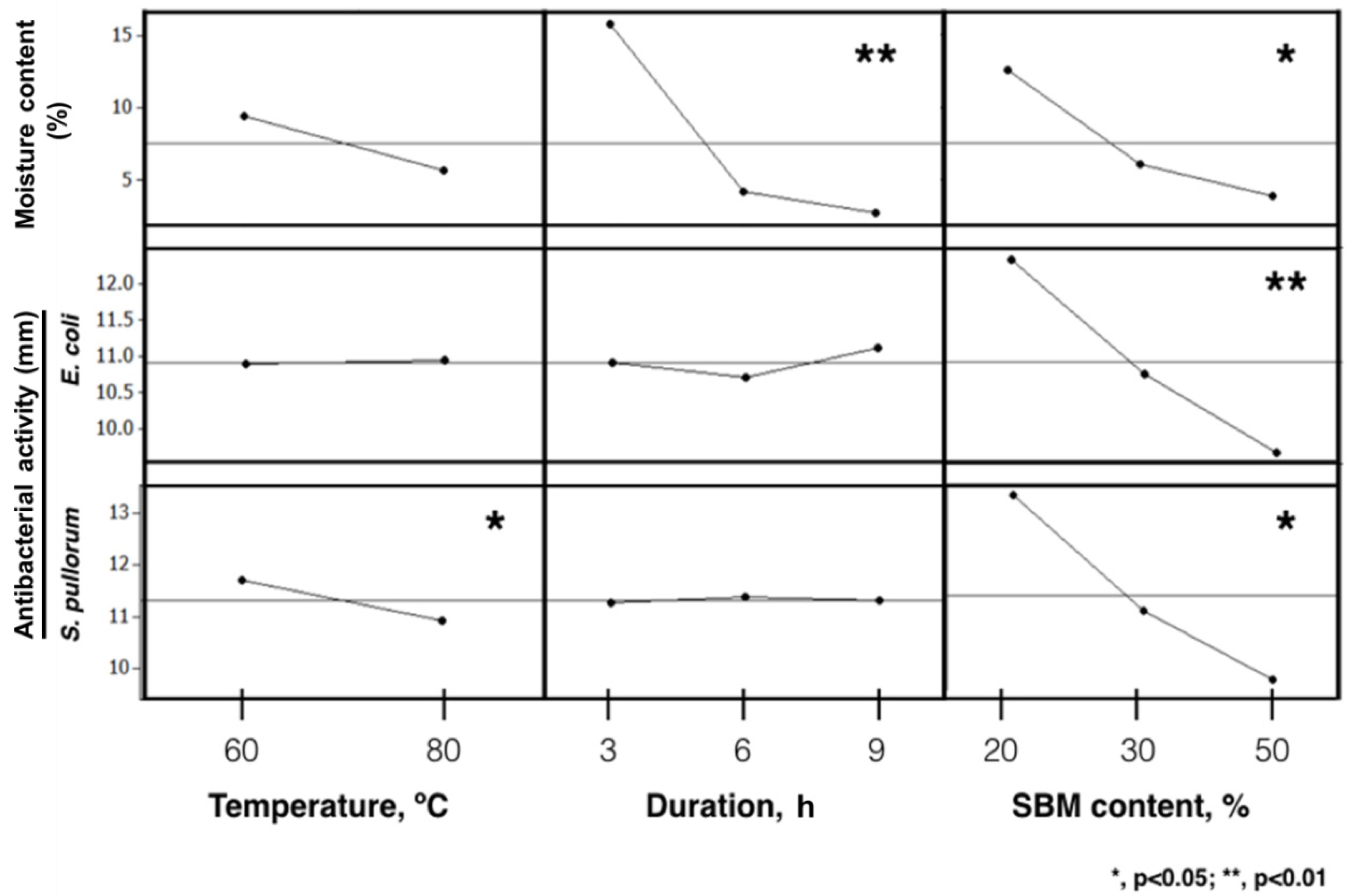
3.3. Fractional Factorial Design for Optimized CC Products
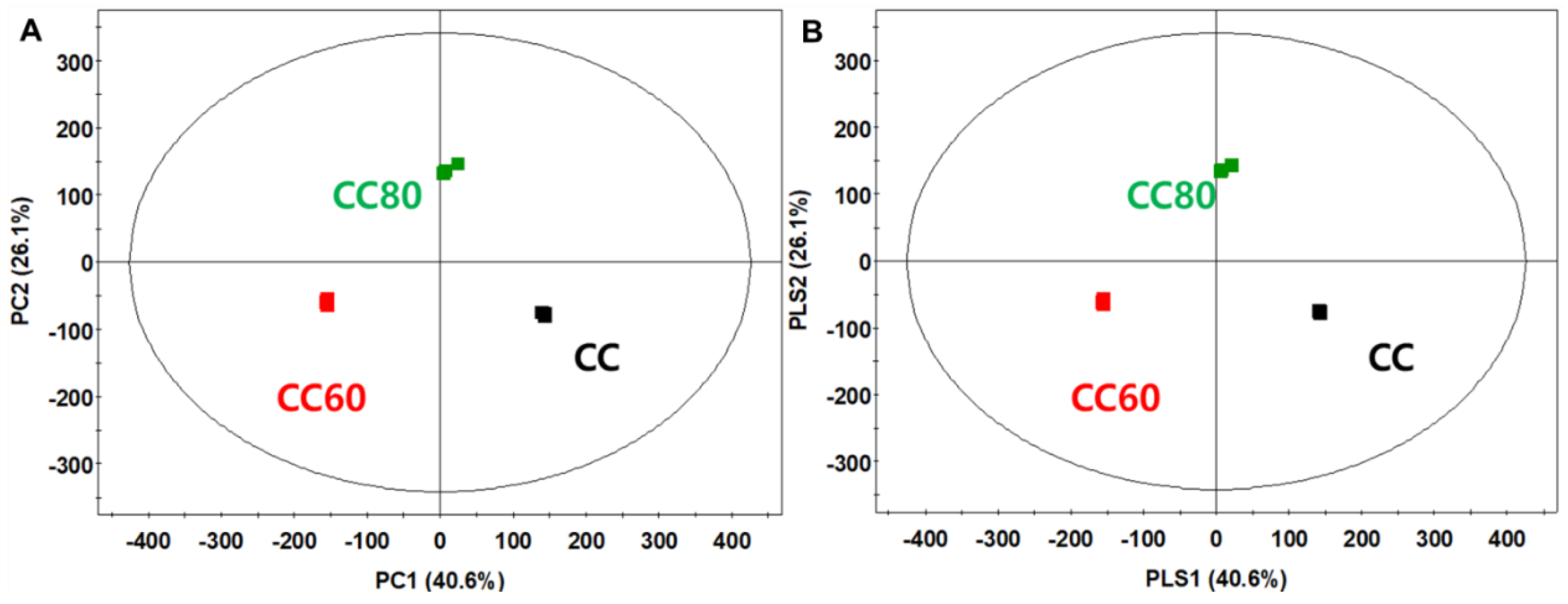
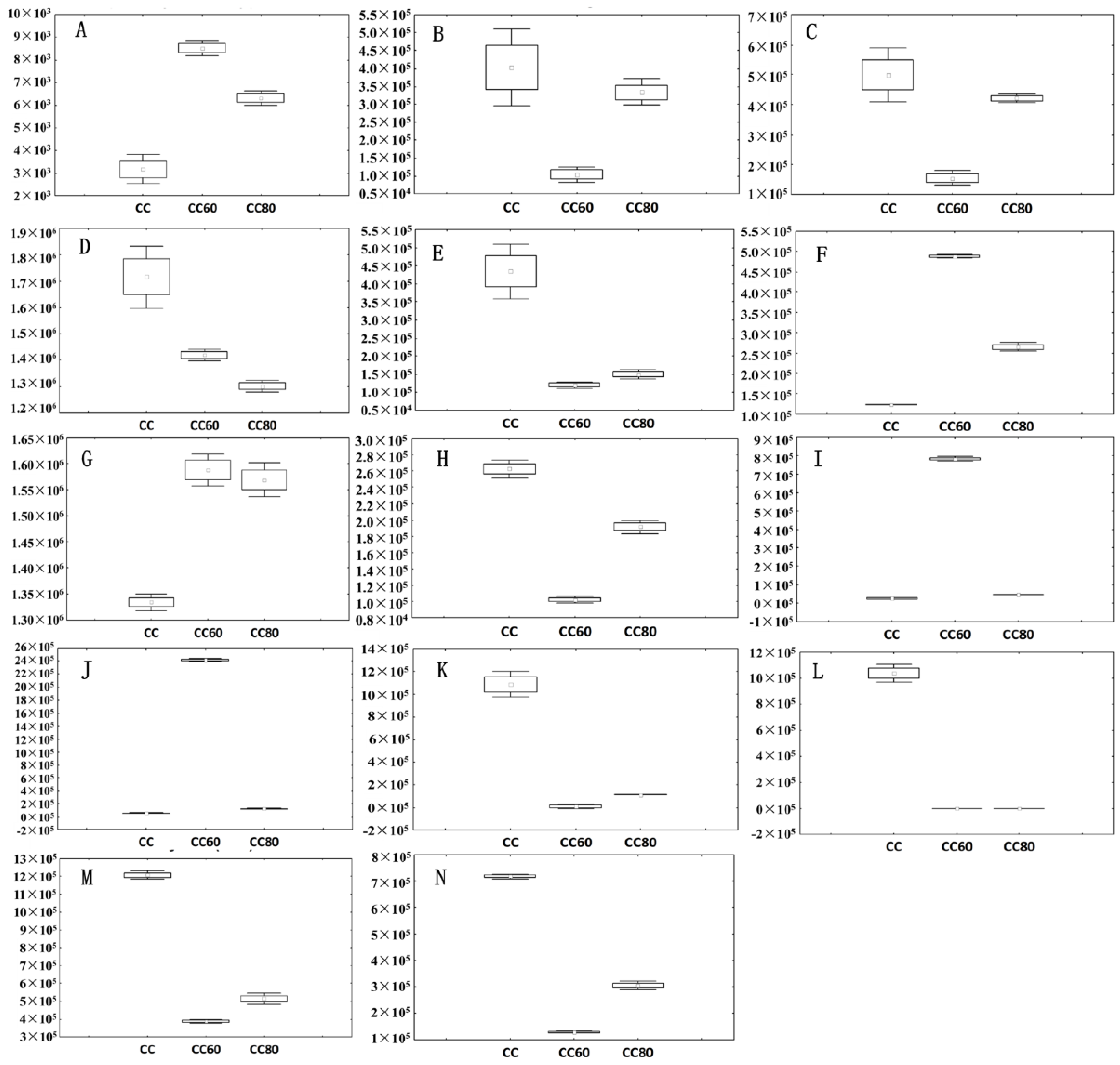
3.4. Biochemical Changes with Optimized Drying Conditions
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Wang, J.; Han, M.; Zhang, G.; Qiao, S.; Li, D.; Ma, X. The signal pathway of antibiotic alternatives on intestinal microbiota and immune function. Curr. Protein Pept. Sci. 2016, 17, 785–796. [Google Scholar] [CrossRef] [PubMed]
- Lawal, B.; Shittu, O.K.; Oibiokpa, F.I.; Mohammed, H.; Umar, S.I.; Haruna, G.M. Antimicrobial evaluation, acute and sub-acute toxicity studies of Allium sativum. J. Acute Dis. 2016, 5, 296–301. [Google Scholar] [CrossRef]
- Qin, S.; Hou, D.X. The biofunctions of phytochemicals and their applications in farm animals: The Nrf2/Keap1 system as a target. Engineering 2017, 3, 738–752. [Google Scholar] [CrossRef]
- Kothari, D.; Lee, W.D.; Niu, K.M.; Kim, S.K. The genus Allium as poultry feed additive: A review. Animals 2019, 9, 1032. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.Z.; He, M.L. Effects of feeding garlic and juniper berry essential oils on milk fatty acid composition of dairy cows. Nutr. Metab. Insights 2016, 9, 19–24. [Google Scholar] [CrossRef]
- Aji, S.B.; Ignatius, K.; Asha’Adatu, Y.; Nuhu, J.B.; Abdulkarim, A.; Aliyu, U.; Gambo, M.B.; Ibrahim, M.A.; Abubakar, H.; Bukar, M.M.; et al. Effects of feeding onion (Allium сера) and garlic (Allium sativum) on some performance characteristics of broiler chickens. Res. J. Poult. Sci. 2011, 4, 22–27. [Google Scholar]
- Alagawany, M.; Ashour, E.A.; Reda, F.M. Effect of dietary supplementation of garlic (Allium sativum) and turmeric (Curcuma longa) on growth performance, carcass traits, blood profile and oxidative status in growing rabbits. Ann. Anim. Sci. 2016, 16, 489–505. [Google Scholar] [CrossRef]
- Mau, J.L.; Chen, C.P.; Hsieh, P.C. Antimicrobial effect of extracts from Chinese chive, cinnamon, and corni fructus. J. Agric. Food Chem. 2001, 49, 183–188. [Google Scholar] [CrossRef]
- Lee, C.F.; Han, C.K.; Tsau, J.L. In vitro inhibitory activity of Chinese leek extract against Campylobacter species. Int. J. Food Microbiol. 2004, 94, 169–174. [Google Scholar] [CrossRef]
- Venâncio, P.C.; Figueroba, S.; Nani, B.; Ferreira, L.E.N.; Muniz, B.V.; Del Fiol, F.S.; Sartoratto, A.; Ribeiro Rosa, E.A.; Groppo, F.C. Antimicrobial activity of two garlic species (Allium sativum and A. tuberosum) against Staphylococci infection. In vivo study in rats. Adv. Pharm. Bull. 2017, 7, 115–121. [Google Scholar] [CrossRef]
- Mnayer, D.; Fabiano-Tixier, A.S.; Petitcolas, E.; Hamieh, T.; Nehme, N.; Ferrant, C.; Fernandez, X.; Chemat, F. Chemical composition, antibacterial and antioxidant activities of six essentials oils from the Alliaceae family. Molecules 2014, 19, 20034–20053. [Google Scholar] [CrossRef] [PubMed]
- Kothari, D.; Lee, W.D.; Jung, E.S.; Niu, K.M.; Lee, C.H.; Kim, S.K. Controlled fermentation using autochthonous Lactobacillus plantarum improves antimicrobial potential of Chinese chives against poultry pathogens. Antibiotics 2020, 9, 386. [Google Scholar] [CrossRef] [PubMed]
- Rattanachaikunsopon, P.; Phumkhachorn, P. Potential of Chinese chive oil as a natural antimicrobial for controlling Flavobacterium columnare infection in Nile tilapia Oreochromis niloticus. Fish Sci. 2009, 75, 1431–1437. [Google Scholar] [CrossRef]
- Sumonsiri, N.; Barringer, S.A. Fruits & vegetables-processing technologies and applications. In Food Processing: Principles and Applications, 2nd ed.; Clark, S., Jung, S., Lamsal, B., Eds.; Wiley: Chichester, UK, 2014; pp. 363–382. [Google Scholar]
- Poojary, M.M.; Putnik, P.; Kovačević, D.B.; Barba, F.J.; Lorenzo, J.M.; Dias, D.A.; Shpigelman, A. Stability and extraction of bioactive sulfur compounds from Allium genus processed by traditional and innovative technologies. J. Food Compos. Anal. 2017, 61, 28–39. [Google Scholar] [CrossRef]
- Kamiloglu, S.; Toydemir, G.; Boyacioglu, D.; Beekwilder, J.; Hall, R.D.; Capanoglu, E. A review on the effect of drying on antioxidant potential of fruits and vegetables. Crit. Rev. Food Sci. 2016, 56, S110–S129. [Google Scholar] [CrossRef] [PubMed]
- Alam, M.J.; Mamuad, L.L.; Kim, S.H.; Jeong, C.D.; Sung, H.G.; Cho, S.B.; Jeon, C.O.; Lee, K.; Lee, S.S. Effect of phytogenic feed additives in soybean meal on in vitro swine fermentation for odor reduction and bacterial community comparison. Asian Austral. J. Anim. Sci. 2013, 26, 266–274. [Google Scholar] [CrossRef]
- Tang, X.; Dai, Y.; Sun, P.; Meng, S. Interaction-based feature selection using factorial design. Neurocomputing 2017, 281, 47–54. [Google Scholar] [CrossRef]
- Won, J.; Son, S.; Lee, S.; Singh, D.; Lee, S.; Lee, J.; Lee, C. Strategy for screening of antioxidant compounds from two ulmaceae species based on liquid chromatography-mass spectrometry. Molecules 2018, 23, 1830. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT-Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Ainsworth, E.A.; Gillespie, K.M. Estimation of total phenolic content and other oxidation substrates in plant tissues using Folin–Ciocalteu reagent. Nat. Protoc. 2007, 2, 875–877. [Google Scholar] [CrossRef]
- Herald, T.J.; Gadgil, P.; Tilley, M. High-throughput micro plate assays for screening flavonoid content and DPPH-scavenging activity in sorghum bran and flour. J. Sci. Food Agric. 2012, 92, 2326–2331. [Google Scholar] [CrossRef] [PubMed]
- Firestone, D. Official Methods of Analysis of the Association of Official Analytical Chemists; The Association: Arlington, VA, USA, 1990. [Google Scholar]
- Dubreuil, J.D.; Isaacson, R.E.; Schifferli, D.M. Animal enterotoxigenic Escherichia coli. EcoSal Plus 2016, 7, 1–80. [Google Scholar] [CrossRef] [PubMed]
- Cosby, D.E.; Cox, N.A.; Harrison, M.A.; Wilson, J.L.; Buhr, R.J.; Fedorka-Cray, P.J. Salmonella and antimicrobial resistance in broilers: A review. J. Appl. Poult. Res. 2015, 24, 408–426. [Google Scholar] [CrossRef]
- Yang, M.Y.; Zheng, F.P.; Duan, Y.; Xie, J.C.; Huang, M.Q.; Ren, T.L.; Sun, B.G. Analysis of volatiles in wild Chinese chive flowers by solvent extraction/solvent-assisted flavor evaporation coupled with gas chromatography-mass spectrometry. Food Sci. 2011, 32, 211–216. [Google Scholar]
- Wang, X.; Wu, R.; Zhang, L.; Liu, L.; Guan, H.; Luo, P. GC-MS analysis of chemical compositions and antimicrobial activity of volatile oil from Allium tuberosum against common pathogenic bacteria. Chin. Vet. Sci. 2012, 42, 201–204. [Google Scholar]
- Woo, K.S.; Yoon, H.S.; Lee, Y.R.; Lee, J.; Kim, D.J.; Hong, J.T.; Jeong, H.S. Characteristics and antioxidative activity of volatile compounds in heated garlic (Allium sativum). Food Sci. Biotechnol. 2007, 16, 822–827. [Google Scholar]
- Górniak, I.; Bartoszewski, R.; Króliczewski, J. Comprehensive review of antimicrobial activities of plant flavonoids. Phytochem. Rev. 2018, 18, 241–272. [Google Scholar] [CrossRef]
- Kim, J.S.; Kang, O.J.; Gweon, O.C. Comparison of phenolic acids and flavonoids in black garlic at different thermal processing steps. J. Funct. Foods 2013, 5, 80–86. [Google Scholar] [CrossRef]
- Bourdoux, S.; Li, D.; Rajkovic, A.; Devlieghere, F.; Uyttendaele, M. Performance of drying technologies to ensure microbial safety of dried fruits and vegetables. Compr. Rev. Food Sci. Food Saf. 2016, 15, 1056–1066. [Google Scholar] [CrossRef]
- Kim, D.H.; Kim, Y.C.; Choi, U.K. Optimization of antibacterial activity of Perilla frutescens var. acuta leaf against Staphylococcus aureus using evolutionary operation factorial design technique. Int. J. Mol. Sci. 2011, 12, 2395–2407. [Google Scholar] [CrossRef]
- Lima, C.A.; Campos, J.F.; Filho, J.L.; Converti, A.; da Cunha, M.G.; Porto, A.L. Antimicrobial and radical scavenging properties of bovine collagen hydrolysates produced by Penicillium aurantiogriseum URM 4622 collagenase. J. Food Sci. Technol. 2015, 52, 4459–4466. [Google Scholar] [CrossRef] [PubMed]
- Shah, M.; Garg, S.K. Application of 2k full factorial design in optimization of solvent-free microwave extraction of ginger essential oil. J. Eng. 2014, 2014, 1–5. [Google Scholar] [CrossRef]
- Farag, M.A.; Ali, S.E.; Hodaya, R.H.; El-Seedi, H.R.; Sultani, H.N.; Laub, A.; Eissa, T.F.; Abou-Zaid, F.O.F.; Wessjohann, L.A. Phytochemical profiles and antimicrobial activities of Allium cepa red cv. and A. sativum subjected to different drying methods: A comparative MS-based metabolomics. Molecules 2017, 22, 761. [Google Scholar] [CrossRef] [PubMed]
- Farhadi, F.; Khameneh, B.; Iranshahi, M.; Iranshahy, M. Antibacterial activity of flavonoids and their structure-activity relationship: An update review. Phytother. Res. 2019, 33, 13–40. [Google Scholar] [CrossRef]
- Wang, S.; Yao, J.; Zhou, B.; Yang, J.; Chaudry, M.T.; Wang, M.; Xiao, F.L.; Li, Y.; Yin, W.Z. Bacteriostatic effect of quercetin as an antibiotic alternative in vivo and its antibacterial mechanism in vitro. J. Food Protect. 2018, 81, 68–78. [Google Scholar] [CrossRef] [PubMed]
- Al-Yousef, H.M.; Ahmed, A.F.; Al-Shabib, N.A.; Laeeq, S.; Khan, R.A.; Rehman, M.T.; Alsalme, A.; Al-Ajmi, M.F.; Khan, M.S.; Husain, F.M. Onion peel ethylacetate fraction and its derived constituent quercetin 4′-O-D glucopyranoside attenuates quorum sensing regulated virulence and biofilm formation. Front. Microbiol. 2017, 8, 1675. [Google Scholar] [CrossRef]
- Nićiforović, N.; Abramovič, H. Sinapic acid and its derivatives: Natural sources and bioactivity. Compr. Rev. Food Sci. Food Saf. 2014, 13, 34–51. [Google Scholar] [CrossRef]
- Gao, Q.; Li, X.B.; Sun, J.; Xia, E.D.; Tang, F.; Cao, H.Q.; Xun, H. Isolation and identification of new chemical constituents from Chinese chive (Allium tuberosum) and toxicological evaluation of raw and cooked Chinese chive. Food Chem. Toxicol. 2017, 112, 400–411. [Google Scholar] [CrossRef]
- Grzelczyk, A.; Gendaszewska-Darmach, E. Novel bioactive glycerol-based lysophospholipids: New data–new insight into their function. Biochimie 2013, 95, 667–679. [Google Scholar] [CrossRef]
- Meylaers, K.; Clynen, E.; Daloze, D.; DeLoof, A.; Schoofs, L. Identification of 1-lysophosphatidylethanolamine (C16: 1) as an antimicrobial compound in the housefly, Musca domestica. Insect Biochem. Mol. Biol. 2004, 34, 43–49. [Google Scholar] [CrossRef]
- Schrimpe-Rutledge, A.C.; Codreanu, S.G.; Sherrod, S.D.; McLean, J.A. Untargeted metabolomics strategies-challenges and emerging directions. J. Am. Soc. Mass Spectrom. 2016, 27, 1897–1905. [Google Scholar] [CrossRef] [PubMed]
| Pathogens | Antibacterial Activity (1) |
|---|---|
| Clostridium perfringens Type E | ++ |
| Streptococcus iniae | +++ |
| Lactococcus garvieae | - |
| Enterotoxigenic E. coli O157:H7 | ++ |
| Pantoea agglomerans | ++ |
| Haemopillus parsuis | ++ |
| Haemopillus somnus | +++ |
| Burkholderia sp. | +++ |
| Salmonella gallinarum | ++ |
| Salmonella pullorum | +++ |
| Edwardsiella tarda | +++ |
| Photobacterium damselae subsp. damselae | +++ |
| Vibrio ichthyoenteri | +++ |
| Vibrio harveyi | + |
| Pathogens | Heating Time at 60 °C (h) | Heating Time at 80 °C (h) | SEM | p-Value | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | 0 | 1 | 2 | 3 | Temp (1) | Time | Temp * Time | ||
| ---------- Zone of inhibition (mm) ---------- | ||||||||||||
| C. perfringens | 16.2 a | 14.5 a,b | 14.3 b | 13.5 b | 15.2 a | 12.3 b | 9.8 c | 10.7 c | 0.45 | <0.01 | <0.01 | 0.01 |
| S. iniae | 21.7 a | 23.7 b | 19.5 c | 18.0 c | 20.7 a | 20.7 a | 16.0 b | 14.0 c | 0.63 | <0.01 | <0.01 | 0.09 |
| E. coli O157:H7 | 18.7 a | 18.8 a | 16.7 b | 16.5 b | 16.0 a | 14.5 b | 13.3 c | 12.3 d | 0.47 | <0.01 | <0.01 | 0.14 |
| P. agglomerans | 14.3 a | 13.0 a,b | 11.7 b,c | 11.0 c | 12.3 a | 11.3 a | 10.5 a,b | 8.7 b | 0.36 | <0.01 | <0.01 | 0.71 |
| H. parsuis | 22.5 a | 22.5 a | 21.0 b | 20.0 b | 19.8 a | 17.3 b | 15.0 c | 13.7 c | 0.66 | <0.01 | <0.01 | <0.01 |
| H. somnus | 28.5 a | 29.7 a | 27.3 a,b | 24.5 b | 25.3 a | 24.3 a | 22.7 a,b | 20.2 b | 0.66 | <0.01 | <0.01 | 0.65 |
| Burkholderia sp. | 30.7 a | 31.0 a | 28.7 b | 26.5 c | 28.5 a | 25.0 b | 23.2 b | 18.2 c | 0.91 | <0.01 | <0.01 | 0.11 |
| S. gallinarum | 16.5 a | 15.0 b | 13.8 c | 13.8 c | 14.5 a | 13.0 a | 11.2 b | 10.0 b | 0.42 | <0.01 | <0.01 | 0.06 |
| S. pullorum | 20.7 a | 20.7 a | 18.3 a,b | 17.0 b | 21.3 a | 17.3 b | 14.7 c | 11.8 d | 0.67 | <0.01 | <0.01 | <0.01 |
| E. tarda | 25.8 a | 23.8 b | 23.8 b | 22.5 c | 29.8 a | 21.5 b | 20.3 b | 16.5 c | 0.79 | <0.01 | <0.01 | <0.01 |
| P. damselae subsp. damselae | 24. 8 a | 23.0 b | 23.3 b | 23.5 b | 24.6 a | 22.9 b | 13.0 c | 12.9 c | 0.96 | <0.01 | <0.01 | <0.01 |
| V. ichthyoenteri | 27.3 a | 22.8 b | 23.0 b | 20.5 c | 25.8 a | 20.3 b | 16.5 c | 15.8 c | 0.80 | <0.01 | <0.01 | <0.01 |
| Items (1) | Heating Time at 60℃ (h) | Heating Time at 80℃ (h) | SEM | p-Value | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | 6 | 9 | 24 | 0 | 1 | 2 | 3 | 6 | 9 | 24 | Temp (2) | Time | Temp * Time | ||
| DPPH inhibition (%) | 69.4 a | 69.3 a,b | 71.0 a,b,c | 70.4 a,b | 71.1 a,b,c | 72.0 b,c | 73.2 c | 69.4 a | 70.3 a,b | 71.5 b,c | 71.1 b | 71.9 b,c,d | 72.7 c,d | 72.9 d | 0.2 | 0.42 | <0.01 | 0.97 |
| AEAC (µg/mL) | 35.3 a | 35.2 a,b | 36.1 a,b | 35.8 a,b | 36.1 a,b | 36.6 b,c | 37.1 c | 35.3 a | 35.7 a,b | 36.3 b,c,d | 36.1 a,b | 36.5 b,c,d | 36.9 c,d | 37.0 d | 0.1 | 0.35 | <0.01 | 0.96 |
| QEAC (µg/mL) | 13.4 a,b | 13.3 a | 13.7 b,c,d | 13.6 a,b,c | 13.7 b,c,d | 13.9 c,d | 14.1 d | 13.4 a | 13.5 a,b | 13.8 b,c | 13.7 a,b,c | 13.8 b,c | 14.0 c | 14.0 c | 0.0 | 0.15 | <0.01 | 0.88 |
| TPC (GAC, µg/mL) | 375.5 | 360.0 | 366.1 | 371.1 | 373.1 | 371.1 | 366.8 | 375.5 | 373.5 | 373.1 | 373.7 | 372.0 | 379.9 | 371.9 | 6.8 | 0.38 | 0.50 | 0.46 |
| TFC (QE, µg/mL) | 164.6 c | 129.0 b | 112.8 a | 115.3 a | 112.8 a | 115.3 b | 110.9 a | 164.6 a | 142.8 b | 129.0 c | 131.5 c | 127.1 c | 127.8 c | 129.6 c | 2.7 | <0.01 | <0.01 | 0.03 |
| Runs | Variables (1) | Responses (2) | ||||
|---|---|---|---|---|---|---|
| X1 | X2 | X3 | Y1 | Y2 (E. coli) | Y2 (S. pullorum) | |
| 1 | 60 | 3 | 20 | 28.69 ± 9.51 | 14.00 ± 0.81 | 15.00 ± 0.47 |
| 2 | 60 | 3 | 30 | 18.97 ± 6.61 | 11.33 ± 0.54 | 12.00 ± 0.94 |
| 3 | 60 | 3 | 50 | 9.56 ± 2.12 | 8.67 ± 0.27 | 10.17 ± 0.59 |
| 4 | 60 | 6 | 20 | 8.82 ± 1.62 | 10.33 ± 0.98 | 12.67 ± 0.72 |
| 5 | 60 | 6 | 30 | 4.45 ± 0.31 | 10.33 ± 0.27 | 12.00 ± 0.00 |
| 6 | 60 | 6 | 50 | 3.59 ± 0.18 | 8.83 ± 0.36 | 9.50 ± 0.24 |
| 7 | 60 | 9 | 20 | 3.78 ± 0.17 | 11.50 ± 0.70 | 12.83 ± 0.89 |
| 8 | 60 | 9 | 30 | 3.41 ± 0.18 | 9.33 ± 0.72 | 10.33 ± 0.72 |
| 9 | 60 | 9 | 50 | 3.17 ± 0.18 | 9.00 ± 0.47 | 9.33 ± 0.36 |
| 10 | 80 | 3 | 20 | 28.34 ± 4.77 | 10.33 ± 0.72 | 12.00 ± 1.25 |
| 11 | 80 | 3 | 30 | 5.73 ± 0.16 | 9.67 ± 0.72 | 11.00 ± 0.41 |
| 12 | 80 | 3 | 50 | 3.22 ± 0.19 | 9.00 ± 0.47 | 9.17 ± 0.36 |
| 13 | 80 | 6 | 20 | 3.93 ± 0.39 | 10.67 ± 1.18 | 11.67 ± 1.09 |
| 14 | 80 | 6 | 30 | 2.47 ± 0.09 | 10.33 ± 0.95 | 10.33 ± 0.59 |
| 15 | 80 | 6 | 50 | 1.74 ± 0.03 | 9.00 ± 0.47 | 9.33 ± 0.27 |
| 16 | 80 | 9 | 20 | 2.23 ± 0.10 | 11.17 ± 1.30 | 11.33 ± 1.38 |
| 17 | 80 | 9 | 30 | 1.60 ± 0.12 | 10.00 ± 0.94 | 9.50 ± 0.85 |
| 18 | 80 | 9 | 50 | 1.83 ± 0.05 | 10.17 ± 0.89 | 9.67 ± 0.72 |
| Drying Time (h) | Moisture Content (%) | Zone of Inhibition (mm) | |
|---|---|---|---|
| E. coli | S. pullorum | ||
| 6 | 25.71 c | 12.2 | 14.0 |
| 7 | 14.96 b | 13.7 | 15.0 |
| 8 | 4.68 a | 12.7 | 13.8 |
| 9 | 5.51 a | 12.0 | 13.0 |
| 10 | 2.70 a | 11.7 | 13.8 |
| SEM | 2.45 | 0.27 | 0.24 |
| p-value | <0.001 | 0.122 | 0.118 |
| No. | Tentatively Identified Metabolites (1) | RT (2) (min) | MW (3) | Measured Mass | MS/MS Fragments | Class of Compounds |
|---|---|---|---|---|---|---|
| Negative Mode (m/z) | ||||||
| 1 | N-(1-Deoxy-1-fructosyl)leucine | 1.00 | 293 | 292.1377 [M − H]− | 292 > 274/202/172/130 | Amino acid |
| 2 | Quercetin-glucosylgalactoside-glucoside or Quercetin-triglucoside | 3.35 | 788 | 787.1852 [M − H]− | 787 > 625/463/301 | Flavonol |
| 3 | Kaempferol-di-glucoside or Kaempferol-sophoroside | 3.59/4.05/4.37/4.50 | 610 | 609.1404 [M − H]− | 609 > 447/285/489/581 | Flavonol |
| 4 | Feruloyl-galactaric acid | 3.94 | 386 | 385.0739 [M − H]− | 385 > 191/209/367 | Hexaric acid |
| 5 | Kaempferol-sophoroside-glucoside | 3.65/4.01 | 772 | 771.1522 [M − H]− | 609 > 429/447/489 | Flavonol |
| 6 | Quercetin-di-glucoside | 4.39 | 626 | 625.1349 [M − H]− | 625 > 463/300/445/505/607 | Flavonol |
| 7 | Sinapinic acid-O-glucuronide isomer | 4.55 | 400 | 399.0897 [M − H]− | 399 > 381/355/223/205/187 | Phenolic acid |
| 8 | Kaempferol diglucoside-(feruloylglucoside) | 4.55/4.80 | 948 | 947.2362 [M − H]− | 947 > 623/785/447/609/285 | Flavonol |
| 9 | Quercetin-hexoside | 4.81 | 464 | 463.0843 [M − H]− | 463 > 301 | Flavonol |
| 10 | Kaempferol-glucoside | 5.04 | 448 | 447.0893 [M − H]− | 447 > 284/255 | Flavonol |
| 11 | LysoPE(18:2) | 8.22/8.34 | 477 | 476.2734 [M − H]− | 476 > 279/402/214 | Lipid |
| 12 | LysoPC(18:2) | 8.28/8.40 | 519 | 564.3246 [M + FA − H]− | 564 > 504/279 | Lipid |
| 13 | LysoPE(16:0) | 8.61 | 453 | 452.2737 [M − H]− | 452 > 255/214/323/378 | Lipid |
| 14 | LysoPC(16:0) | 8.68 | 495 | 540.3253 [M + FA − H]− | 540 > 480/255/391/224 | Lipid |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Niu, K.-M.; Kothari, D.; Lee, W.-D.; Cho, S.; Wu, X.; Kim, S.-K. Optimization of Chinese Chive Juice as a Functional Feed Additive. Appl. Sci. 2020, 10, 6194. https://doi.org/10.3390/app10186194
Niu K-M, Kothari D, Lee W-D, Cho S, Wu X, Kim S-K. Optimization of Chinese Chive Juice as a Functional Feed Additive. Applied Sciences. 2020; 10(18):6194. https://doi.org/10.3390/app10186194
Chicago/Turabian StyleNiu, Kai-Min, Damini Kothari, Woo-Do Lee, Sangbuem Cho, Xin Wu, and Soo-Ki Kim. 2020. "Optimization of Chinese Chive Juice as a Functional Feed Additive" Applied Sciences 10, no. 18: 6194. https://doi.org/10.3390/app10186194
APA StyleNiu, K.-M., Kothari, D., Lee, W.-D., Cho, S., Wu, X., & Kim, S.-K. (2020). Optimization of Chinese Chive Juice as a Functional Feed Additive. Applied Sciences, 10(18), 6194. https://doi.org/10.3390/app10186194







