Enhanced Bactericidal Efficacy of NaOCl at pH 12 Followed by Acidified NaOCl at pH 6.5 on Enterococcus faecalis Biofilm
Abstract
1. Introduction
2. Materials and Methods
2.1. Dentine Specimen Preparation
2.2. Bacterial Biofilm Formation
2.3. Disinfection of Dentine Covered with Biofilm
2.4. Disinfection Assessment
2.5. Statistical Analysis
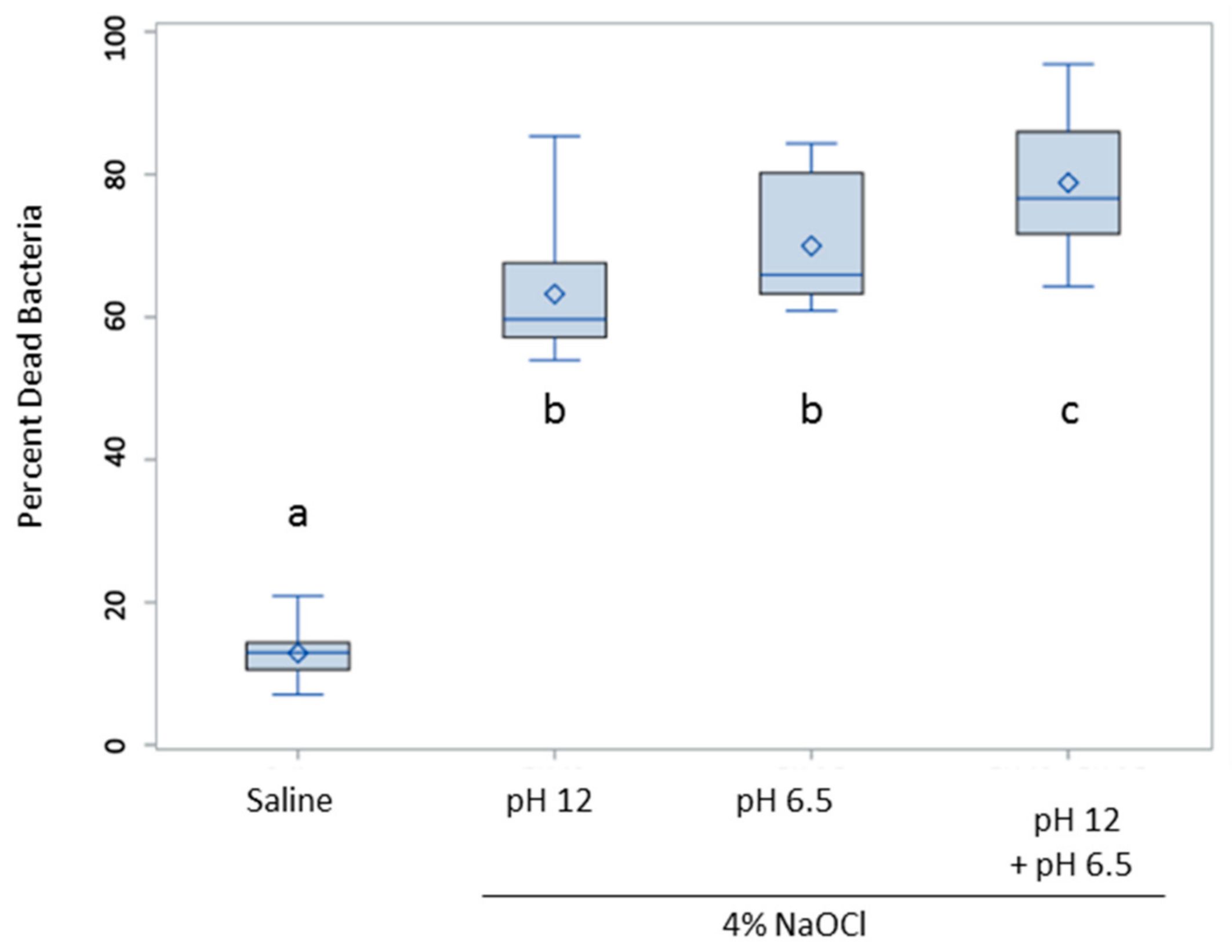
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Ruiz, M.; Aguado-Pérez, B.; Baca, P.; Arias-Moliz, M.T.; Ferrer-Luque, C.M. Efficacy of antimicrobial solutions against polymicrobial root canal biofilm. Int. Endod. J. 2017, 50, 77–83. [Google Scholar] [CrossRef] [PubMed]
- Stewart, P.S.; Costerton, J.W. Antibiotic resistance of bacteria in biofilms. Lancet 2001, 358, 135–138. [Google Scholar] [CrossRef]
- Li, W.; Liu, H.; Xu, Q. Extracellular dextran and dna affect the formation of enterococcus faecalis biofilms and their susceptibility to 2% chlorhexidine. J. Endod. 2012, 38, 894–898. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi, Z.; Palazzi, F.; Giardino, L.; Shalavi, S. Microbial biofilms in endodontic infections: An update review. Biomed. J. 2013, 36, 59–70. [Google Scholar] [CrossRef]
- Niazi, S.A.; Al-Ali, W.M.; Patel, S.; Foschi, F.; Mannocci, F. Synergistic effect of 2% chlorhexidine combined with proteolytic enzymes on biofilm disruption and killing. Int. Endod. J. 2015, 48, 1157–1167. [Google Scholar] [CrossRef]
- Zehnder, M. Root canal irrigants. J. Endod. 2006, 32, 389–398. [Google Scholar] [CrossRef]
- Estrela, C.; Estrela, C.R.; Barbin, E.L.; Spanó, J.C.; Marchesan, M.A.; Pécora, J.D. Mechanism of action of sodium hypochlorite. Braz. Dent. J. 2002, 13, 113–117. [Google Scholar] [CrossRef]
- Kuroiwa, K.; Nakayama, H.; Kuwahara, T.; Tamagawa, K.; Hattori, K.; Murakami, K.; Korai, H.; Ohnishi, Y. Augmenting effect of acetic acid for acidification on bactericidal activity of hypochlorite solution. Lett. Appl. Microbiol. 2003, 36, 46–49. [Google Scholar] [CrossRef]
- Mercadé, M.; Durán-Sindreu, F.; Kuttler, S.; Roig, M.; Durany, N. Antimicrobial efficacy of 4.2% sodium hypochlorite adjusted to pH 12, 7.5, and 6.5 in infected human root canals. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontol. 2009, 107, 295–298. [Google Scholar] [CrossRef]
- Aubut, V.; Pommel, L.; Verhille, B.; Orsière, T.; Garcia, S.; About, I.; Camps, J. Biological properties of a neutralized 2.5% sodium hypochlorite solution. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontol. 2010, 109, e120–e125. [Google Scholar] [CrossRef] [PubMed]
- Tawakoli, P.N.; Ragnarsson, K.T.; Rechenberg, D.K.; Mohn, D.; Zehnder, M. Effect of endodontic irrigants on biofilm matrix polysaccharides. Int. Endod. J. 2016, 50, 153–160. [Google Scholar] [CrossRef] [PubMed]
- Wright, P.P.; Kahler, B.; Walsh, L.J. Alkaline Sodium Hypochlorite Irrigant and Its Chemical Interactions. Materials (Basel) 2017, 10, 1147. [Google Scholar] [CrossRef] [PubMed]
- Fukuzaki, S. Mechanisms of actions of sodium hypochlorite in cleaning and disinfection processes. Biocontrol Sci. 2006, 11, 147–157. [Google Scholar] [CrossRef] [PubMed]
- Aldo, D.C.P.; Clovis, M.B.; Marco, H.D.; Flaviana, B.D.A.; Marcia, Z.G.; Marina, M.D.S.; Bruno, C.C.; Samuel, L.F. Effect of Temperature, Concentration and Contact Time of Sodium Hypochlorite on the Treatment and Revitalization of Oral Biofilms. J. Dent. Res. Dent. Clin. Dent. Prospect. 2015, 9, 209–215. [Google Scholar] [CrossRef]
- Camps, J.; Pommel, L.; Aubut, V.; Verhille, B.; Satoshi, F.; Lascola, B. About I. Shelf life, dissolving action, and antibacterial activity of a neutralized 2.5% sodium hypochlorite solution. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontol. 2009, 108, e66–e73. [Google Scholar] [CrossRef]
- Azim, A.A.; Piasecki, L.; da Silva Neto, U.X.; Cruz, A.T.G.; Azim, K.A. XP Shaper, A Novel Adaptive Core Rotary Instrument: Micro–computed Tomographic Analysis of Its Shaping Abilities. J. Endod. 2017, 43, 1532–1538. [Google Scholar] [CrossRef]
- Lacerda, M.F.; Marceliano-Alves, M.F.; Pérez, A.R.; Provenzano, J.C.; Neves, M.A.; Pires, F.R.; Gonçalves, L.S.; Rôças, I.N.; Siqueira, J.F., Jr. Cleaning and Shaping Oval Canals with 3 Instrumentation Systems: A Correlative Micro–computed Tomographic and Histologic Study. J. Endod. 2017, 43, 1878–1884. [Google Scholar] [CrossRef]
- Verma, N.; Sangwan, P.; Tewari, S.; Duhan, J. Effect of different concentrations of sodium hypochlorite on outcome of primary root canal treatment: A randomized controlled trial. J. Endod. 2019, 45, 357–363. [Google Scholar] [CrossRef]
- Love, R. Enterococcus faecalis—A mechanism for its role in endodontic failure. Int. Endod. J. 2001, 34, 399–405. [Google Scholar] [CrossRef]
- Narayanan, L.L.; Vaishnavi, C. Endodontic microbiology. J. Conserv. Dent. 2010, 13, 233–239. [Google Scholar] [CrossRef]
- Rôças, I.N.; Siqueira, J.F., Jr.; Santos, K.R. Association of Enterococcus faecalis with different forms of periradicular diseases. J. Endod. 2004, 30, 315–320. [Google Scholar] [CrossRef]
- Prada, I.; Micó-Muñoz, P.; Giner-Lluesma, T.; Micó-Martínez, P.; Collado-Castellano, N.; Manzano-Saiz, A. Influence of microbiology on endodontic failure. Literature review. Med. Oral Patol. Oral y Cir. Bucal 2019, 24, e364–e372. [Google Scholar] [CrossRef] [PubMed]
- Watson, T.F. Fact and artefact in confocal microscopy. Adv. Dent. Res. 1997, 11, 433–441. [Google Scholar] [CrossRef] [PubMed]
- Ordinola-Zapata, R.; Bramante, C.M.; Cavenago, B.; Graeff, M.S.Z.; Gomes de Moraes, I.; Marciano, M.; Duarte, M.A.H. Antimicrobial effect of endodontic solutions used as final irrigants on a dentine biofilm model. Int. Endod. J. 2011, 45, 162–168. [Google Scholar] [CrossRef] [PubMed]

| Irrigant | Log10 Total Biovolume (µm3) | ||||
|---|---|---|---|---|---|
| Mean | SD | Min. | Max. | Median | |
| Saline | 7.35 | 0.22 | 7.03 | 7.40 | 7.68 |
| NaOCl pH 12 | 7.28 | 0.23 | 6.94 | 7.36 | 7.74 |
| NaOCl pH 6.5 | 7.16 | 0.29 | 6.73 | 7.14 | 7.61 |
| NaOCl pH 12 + pH 6.5 | 7.32 | 0.22 | 7.03 | 7.36 | 7.62 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wigler, R.; Matalon, S.; Goldberger, T.; Lerner, A.O.; Kfir, A. Enhanced Bactericidal Efficacy of NaOCl at pH 12 Followed by Acidified NaOCl at pH 6.5 on Enterococcus faecalis Biofilm. Appl. Sci. 2020, 10, 6096. https://doi.org/10.3390/app10176096
Wigler R, Matalon S, Goldberger T, Lerner AO, Kfir A. Enhanced Bactericidal Efficacy of NaOCl at pH 12 Followed by Acidified NaOCl at pH 6.5 on Enterococcus faecalis Biofilm. Applied Sciences. 2020; 10(17):6096. https://doi.org/10.3390/app10176096
Chicago/Turabian StyleWigler, Ronald, Shlomo Matalon, Tomer Goldberger, Anat Or Lerner, and Anda Kfir. 2020. "Enhanced Bactericidal Efficacy of NaOCl at pH 12 Followed by Acidified NaOCl at pH 6.5 on Enterococcus faecalis Biofilm" Applied Sciences 10, no. 17: 6096. https://doi.org/10.3390/app10176096
APA StyleWigler, R., Matalon, S., Goldberger, T., Lerner, A. O., & Kfir, A. (2020). Enhanced Bactericidal Efficacy of NaOCl at pH 12 Followed by Acidified NaOCl at pH 6.5 on Enterococcus faecalis Biofilm. Applied Sciences, 10(17), 6096. https://doi.org/10.3390/app10176096





