Abstract
The intensity and frequency of harmful algal blooms (HABs) have increased, posing a threat to human seafood resources due to massive kills of cultured fish and toxin contamination of bivalves. In recent years, bacteria that inhibit the growth of HAB species were found to be densely populated on the biofilms of some macroalgal species, indicating the possible biological control of HABs by the artificial introduction of macroalgal beds. In this study, an artificially created Ulva pertusa bed using mobile floating cages and a natural macroalgal bed were studied to elucidate the distribution of algal growth-limiting bacteria (GLB). The density of GLB affecting fish-killing raphidophyte Chattonella antiqua, and two harmful dinoflagellates, were detected between 106 and 107 CFU g−1 wet weight on the biofilm of artificially introduced U. pertusa and 10 to 102 CFU mL−1 from adjacent seawater; however, GLB found from natural macroalgal species targeted all tested HAB species (five species), ranging between 105 and 106 CFU g−1 wet weight in density. These findings provide new ecological insights of GLB at macroalgal beds, and concurrently demonstrate the possible biological control of HABs by artificially introduced Ulva beds.
1. Introduction
Harmful algal blooms (HABs) are naturally-occurring phenomena in fresh and marine water systems, often causing severe damage to the fishery industry worldwide, through massive kills of commercially cultured fishes and toxin-contamination of shellfish leading to closures of commercial shellfish harvests, and, in some cases, consumption of contaminated shellfish results in human illness and even death [1,2,3]. Lately, the acceleration of geographic expansion and intensity of HABs on a global scale associated with climate change have been reported [4,5,6,7]. Hence, developments of effective countermeasures to control the HAB events are even more urgent and crucial concerns. Mechanical, chemical, genetic, biological, and environmental measures to prevent HAB have been proposed [4], and clay dispersion is the only practical measure confirmed to control HABs formed by Cochlodinium polykrikoides in Korean coastal waters [8]. However, the negative impacts of clay on non-target organisms living in the seafloor and water column are also pointed out [9,10,11,12]. The control of HABs directly intervenes with surroundings in dynamic ocean environments, demanding an establishment of measures as environmentally friendly as possible. Among all proposed strategies to mitigate HABs, biological controls using naturally living organisms may have the least impact on ecosystems in the present circumstances. Numbers of biological controls using microbes to bivalves have been proposed and tested extensively for several decades [13,14,15,16,17,18,19,20]. In particular, bacteria that kill and/or inhibit the growth of HAB species often associated with bloom termination have received worldwide attention as possible biological control of HABs [21,22,23,24,25,26,27,28,29,30,31].
Although the use of natural pathogenicity could be environmentally friendly and cost-effective to control HABs, introducing potentially invasive bacteria may require a careful manner [32,33]. Recently, a biofilm of seagrass and macroalgae were found to be densely populated by algal growth-limiting bacteria (GLB), including both algicidal and growth-inhibiting bacteria [34,35,36,37], indicating their function as an enormous source of GLB for the adjacent seawater [31,38]. The latest microcosm study demonstrated that seawater in the Zostera marina bed successfully suppressed artificial Chattonella blooms [31], indicating that protection and restoration of seagrass and macroalgal beds prevent the outbreak of HABs through providing the specific microbial communities. However, these holistic approaches may sometimes not be flexible in actual field applications in that they often require specific habitat characteristics such as light, sediment or substrate types, currents, bioturbation, etc. [39,40].
In this study, Ulva pertusa beds using mobile floating cages were artificially created to study the distribution of algal growth-limiting bacteria (GLB), seeking flexible and widely applicable technologies to control HABs. A natural macroalgal bed was also studied for comparison.
2. Materials and Methods
2.1. Installation of Artificial Ulva Bed
Yellowish-green reproductively matured Ulva pertusa thalli were provided from Wakayama prefectural fisheries experiment station, Wakayama Prefecture, Japan. After the thallus of U. pertusa was gently wiped and dried in the shade for 1–2 h, it was placed into a water tank filled with autoclaved-filtered seawater [41]. Pumice stones were carefully rinsed with autoclaved seawater and gently set to promote attachments of motile zoospore of U. pertusa [42]. Then, the pumice stones were placed into mobile floating cages and installed on June 20, 2017, in a nearshore area by an upright seawall at Station 1 (Stn. 1), Kushimoto-cho, Wakayama Prefecture, Japan (Figure 1). The location of installation was carefully selected after confirming the absence of any macroalgal species in the area. However, this may not guarantee attachments of naturally-occurring macroalgal propagules to the tested pumice stone and cages after installation.
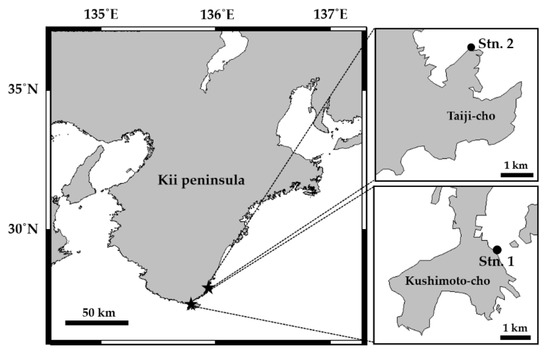
Figure 1.
Location of the installation of artificial Ulva bed (Stn. 1) and the natural macroalgal bed (Stn. 2) at Kushimoto-cho and Taiji-cho, Wakayama Prefecture, Japan.
2.2. Sampling
Samplings were performed on 7 to 8 September 2017, at Stn. 1 (artificial Ulva bed) and Stn. 2 (a natural macroalgal bed), Taiji-cho, Wakayama Prefecture, Japan (Figure 1). The weather was mostly cloudy during the sampling on 7 September 2017, with surface seawater temperature and salinity of 27.9 °C, 34.0 PSU at Stn. 1. On 8 September 2017, it was sunny with surface seawater temperature and salinity of 29.2 °C, 34.7 PSU at Stn. 2. The green alga, U. pertusa, successfully grown on the pumice stone and floating cage at Stn. 1 (Figure 2) and four dominant macroalgae (one brown alga: Sargassum dupulicatum, one red alga: Gelidium elegans, two green algae: Cladophora ohkuboana and U. pertusa) at Stn. 2 were sampled using sterile instruments in a sterile manner. Adjacent surface seawater samples were also collected from both sites. Samplings were conducted at low tide, and all the samples were collected in an autoclaved polycarbonate bottle (500 mL) filled with autoclaved, filtered (0.7 µm GF/F) seawater. All the samples were immediately transported to the laboratory and processed on the same day.
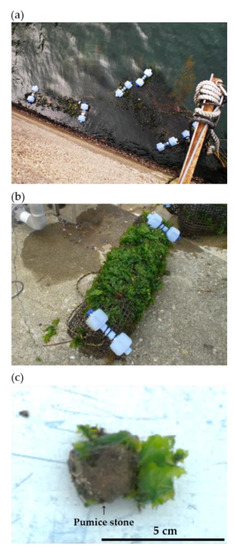
Figure 2.
Pictures of artificially introduced (a) Ulva beds using floating cages at Stn. 1; (b) a landed floating cage U. pertusa is well grown; (c) U. pertusa grew on the pumice stone.
2.3. Sample Processing and Bacterial Culturing
Macroalgal samples using bottles filled with 200 mL autoclaved filtered seawater were shaken 600 times by hand using a hand tally counter to detach a biofilm formed on the surface of the macroalgae. Biofilm-suspended samples were serially diluted 10-fold to 10−4 with autoclaved filtered seawater, and an aliquot (0.1 mL) of each dilution was spread onto a ST10−1 marine agar plate (Trypticase peptone 0.5 g, Yeast extract 0.05 g and agar 15 g in 1 L seawater [43]). The adjacent seawater, also collected from both sites, were serially diluted 10-fold to 10−4, and an aliquot (1 mL) was filtered through autoclaved 3 µm pore size Nucleopore Track-Etch membrane filters (Whatman, GE Healthcare, Parramatta, Australia). The filter was gently placed on a ST10−1 marine agar plate to culture the particle-associated bacteria (PAB). An aliquot of 0.1 mL from the filtrate was also used to culture the free-living bacteria (FLB) on the same medium above [44]. All the plates were kept for two weeks in the dark at 20 °C and used for the culturable bacterial enumeration and isolation. For the isolation, colonies were randomly picked by sterilized toothpicks from the agar plate, inoculated onto a 48-well filled with the same bacterial medium and kept at 20 °C in the dark until the following co-culture experiment. Aliquots (10 mL) from all samples were fixed with glutaraldehyde (1% final concentration) and stored at 4 °C for later determination of total bacterial abundance by DAPI staining [45].
2.4. Targeted Harmful Algal Bloom (HAB) Species
Two raphidophycean flagellates, Chattonella antiqua NIES-1 and Heterosigma akashiwo 893, two armored dinoflagellates, Alexandrium catenella, and Heterocapsa circularisquama and one unarmored dinoflagellate, Karenia mikimotoi, were axenically grown and kept in modified SWM-3 medium [46,47]. The cultured temperature was 15 °C for A. catenella, 20 °C for H. akashiwo and 25 °C for C. antiqua, H. circularisquama and K. mikimotoi under a light intensity of 50–100 µmol photons m−2 s−1 using a 14:10 h light–dark photocycle in algal growth chambers. Early stationary growth phase cultures were used for following co-culture experiments to test bacterial growth inhibitory activities.
2.5. Co-Culture Experiment Using Bacterial Isolates
Cultured bacteria isolated from the biofilm of U. pertusa grown on the pumice stone and floating cage (Stn. 1) and four macroalgal species (Stn. 2), as well as PAB and FLB from the surrounding seawater at both sites, had their growth inhibitory properties determined through co-culturing experiments (a single axenic HAB species cultured in the algal medium with a single bacterium) toward five targeted HAB species [37]. Cell densities were diluted with the same algal medium to approximately 103 cells mL−1 for C. antiqua, A. catenella, and K. mikimotoi and 104 cells mL−1 for H. akashiwo and H. circularisquama, then 0.8 mL aliquots were incubated for two days in sterilized disposable 48-well microplates to check for any abnormal appearance of cells. Individual bacterial isolates were inoculated into the algal cultures with an approximate density of 105 cells mL−1, and the plates were incubated for another two weeks with the conditions described above for the algal cultures. Bacteria-free quadruplicate wells (Controls) were also prepared in each microplate. A Nikon ECLIPSE TE200 inverted microscope was used for daily checks of algal cell mortality and/or any abnormalities of algal cells. The wells with 90% or more of algal cells killed were labeled as algicidal bacteria (AB), and the wells with 90% or more of motility reduced algal cells simultaneously were labeled as growth-inhibiting bacteria (GIB). In this study, the term algal growth-limiting bacteria (GLB) was mainly applied to express a set of bacteria limiting algal growth, including both AB and GIB, to eliminate repeated usage of those terms. The density of AB and GIB was estimated using the following formula: NAG = NV × SAG/NT (NAG is the number of AB or GIB (CFU mL−1 or CFU g−1 wet weight of macroalgae), NV is the number of viable bacteria (CFU mL−1 or CFU g−1 wet weight of macroalgae), SAG is the number of bacterial strains that expressed growth inhibitory properties, NT is the number of bacterial strains examined). All of the GLB were isolated aseptically into a 48-well plate after confirming a pure culture by the streak plate method [48].
2.6. 16S rRNA Gene Sequencing for Identification
A total of 47 bacterial strains (12 strains from Stn. 1 and 35 strains from Stn. 2) were identified by partial 16S rRNA gene sequencing (500 bp). All strains were cultured in ST10−1 liquid medium for two weeks then centrifuged (8000 rpm) for 5 min to obtain bacterial pellets. Pellets were suspended in phosphate-buffered saline and washed three times to remove the medium. Bacterial DNA was extracted using NucleoSpin Tissue XS (TaKaRa BIO Inc., Japan) and stored at −20 °C. The universal primers 27F (5′-AGAGTTTGATCMTGGCTCAG-3′ [49]) and 519R (5′-GWATTACCGCGGCKGCTG-3′ [50]) were applied to amplify the 16S rRNA gene fragments using Blend Taq®-Plus- (TOYOBO, Japan). The PCR temperature cycling conditions were: initial denaturation at 94 °C for 1 min, followed by 30 cycles of denaturation at 94 °C for 30 s, annealing at 52 °C for 30 s, and extension at 72 °C for 1 min; final elongation was for 7 min at 72 °C. PCR products were purified using ISOSPIN PCR Product (NIPPON GENE CO., LTD., Japan) before sequencing. Both strands of the PCR products were sequenced using the same primers in a cycle sequencing reaction using a sequencing kit (Big Dye Terminator Cycle version 3.1, Applied Biosystems, USA) using a DNA sequencer (ABI 3130, Applied Biosystems, USA). Nucleotide sequences obtained were aligned using Chromas Pro (ver. 1.7.1). Approximately 500 bp of each consensus sequence were analyzed by A BLAST (http://blast.ncbi.nlm.nih.gov/Blast.cgi) sequence similarity search to identify similarity with previously reported bacteria.
3. Results
3.1. Culturable and Total Bacterial Enumeration
The densities of culturable and total bacteria from seawater and macroalgal biofilms are shown in Table 1 and Figure 3. The densities of culturable free-living bacteria (FLB) at both sites were higher than particle-associated bacteria (PAB) with values of 1.7 × 103 CFU mL−1 at Stn. 1 and 2.3 × 103 CFU mL−1 at Stn. 2, respectively (Figure 3a). The density of culturable Ulva pertusa biofilm-associated bacteria on the pumice stone (2.4 × 108 CFU g−1 wet weight) was about ten times higher than U. pertusa collected from the floating cage (1.9 × 107 CFU g−1 wet weight) (Figure 3b). Sargassum dupulicatum associated culturable bacteria showed the lowest density with 7.9 × 106 CFU g−1 wet weight, and the density of U. pertusa-associated culturable bacteria was the highest with 4.0 × 107 CFU g−1 wet weight at natural macroalgal bed (Stn. 2, Figure 3b). The density of total bacteria in seawater at Stn. 1 was 2.9 × 106 cells mL−1 (PAB: 2.6 × 106 cells mL−1, FLB: 3.1 × 105 cells mL−1) higher than the density of 6.7 × 105 cells mL−1 (PAB: 3.9 × 105 cells mL−1, FLB: 2.8 × 105 cells mL−1) at Stn.2 (Figure 3c). In contrast to the culturable bacterial densities, the total PAB densities were higher than those of FLB at both sites (Figure 3a,c). The total bacterial density of U. pertusa on the pumice stone showed the maximum value of 2.7 × 109 cells g−1 wet weight at Stn. 1 (Figure 3d) as well as culturable bacteria. Total bacterial density of U. pertusa was the highest with the density of 1.2 × 109 cells g−1 wet weight at Stn.2 as well, followed by Cladophora ohkuboana (9.9 × 108 cells g−1 wet weight), Gelidium elegans (6.0 × 108 cells g−1 wet weight) and S. dupulicatum (3.9 × 108 cells g−1 wet weight) (Figure 3d). U. pertusa grown on the pumice stone appeared to be unhealthy, turning partly black in decay, and resulted in the highest values of both the densities of culturable and total bacteria at Stn. 1.

Table 1.
Summary of culturable and total bacterial densities enumerated from seawater and macroalgal biofilms at Stns. 1 and 2. (FLB: free-living bacteria, PAB: particle-associated bacteria)
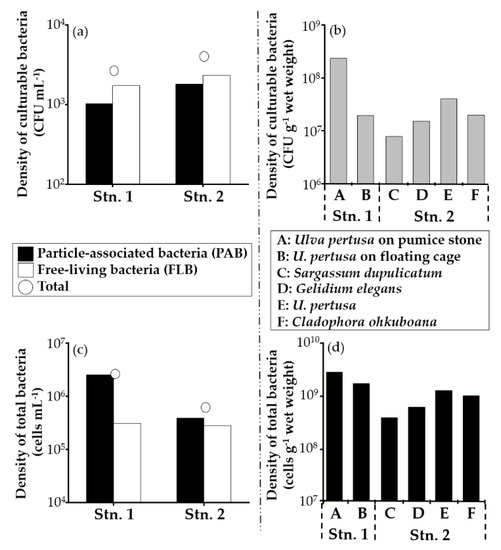
Figure 3.
The densities of culturable bacteria (a): seawater, (b): macroalgal biofilms and total bacteria directly counted with DAPI staining (c): seawater, (d): macroalgal biofilms and at Stns. 1 and 2. Total (○) shown on (a) and (c) are expressed as the sum of particle-associated bacteria (PAB) and free-living bacteria (FLB). A to F are macroalgal samples used to enumerate the densities.
3.2. Density of Growth-Limiting Bacteria
The densities of growth-limiting bacteria (GLB: the sum of algicidal bacteria (AB) and growth-inhibiting bacteria (GIB)) detected from seawater, and macroalgal samples at Stns.1 and 2, were shown in Table 2 and Figure 4 and Figure 5. GLB targetting at least one HAB species were detected both in seawater and U. pertusa samples collected at Stn. 1 (Figure 4). The densities of GLB showed that activities against Chattonella antiqua were 3.5 × 102 CFU mL−1 in FLB and 1.9 × 102 CFU mL−1 in PAB (Figure 4a,b). GLB against Heterocapsa circularisquama and Karenia mikimotoi were detected only in PAB with densities of 1.9 × 102 CFU mL−1 and 93 CFU mL−1, respectively (Figure 4b). AB targeted C. antiqua, and H. circularisquama were detected from U. pertusa on the pumice stone with the same density of 5.9 × 106 CFU g−1 wet weight (Figure 4c). GIB targeted H. circularisquama was detected from U. pertusa on the floating cage at a concentration of 1.5 × 106 CFU g−1 wet weight (Figure 4d). There were no GLB negatively affecting the growth of Alexandrium catenella and Heterosigma akashiwo at Stn. 1. GLB targeting at least one HAB species were also detected from all samples collected at Stn. 2 (Figure 5). The density of GLB showed that the activity against H. circularisquama was detected in FLB with a density of 69 CFU mL−1 (Figure 5a). GLB isolated from PAB at Stn. 2 had a wide range of activities affecting four HAB species except for A. catenella with densities ranging between 1.1 × 102 CFU mL−1 and 7.9 × 102 CFU mL−1 (Figure 5b). GLB affecting the growth of A. catenella, H. circularisquama, and K. mikimotoi were found from the S. dupulicatum biofilm with densities of 4.0 × 105 CFU g−1 wet weight, 2.4 × 106 CFU g−1 wet weight and 4.0 × 105 CFU g−1 wet weight, respectively (Figure 5c). GLB affecting C. antiqua, and H. circularisquama were detected from G. elegans biofilm with densities of 7.6 × 105 CFU g−1 wet weight and 1.5 × 106 CFU g−1 wet weight, respectively (Figure 5d). GLB isolated from C. ohkuboana targetted four HAB species, except K. mikimotoi, with densities of 1.0 × 106 CFU g−1 wet weight for A. catenella, 5.0 × 105 CFU g−1 wet weight for C. antiqua, 5.0 × 105 CFU g−1 wet weight for H. akashiwo, and 4.0 × 106 CFU g−1 wet weight for H. circularisquama, respectively (Figure 5e). GLB were found in U. pertusa with densities of 3.0 × 106 CFU g−1 wet weight for A. catenella, 6.0 × 106 CFU g−1 wet weight for H. circularisquama and 2.0 × 106 CFU g−1 wet weight for K. mikimotoi, respectively (Figure 5f). GLB negatively affecting the growth of all five HAB species were detected at the natural macroalgal bed (Stn. 2).

Table 2.
Summary of growth-limiting bacteria (GLB) densities against five different harmful algal bloom (HAB) species estimated through co-culture experiments from seawater and macroalgal biofilms at Stns. 1 and 2.
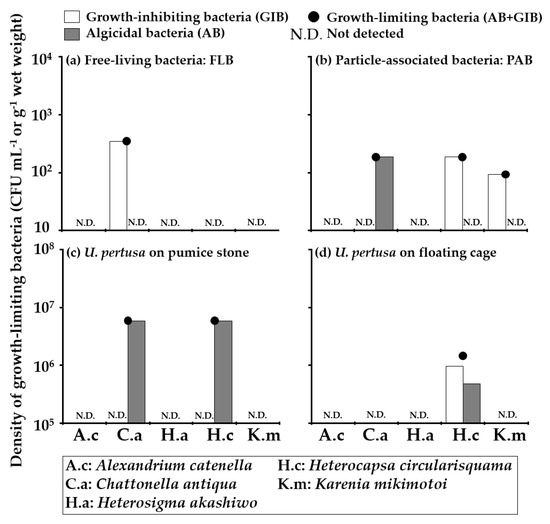
Figure 4.
The densities of growth-limiting bacteria detected from seawater (a): FLB, (b): PAB and U. pertusa biofilms (c): on pumice stone, (d): on floating cage collected at Stn.1. The densities of growth-limiting bacteria (●) are expressed as the sum of algicidal bacteria (AB:  ) and growth-inhibiting bacteria (GIB: ▭). The tested HAB species and their abbreviations are displayed at the bottom.
) and growth-inhibiting bacteria (GIB: ▭). The tested HAB species and their abbreviations are displayed at the bottom.
 ) and growth-inhibiting bacteria (GIB: ▭). The tested HAB species and their abbreviations are displayed at the bottom.
) and growth-inhibiting bacteria (GIB: ▭). The tested HAB species and their abbreviations are displayed at the bottom.
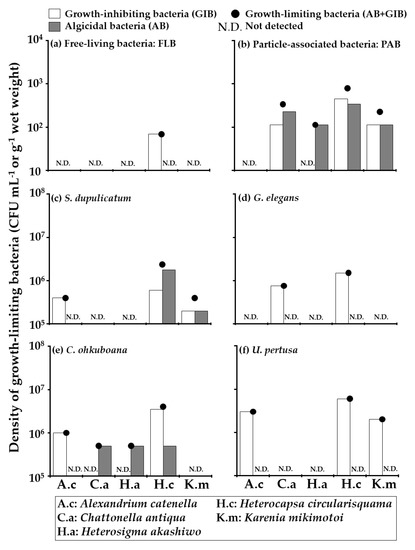
Figure 5.
The densities of growth-limiting bacteria detected from seawater (a): FLB, (b): PAB and four macroalgal species (c): S. dupulicatum, (d): G. elegans, (e): C. ohkuboana and (f): U. pertusa collected at Stn.2. The densities of growth-limiting bacteria (●) are expressed as the sum of algicidal bacteria (AB:  ) and growth-inhibiting bacteria (GIB: ▭). The tested HAB species and their abbreviations are displayed at the bottom.
) and growth-inhibiting bacteria (GIB: ▭). The tested HAB species and their abbreviations are displayed at the bottom.
 ) and growth-inhibiting bacteria (GIB: ▭). The tested HAB species and their abbreviations are displayed at the bottom.
) and growth-inhibiting bacteria (GIB: ▭). The tested HAB species and their abbreviations are displayed at the bottom.
3.3. Composition of Growth-Limiting Bacteria
The genus and class of GLB identified from different seawater fractions (FLB and PAB), and each of the host macroalgae at both sites are shown in Figure 6. All of the GLB isolated from seawater and macroalgal biofilms were comprised of the classes Alphaproteobacteria, Gammaproteobacteria, and Flavobacteria at Stns. 1 and 2. Seven GLB isolated from seawater at Stn. 1 were classified as the genera of Nautella (Alphaproteobacteria) in FLB, Alteromonas, Pseudoalteromonas (Gammaproteobacteria) and Tenacibaculum (Flavobacteria) in PAB (Figure 6a). In total, five GLB isolated from U. pertusa grown on the pumice stone and the floating cage at Stn. 1 were found to belong to the genera of Pseudoalteromonas, Vibrio (Gammaproteobacteria) and Aquimarina (Flavobacteria), respectively (Figure 6b,c). The eight GLB detected in seawater at Stn. 2 were one Winogradskyella (Flavobacteria) as FLB, Roseobacter (Alphaproteobacteria), Alteromonas, Pseudoalteromonas and Vibrio (Gammaproteobacteria) and Aquimarina, Winogradskyella (Flavobacteria) as PAB (Figure 6d). The twenty-seven GLB strains isolated from four macroalgal biofilms consisted of two genera from Alphaproteobacteria, Phaeobacter, and Roseovarius, and three genera from Gammaproteobacteria, Alteromonas, Pseudoalteromonas, and Vibrio plus three genera of Flavobacteria, Aquimarina, Dokdonia and Winogradskyella (Figure 6e–h). About 67% of GLB found from four macroalgal biofilms at the natural bed (Stn. 2) were the bacteria classified into the class Flavobacteria. Aquimarina was the only GLB found from all four macroalgae at the natural bed (Stn. 2).
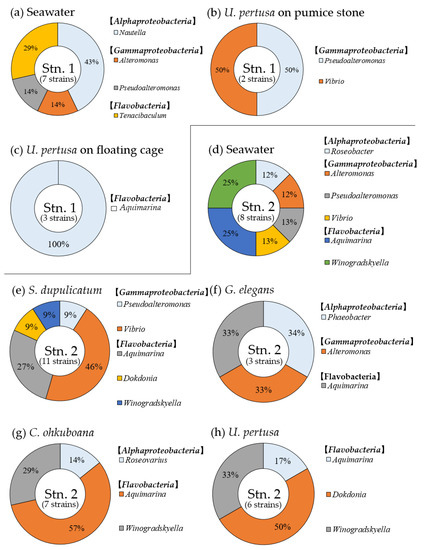
Figure 6.
The composition of growth-limiting bacteria (class and genus) isolated from seawater and the different host macroalgae at Stn. 1 and 2. (Stn. 1: surface seawater (a), Ulva pertusa on pumice stone (b), U. pertusa on floating cage (c), Stn. 2: surface seawater (d), Sargassum dupulicatum (e), Gelidium elegans (f), Cladophora ohkuboana (g), U. pertusa (h)).The number in parenthesis shows the number of strains used for 16S rRNA gene sequencing for identification.
4. Discussion
Recent findings of growth-limiting bacteria densely living in a biofilm of seagrass and macroalgae have offered an insight into possible future HAB control by harnessing ecosystem services [51]. Meanwhile, the loss of seagrass and macroalgal beds due to human impacts have been accelerated over the last half-century [52,53,54]. Such deterioration of coastal vegetation coincided with the increasing occurrences of HAB [51,55]. The increasing toxic blooms of the dinoflagellate A. minutum accompanied by the large-scale decline of seagrass beds around the Mediterranean coast is one of the specific cases [56]. Inaba et al. [37] documented that H. akashiwo cysts were found only from the sediments where eelgrass had disappeared and studied sites where the abundant growth-limiting bacteria detected rarely experienced blooms of paralytic shellfish toxin-producing dinoflagellate Alexandrium in Puget Sound, WA, USA. On the positive side, the efforts to protect and restore seagrass and macroalgal beds are underway worldwide [57,58]; for instance, vigorous restoration activities of seagrass bed in Hinase, Okayama, Japan increased the natural bed more than twenty times since 1985 [57]. The efforts will enhance the resistance of coastal areas against HAB events in the long run. However, this holistic approach to prevent HABs may not be as applicable as direct controls, such as the use of flocculants [59]. Thus, it is crucial to develop an environmentally friendly measure that can be widely applicable and cost-effective.
Ulva can be attractive candidate organisms revealed to harbor GLB [34,37], growing fast compared to other macroalgae or seagrass [60,61], relatively easy to culture [62], restoring water quality through the uptake of excess nutrients [63,64], releasing allelochemicals negatively affecting the growth of HAB species [65,66], and having commercial importance in that they are frequently consumed under the name “Aonori” in Asia [67,68]. Several Ulva species have already been tested to be organisms composing large scale integrated multi-trophic aquaculture (IMTA) systems [67,69]. In the present study, harmful algal growth-limiting bacteria (GLB) were found in the order of 106 CFU per 1 g of U. pertusa grown on the pumice stone and the mobile floating cage at Stn. 1, showing the equivalent value to the natural growing macroalgal species at Stn. 2 as well as previous studies estimating the densities of GLB on Ulva sp. and Gelidium sp. in Osaka Bay, Japan [34] and on U. lactuca in Puget Sound, USA [37]. To our best knowledge, this is the first report to reveal that algal growth-limiting bacteria can be harbored within a few months on the newly introduced Ulva thallus, suggesting the possible future field application to control HABs by introducing Ulva beds as a source of GLB.
Previous studies have shown that a higher proportion of GLB was often associated with particles [28,44]. In the study in Puget Sound, most isolated bacteria from seawater having more extensive activity ranges were in the particle-associated form [37]. In the present study, the density of PAB (Particle-associated bacteria) as GLB was higher than FLB (Free-living bacteria) at the natural macroalgal bed (Stn. 2), killing most of the tested HAB species except A. catenella (Figure 5b). Moreover, 88% of GLB detected from seawater at the natural macroalgal bed was the same bacterial genera as GLB detected from macroalgal biofilms (Figure 6). It is known that macroalgal beds are one of the essential sources releasing particulate and dissolved organic matter (POM and DOM) to the surrounding environments [70,71,72]. The POM derived from the macroalgae at Stn. 2 may have increased substrate availability for specific bacterial communities with high exoenzymatic activities [73,74,75] that resulted in frequent detection of GLB as PAB forms in the water. This could be reasonably explained by highly dense GLB harbored on a macroalgal surface being provided synchronously with macroalgal-derived POM to the surrounding water.
Many bacteria previously reported as algicidal and/or growth-inhibiting bacteria were among the Cytophaga/Flavobacterium/Bacteroidetes (CFB) group [27]. They are also critical players for the degradation of organic matter in aquatic environments [76]. Ecologically important roles toward macroalgae of the CFB group have been demonstrated, such as controlling morphogenesis [77,78], promotion and/or inhibition of spore germination, and colonization [79,80], close association with diseases [81], etc. In the present study, more than half of GLB were identified as bacteria belonging to the class Flavobacteria. Notably, the genus Aquimarina within the class Flavobacteria were found from all of the macroalgae and 25% of GLB in seawater samples at Stn. 2 (Figure 6). With regard to the growth-limiting activity range, all GLB isolated from seawater and Ulva samples at Stn. 1 were found to exhibit specific activities in that 92% of GLB negatively affected the growth of only one HAB species (C. antiqua or H. circularisquama) (Figure 7a); however, GLB isolated from natural macroalgae bed showed a much broader range of activities on tested HAB species (Figure 7b). About 90% of GLB affecting the growth of more than one HAB species were found to be Flavobacteria, which consisted of Aquimarina and Dokdonia (Figure 7b). Aquimarina isolated from red alga on the coast of China is shown to exhibit diverse agarase activities, enhancing the degradation of host algal tissue [82]. These results suggest the multiple roles of the members of Flavobacteria diffusing around macroalgal beds. The physiological and biochemical characteristics of macroalgae predetermine the composition of their biofilm microbial communities [83], and the host species-specific microbial association in aquatic angiosperms is known [84]. It is worth noting that natural macroalgal beds with higher diversity may offer various inhabiting substrates for diverse GLB, functioning effectively as bloom control for multiple harmful algal species (Figure 7b).
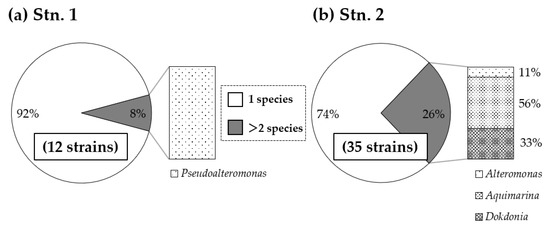
Figure 7.
The growth-limiting activity ranges of GLB against all five harmful algal species (A. catenella, C. antiqua, H. akashiwo, H. circularisquama, and K. mikimotoi) isolated from (a) Stn. 1 and (b) Stn. 2. It includes all GLB strains isolated from seawater and macroalgal samples at each site. Total strains tested are shown in parenthesis in the lower center of the pie chart.
5. Conclusions
The present study illustrated that GLB with equivalent value as naturally growing macroalgae could be harbored on the surface of artificially introduced U. pertusa for less than three months, providing novel insight into the potential use of the artificial introduction of U. pertusa beds as biological control of HABs. Results from the natural macroalgal bed suggested that higher macroalgal diversity provide diverse inhabiting substrates for GLB, functioning effectively as bloom control for multiple harmful algal species. It should be also emphasized that the protection and restoration of natural macroalgal beds may serve as not only critical habitats for numerous marine and estuary organisms but also one of the original hotspots of GLB, contributing to mitigating HABs in coastal environments. Further investigations to understand the diffusive behavior of GLB are needed to achieve the practical application.
Author Contributions
All authors contributed the project. N.I. wrote the manuscript. I.K. supported the sampling and performed the algal and part of the molecular analyses. S.N. supported molecular analyses. T.S. supported sampling and zoospore preparation. N.I., K.M., A.Y. and I.I. conceived and designed the experiments. All co-authors participated in discussions and review of the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported financially in part by a Grant-in-Aid for Scientific Research grants 18K14506 (Early Career Scientists) from the Japanese Society for the Promotion of Science (JSPS) and a Grant-in-Aid (Technological developments for characterization of harmful plankton in the seawater: No. 16808839) from the Ministry of Agriculture, Forestry and Fisheries of Japan.
Acknowledgments
We would like to show sincere thanks to the members of Fisheries Engineering Research Team, Civil Engineering Research Institute for Cold Region (CERI), Japan for administrative support, especially, Sawako Shirai, technical assistant. We also express our gratitude to Masaru Okamoto, Shinkotec co., ltd for the suggestions on pumice stone.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Trainer, V.L.; Eberhart, B.-T.L.; Wekell, J.C.; Adams, N.G.; Hanson, L.; Cox, F.; Dowell, J. Paralytic shellfish toxins in Puget Sound, Washington State. J. Shellfish Res. 2003, 22, 213–223. [Google Scholar]
- Trainer, V.L.; Bates, S.S.; Lundholm, N.; Thessen, A.E.; Cochlan, W.P.; Adams, N.G.; Trick, C.G. Pseudo-nitzschia physiological ecology, phylogeny, toxicity, monitoring and impacts on ecosystem health. Harmful Algae 2012, 14, 271–300. [Google Scholar] [CrossRef]
- Imai, I.; Yamaguchi, M. Life cycle, physiology, ecology and red tide occurrences of the fish-killing raphidophyte Chattonella. Harmful Algae 2012, 14, 46–70. [Google Scholar] [CrossRef]
- Anderson, D.M.; Cembella, A.D.; Hallegraeff, G.M. Progress in understanding harmful algal blooms: Paradigm shifts and new technologies for research, monitoring, and management. Annu. Rev. Mar. Sci. 2012, 4, 143–176. [Google Scholar] [CrossRef]
- Wells, M.L.; Trainer, V.L.; Smayda, T.J.; Karlson, B.S.O.; Trick, C.G.; Kudela, R.M.; Ishikawa, A.; Bernard, S.; Wulff, A.; Anderson, D.M.; et al. Harmful algal blooms and climate change: Learning from the past and present to forecast the future. Harmful Algae 2015, 49, 68–93. [Google Scholar] [CrossRef]
- Gobler, C.J.; Doherty, O.M.; Hattenrath-Lehmann, T.K.; Griffith, A.W.; Kang, Y.; Litaker, R.W. Ocean warming since 1982 has expanded the niche of toxic algal blooms in the North Atlantic and North Pacific oceans. Proc. Natl. Acad. Sci. USA 2017, 114, 4975–4980. [Google Scholar] [CrossRef]
- Gobler, C.J. Climate change and harmful algal blooms: Insights and perspective. Harmful Algae 2020, 91, 101731. [Google Scholar] [CrossRef]
- Seger, A.; Park, T.G.; Hallegraeff, G. Assessment of the efficacy of clay flocculation in Korean fish farm waters: Cochlodinium cell removal and mitigation of ichthyotoxicity. Harmful Algae 2017, 61, 46–55. [Google Scholar] [CrossRef]
- Archambault, M.-C.; Bricelj, V.M.; Grant, J.; Anderson, D.M. Effects of clay, used to control harmful algal blooms, on juvenile Mercenaria mercenaria. J. Shellfish Res. 2002, 21, 395–396. [Google Scholar]
- Shumway, S.E.; Frank, D.M.; Ewart, L.M.; Ward, J.E. Effect of yellow loess on clearance rate in seven species of benthic, filter-feeding invertebrates. Aquac. Res. 2003, 34, 1391–1402. [Google Scholar] [CrossRef]
- Sengco, M.R.; Anderson, D.M. Controlling harmful algal blooms through clay flocculation. J. Eukaryot. Microbiol. 2004, 51, 169–172. [Google Scholar] [CrossRef] [PubMed]
- Seo, K.S.; Lee, C.K.; Park, Y.T.; Lee, Y. Effect of yellow clay on respiration and phytoplankton uptake of bivalves. Fish. Sci. 2008, 74, 120–127. [Google Scholar] [CrossRef]
- Shirota, A. Red tide problem and countermeasures. Int. J. Aquat. Fish. Technol. 1989, 1, 195–223. [Google Scholar]
- Matsuyama, Y.; Miyamoto, M.; Kotani, Y. Grazing impacts of the heterotrophic dinoflagellate Polykrikos kofoidii on a bloom of Gymnodinium catenatum. Aquat. Microb. Ecol. 1999, 17, 91–98. [Google Scholar] [CrossRef]
- Sakata, T. Control of harmful microalgae by microorganisms. In Mechanisms, Prediction, and Mitigation of Harmful Algal Blooms in Japan; Ishida, Y., Honjo, T., Fukuyo, Y., Imai, I., Eds.; The Japan Fisheries Resource Conservation Association: Tokyo, Japan, 2000; pp. 215–235. (In Japanese) [Google Scholar]
- Jeong, H.J.; Kim, J.S.; Yoo, Y.D.; Kim, S.T.; Kim, T.H.; Park, M.G.; Lee, C.H.; Seong, K.A.; Rang, N.S.; Shim, J.H. Feeding by the heterotrophic dinoflagellate Oxyrrhis marina on the red-tide raphidophyte Heterosigma akashiwo: A potential biological method to control red tides using mass-cultured grazers. J. Eukaryot. Microbiol. 2003, 50, 274–282. [Google Scholar] [CrossRef]
- Brussaard, C.P.D. Viral control of phytoplankton populations—A review. J. Eukaryot. Microbiol. 2004, 51, 125–138. [Google Scholar] [CrossRef]
- Park, M.G.; Yih, W.; Coats, D.W. Parasites and phytoplankton, with special emphasis on dinoflagellate infections. J. Eukaryot. Microbiol. 2004, 51, 145–155. [Google Scholar] [CrossRef]
- Salomon, P.S.; Imai, I. Pathogens of harmful microalgae. In Ecology of Harmful Algae, Ecological Studies Vol 189; Granéli, E., Turner, J.T., Eds.; Springer: Berlin, Germany, 2006; pp. 271–282. [Google Scholar]
- Imai, I.; Kakumu, A.; Ohara, S.; Yuki, T.; Koike, K.; Hagiwara, E.; Ogawa, K.; Yoneyama, H. Feasibility studies on sediment perturbation as control strategies for Chattonella red tides. Bull. Fish. Sci. Hokkaido Univ. 2017, 67, 57–66. [Google Scholar] [CrossRef]
- Imai, I.; Kim, M.C.; Nagasaki, K.; Itakura, S.; Ishida, Y. Relationships between dynamics of red tide-causing raphidophycean flagellates and algicidal micro-organisms in the coastal sea of Japan. Phycol. Res. 1998, 46, 139–146. [Google Scholar] [CrossRef]
- Imai, I.; Sunahara, T.; Nishikawa, T.; Hori, Y.; Kondo, R.; Hiroishi, S. Fluctuations of the red tide flagellates Chattonella spp. (Raphidophyceae) and the algicidal bacterium Cytophaga sp. in the Seto Inland Sea, Japan. Mar. Biol. 2001, 138, 1043–1049. [Google Scholar] [CrossRef]
- Lovejoy, C.; Bowman, J.P.; Hallegraeff, G.M. Algicidal effects of a novel marine Pseudoalteromonas isolate (class proteobacteria gamma subdivision) on harmful algal bloom species of the genera Chattonella, Gymnodinium and Heterosigma. Appl. Environ. Microbiol. 1998, 64, 2806–2813. [Google Scholar] [CrossRef] [PubMed]
- Doucette, G.J.; Kodama, M.; Franca, S.; Gallacher, S. Bacterial interactions with harmful algal bloom species: Bloom ecology, toxigenesis and cytology. In Physiological Ecology of Harmful Algal Blooms, NATO ASI Series; Anderson, D.M., Cembella, A.D., Hallegraeff, G.M., Eds.; Springer: Berlin, Germany, 1998; Volume G41, pp. 619–647. [Google Scholar]
- Doucette, G.J.; McGovern, E.R.; Babinchak, J.A. Algicidal bacteria active against Gymnodinium breve (Dinophyceae). I. Bacterial isolation and characterization of killing activity. J. Phycol. 1999, 35, 1447–1454. [Google Scholar] [CrossRef]
- Skerratt, J.H.; Bowman, J.P.; Hallegraeff, G.; James, S.; Nichols, P.D. Algicidal bacteria associated with blooms of a toxic dinoflagellate in a temperate Australian estuary. Mar. Ecol. Prog. Ser. 2002, 244, 1–15. [Google Scholar] [CrossRef]
- Mayali, X.; Azam, F. Algicidal bacteria in the sea and their impact on algal blooms. J. Eukaryot. Microbiol. 2004, 51, 139–144. [Google Scholar] [CrossRef]
- Park, J.H.; Yoshinaga, I.; Nishikawa, T.; Imai, I. Algicidal bacteria in particle-associated form and in free-living form during a diatom bloom in the Seto Inland Sea, Japan. Aquat. Microb. Ecol. 2010, 60, 151–161. [Google Scholar] [CrossRef][Green Version]
- Paul, C.; Pohnert, G. Interactions of the algicidal bacterium Kordia algicida with diatoms: Regulated protease excretion for specific algal lysis. PLoS ONE 2011, 6, e21032. [Google Scholar] [CrossRef]
- Pokrzywinski, K.L.; Place, A.R.; Warner, M.E.; Coyne, K.J. Investigation of the algicidal exudate produced by Shewanella sp. IRI-160 and its effect on dinoflagellates. Harmful Algae 2012, 19, 23–29. [Google Scholar] [CrossRef]
- Inaba, N.; Trainer, V.L.; Nagai, S.; Kojima, S.; Sakami, T.; Takagi, S.; Imai, I. Dynamics of seagrass bed microbial communities used to control artificial Chattonella blooms: A microcosm study. Harmful Algae 2019, 84, 139–150. [Google Scholar] [CrossRef] [PubMed]
- Sanders, N.J.; Gotelli, N.J.; Heller, N.E.; Gordon, D.M. Community disassembly by an invasive species. Proc. Natl. Acad. Sci. USA 2003, 100, 2474–2477. [Google Scholar] [CrossRef] [PubMed]
- Secord, D. Biological control of marine invasive species: Cautionary tales and land-based lessons. Biol. Invasions 2003, 5, 117–131. [Google Scholar] [CrossRef]
- Imai, I.; Fujimaru, D.; Nishigaki, T. Co-culture of fish with macroalgae and associated bacteria: A possible mitigation strategy for noxious red tides in enclosed coastal sea. Fish. Sci. 2002, 68 (Suppl. S1), 493–496. [Google Scholar] [CrossRef][Green Version]
- Imai, I.; Yamamoto, T.; Ishii, K.I.; Yamamoto, K. Promising prevention strategies for harmful red tides by seagrass beds as enormous sources of algicidal bacteria. In Proceedings of the 5th World Fisheries Congress, Tokyo, Japan, 13 December 2009. 6C_0995_133. [Google Scholar]
- Onishi, Y.; Mohri, Y.; Tuji, A.; Ohgi, K.; Yamaguchi, A.; Imai, I. The seagrass Zostera marina harbors growth-inhibiting bacteria against the toxic dinoflagellate Alexandrium tamarense. Fish. Sci. 2014, 80, 353–362. [Google Scholar] [CrossRef]
- Inaba, N.; Trainer, V.L.; Onishi, Y.; Ishii, K.; Wyllie-Echeverria, S.; Imai, I. Algicidal and growth-inhibiting bacteria associated with seagrass and macroalgae beds in Puget Sounds, WA, USA. Harmful Algae 2017, 62, 136–147. [Google Scholar] [CrossRef] [PubMed]
- Sakami, T.; Sakamoto, S.; Takagi, S.; Inaba, N.; Imai, I. Distribution of three algicidal Alteromonas sp. strains in seagrass beds and surrounding areas in the Seto Inland Sea, Japan. Fish. Sci. 2017, 83, 113–121. [Google Scholar] [CrossRef]
- Green, E.P.; Short, F.T. World Atlas of Seagrasses; University of California Press: Berkeley, CA, USA, 2003; p. 324. [Google Scholar]
- Hurd, C.L.; Harrison, P.J.; Bischof, K.; Lobban, C.S. Seaweed Ecology and Physiology, 2nd ed.; Cambridge University Press: Cambridge, UK, 2014; p. 551. [Google Scholar] [CrossRef]
- Yamada, Y.; Saito, E. On Some Culture Experiments with the Swarmers of Certain Species belonging to the Ulvaceae; Scientific Papers of the Institute of Algological Research; Faculty of Science, Hokkaido Imperial University: Hokkaido, Japan, 1938; Volume 2, pp. 35–51. [Google Scholar]
- Mori, S.; Hidaka, K.; Ushirokawa, T. Aratana Kaiso No Saibyokishitsu No Kento. Bull. Fukuoka Fish. Mar. Technol. Res. Cent. 2017, 27, 19–26. (In Japanese) [Google Scholar]
- Yoshinaga, I.; Kawai, T.; Ishida, Y. Analysis of algicidal ranges of the bacteria killing the marine dinoflagellates Gymnodinium mikimotoi isolated from Tanabe Bay, Wakayama Pref., Japan. Fish. Sci. 1997, 63, 94–98. [Google Scholar] [CrossRef]
- Inaba, N.; Watanabe, T.; Sakami, T.; Nishi, H.; Tahara, Y.; Imai, I. Temporal and spatial distribution of algicidal and growth-inhibiting bacteria in the coastal sea of southwest Japan. J. Plankton Res. 2014, 36, 388–397. [Google Scholar] [CrossRef]
- Porter, K.G.; Feig, Y.S. The use of DAPI for identifying and counting aquatic microflora. Limnol. Oceanogr. 1980, 25, 943–948. [Google Scholar] [CrossRef]
- Chen, L.C.M.; Edelstein, T.; McLachlan, J. Bonnemaisonia hamifera Hariot in nature and in culture. J. Phycol. 1969, 5, 211–220. [Google Scholar] [CrossRef]
- Imai, I.; Itakura, S.; Matsuyama, Y.; Yamaguchi, M. Selenium requirement for growth of a novel red tide flagellate Chattonella verruculosa (Raphidophyceae) in culture. Fish. Sci. 1996, 62, 834–835. [Google Scholar] [CrossRef]
- Holt, J.G.; Krieg, N.R. Enrichment and isolation. In Methods for General and Molecular Bacteriology; Gerhardt, P., Murray, R.G.E., Hood, W.A., Krieg, N.R., Eds.; ASM Press: Washington, DC, USA, 1994; pp. 179–215. [Google Scholar]
- Delong, E.F. Archaea in coastal marine environments. Proc. Natl. Acad. Sci. USA 1992, 89, 5685–5689. [Google Scholar] [CrossRef] [PubMed]
- Lane, D.J. 16S/23S rRNA sequencing. In Nucleic Acid Techniques in Bacterial Systematics; Stackebrandt, E., Goodfellow, M., Eds.; Jon Wiley & Sons: Chichester, UK, 1991; pp. 115–176. [Google Scholar]
- Imai, I. Interactions between harmful algae and algicidal bacteria associated with seaweeds and seagrasses. In Marine Protists; Ohtsuka, S., Suzuki, N., Horiguchi, T., Eds.; Springer: Tokyo, Japan, 2015; pp. 597–619. [Google Scholar]
- Middelboe, A.L.; Sand-Jensen, K. Long-term changes in macroalgal communities in a Danish estuary. Phycologia 2000, 39, 245–257. [Google Scholar] [CrossRef]
- Waycott, M.; Duarte, C.M.; Carruthers, T.J.B.; Orth, R.J.; Dennison, W.C.; Olyarnik, S.; Calladine, A.; Fourqurean, J.W.; Heck, K.L., Jr.; Hughes, A.R.; et al. Accelerating loss of seagrass across the globe threatens coastal ecosystems. Proc. Natl. Acad. Sci. USA 2019, 106, 12377–12381. [Google Scholar] [CrossRef] [PubMed]
- Filbee-Dexter, K.; Wernberg, T. Rise of turfs: A new battlefront for globally declining kelp forests. Bioscience 2018, 68, 64–76. [Google Scholar] [CrossRef]
- Kemp, W.M.; Boynton, W.R.; Adolf, J.E.; Boesch, D.F.; Boicourt, W.C.; Brush, G.; Cornwell, J.C.; Fisher, T.R.; Glibert, P.M.; Hagy, J.D.; et al. Eutrophication of Chesapeake Bay: Historical trends and ecological interactions. Mar. Ecol. Prog. Ser. 2005, 303, 1–29. [Google Scholar] [CrossRef]
- Abdenadher, M.; Hamza, A.; Fekih, W.; Hannachi, I.; Bellaaj, A.Z.; Bradai, M.N.; Aleya, L. Factors determining the dynamics of toxic blooms of Alexandrium minutum during a 10-year study along the shallow southwestern Mediterranean coasts. Estuar. Coast. Shelf. Sci. 2012, 106, 102–111. [Google Scholar] [CrossRef]
- Tanaka, T.; Furukawa, K.; Kuwae, T.; Imai, I. Report of the 32nd Joint symposium of Laiaison council of Academic societies on coastal environment “Road of Zostera bed restoration in Japanese coastal zones” -Past processes and the future prospects. Nippon Suisan Gakkaishi 2017, 83, 1042–1053. [Google Scholar] [CrossRef]
- Tamburello, L.; Papa, L.; Guarnieri, G.; Basconi, L.; Zampardi, S.; Scipione, M.B.; Terlizzi, A.; Zupo, V.; Fraschetti, S. Are we ready for scaling up restoration actions? An insight from Mediterranean macroalgal canopies. PLoS ONE 2019, 14, e0224477. [Google Scholar] [CrossRef]
- Anderson, C.R.; Sellner, K.G.; Anderson, D.M. Bloom prevention and control. In Harmful Algal Blooms (HABs) and Desalination: A Guide to Impacts, Monitoring, and Management; Anderson, D.M., Boerlage, S.F.E., Dixon, M.B., Eds.; Intergovernmental Oceanographic Commission of UNESCO: Paris, France, 2017; pp. 205–222. [Google Scholar]
- Pedersen, M.F.; Borum, J. Nutrient control of algal growth in estuarine waters: Nutrient limitation and the importance of nitrogen requirements and nitrogen storage among phytoplankton and species of macroalgae. Mar. Ecol. Prog. Ser. 1996, 142, 261–272. [Google Scholar] [CrossRef]
- Pedersen, M.F.; Borum, J.; Fotel, F.L. Phosphorus dynamics and limitation of fast- and slow-growing temperate seaweeds in Oslofjord, Norway. Mar. Ecol. Prog. Ser. 2010, 399, 103–115. [Google Scholar] [CrossRef]
- Hirata, H.; Kohirata, E.; Guo, F.; Xu, B.T.; Danakusumah, E. Culture of the sterile Ulva sp. (Chlorophyceae) in a mariculture farm. Suisan Zoshoku 1993, 41, 541–545. [Google Scholar]
- Neori, A.; Krom, M.D.; Ellner, S.P.; Boyd, C.E.; Popper, D.; Rabinovitch, R.; Davison, P.J.; Dvir, O.; Zuber, D.; Ucko, M.; et al. Seaweed biofilters as regulators of water quality in integrated fish-seaweed culture units. Aquaculture 1996, 141, 183–199. [Google Scholar] [CrossRef]
- Neori, A.; Chopin, T.; Troell, M.; Buschmann, A.H.; Kraemer, G.P.; Halling, C.; Shpigel, M.; Yarish, C. Integrated aquaculture: Rationale, evolution and state of the art emphasizing seaweed biofiltration in modern mariculture. Aquaculture 2004, 231, 361–391. [Google Scholar] [CrossRef]
- Wang, Y.; Yu, Z.; Song, X.; Tang, X.; Zhang, S. Effects of macroalgae Ulva pertusa (Chlorophyta) and Gracilaria lemaneiformis (Rhodophyta) on growth of four species of bloom-forming dinoflagellates. Aquat. Bot. 2007, 86, 139–147. [Google Scholar] [CrossRef]
- Tang, Y.Z.; Gobler, C.J. The green macroalga, Ulva lactuca, inhibits the growth of seven common harmful algal bloom species via allelopathy. Harmful Algae 2011, 10, 480–488. [Google Scholar] [CrossRef]
- Bolton, J.J.; Robertson-Andersson, D.V.; Shuuluka, D.; Kandjengo, L. Growing Ulva (Chlorophyta) in integrated systems as a commercial crop for abalone feed in South Africa: A SWOT analysis. J. Appl. Phycol. 2008, 21, 575–583. [Google Scholar] [CrossRef]
- Kawashima, Y.; Akasaki, T.; Matsumoto, Y.; Yamazaki, Y.; Shimada, S. Species identification of imported and Japanese commercial green algal products based on phylogenetic analyses using the nrITS2 and 5S rDNA spacer regions. Fish. Sci. 2013, 79, 521–529. [Google Scholar] [CrossRef]
- Msuya, F.E.; Kyewalyanga, M.S.; Salum, D. The performance of the seaweed Ulva reticulata as a biofilter in a low-tech, low-cost, gravity generated water regime in Zanzibar, Tanzania. Aquaculture 2006, 254, 284–292. [Google Scholar] [CrossRef]
- Duggins, D.O.; Eckman, J.E.; Sewell, A.T. Ecology of understory kelp environments. II. Effects of kelps on recruitment of benthic invertebrates. J. Exp. Mar. Biol. Ecol. 1990, 143, 27–45. [Google Scholar] [CrossRef]
- Leclerc, J.C.; Riera, P.; Leroux, C.; Lévêque, L.; Davoult, D. Temporal variation in organic matter supply in kelp forests: Linking structure to trophic functioning. Mar. Ecol. Prog. Ser. 2013, 494, 87–105. [Google Scholar] [CrossRef]
- Campbell, I.; Macleod, A.; Sahlmann, C.; Neves, L.; Funderud, J.; Øverland, M.; Hughes, A.D.; Stanley, M.S. The environmental risks associated with the development of seaweed farming in Europe—prioritizing key knowledge gaps. Front. Mar. Sci. 2019, 6, 107. [Google Scholar] [CrossRef]
- Smith, D.C.; Simon, M.; Alldredge, A.L.; Azam, F. Intense hydrolytic enzyme activity on marine aggregates and implications for rapid particle dissolution. Nature 1992, 359, 139–142. [Google Scholar] [CrossRef]
- Bidle, K.D.; Azam, F. Accelerated dissolution of diatom silica by marine bacterial assemblages. Nature 1999, 397, 508–512. [Google Scholar] [CrossRef]
- Teeling, H.; Fuchs, B.; Becher, D.; Klockow, C.; Gardebrecht, A.; Bennke, C.; Kassabgy, M.; Huang, S.; Mann, A.; Waldmann, J.; et al. Substrate-controlled succession of marine bacterioplankton populations induced by a phytoplankton bloom. Science 2012, 336, 608–611. [Google Scholar] [CrossRef] [PubMed]
- Kirchman, D. The ecology of Cytophaga-Flavobacteria in aquatic environments. FEMS Microbiol. Ecol. 2002, 39, 91–100. [Google Scholar] [CrossRef]
- Matsuo, Y.; Imagawa, H.; Nishizawa, M.; Shizuri, Y. Isolation of an algal morphogenesis inducer from a marine bacterium. Science 2005, 307, 1598. [Google Scholar] [CrossRef] [PubMed]
- Marshall, K.; Joint, I.; Callow, M.E. Effect of marine bacterial isolates on the growth and morphology of axenic plantlets of the green alga Ulva linza. Microb. Ecol. 2006, 52, 302–310. [Google Scholar] [CrossRef] [PubMed]
- Patel, P.; Callow, M.E.; Joint, I.; Callow, J.A. Specificity in the settlement-modifying response of bacterial biofilms towards zoospores of the marine alga Enteromorpha. Environ. Microbiol. 2003, 5, 338–349. [Google Scholar] [CrossRef]
- Matsuo, Y.; Suzuki, M.; Kasai, H.; Shizuri, Y.; Harayama, S. Isolation and phylogenetic characterization of bacteria capable of inducing differentiation in the green alga Monostroma oxyspermum. Environ. Microbiol. 2003, 5, 25–35. [Google Scholar] [CrossRef]
- Zozaya-Valdes, E.; Egan, S.; Thomas, T. A comprehensive analysis of the microbial communities of healthy and diseased marine macroalgae and the detection of known and potential bacterial pathogens. Front. Microbiol. 2015, 6, 146. [Google Scholar] [CrossRef]
- Lin, B.; Lu, G.; Zheng, Y.; Xie, W.; Li, S.; Hu, Z. Aquimarina agarilytica sp. nov., agarolytic species isolated from a red alga. Int. J. Syst. Evol. Microbiol. 2012, 62, 869–873. [Google Scholar] [CrossRef] [PubMed]
- Beleneva, I.A.; Zhukova, N.V. Bacterial communities of some brown and red algae from Peter the Great Bay, the Sea of Japan. Microbiology 2006, 75, 348–357. [Google Scholar] [CrossRef]
- Crump, B.C.; Koch, E.K. Attached bacterial populations shared by four species of aquatic angiosperms. Appl. Environ. Microbiol. 2008, 74, 5948–5957. [Google Scholar] [CrossRef] [PubMed]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).