Nano-Scale Drinking Water Treatment Residuals Affect Arsenic Fractionation and Speciation in Biosolids-Amended Agricultural Soil
Abstract
1. Introduction
2. Materials and Methods
2.1. Soil, Biosolids and nWTRs
2.2. Soil Stabilization
2.3. Arsenic Sorption and Sorption Efficiency
2.4. Arsenic Fractionation
2.5. Soil Solution Collection
2.6. Arsenic Speciation in Soils
2.6.1. Speciation Modeling Using MINEQL+
2.6.2. X-ray Absorption Near-Edge Structure (XANES) Analysis
2.7. Statistical Analysis
3. Results and Discussion
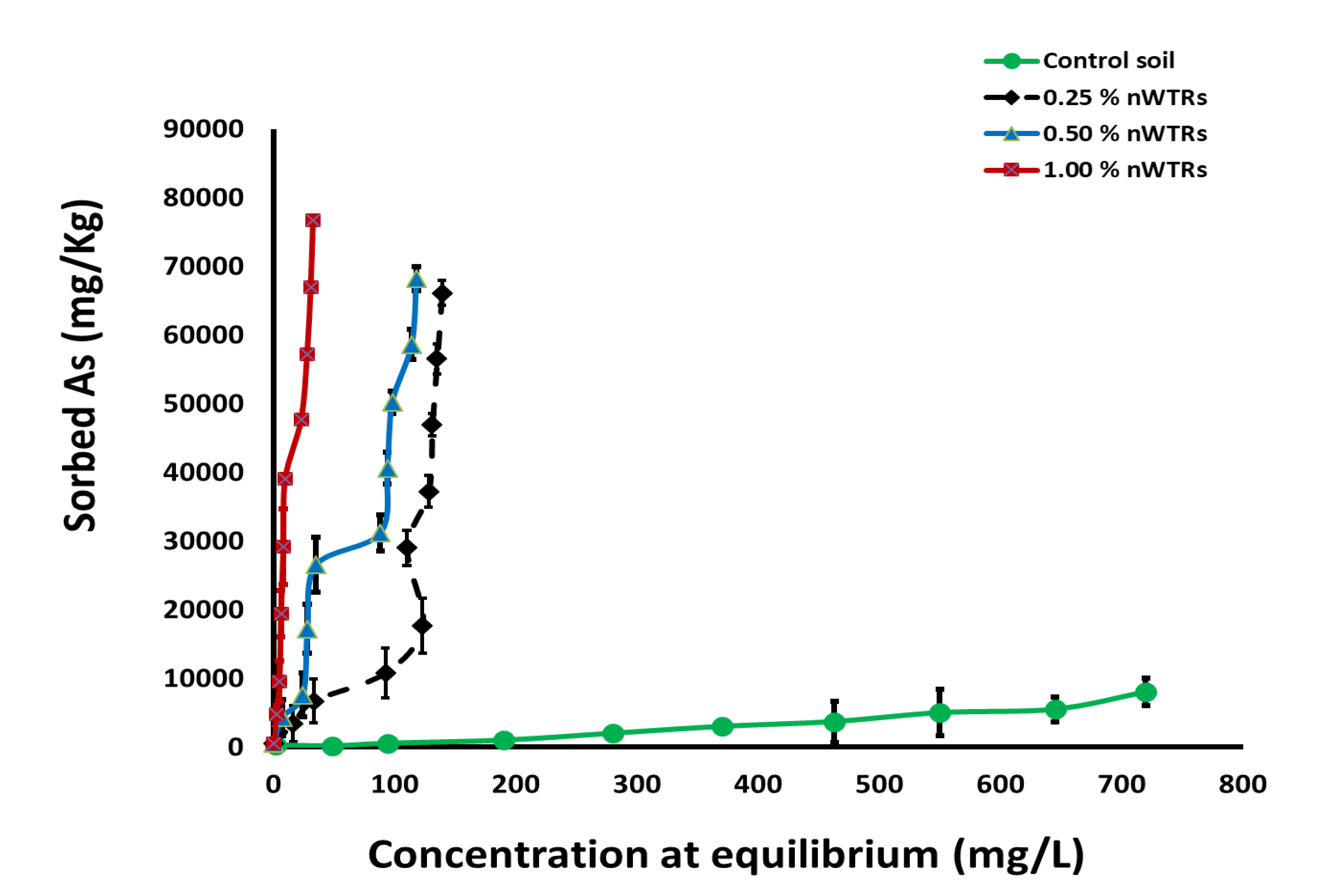
3.1. Sorption Isotherms and Sorption Efficiency of Arsenic
3.2. Investigation of As Removal Mechanism
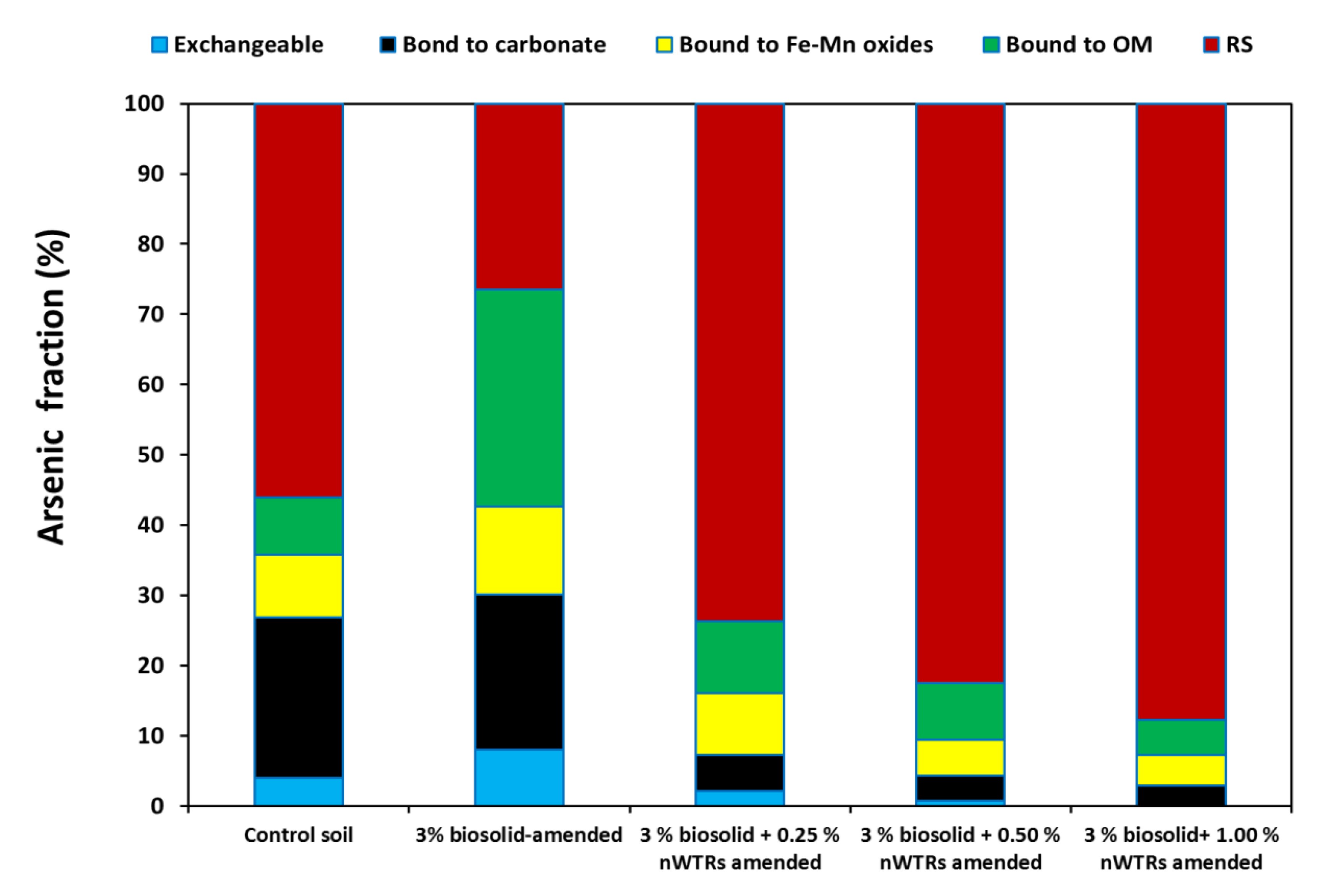
3.3. Effect of nWTRs on As Fractionation in Biosolids-Amended Soil
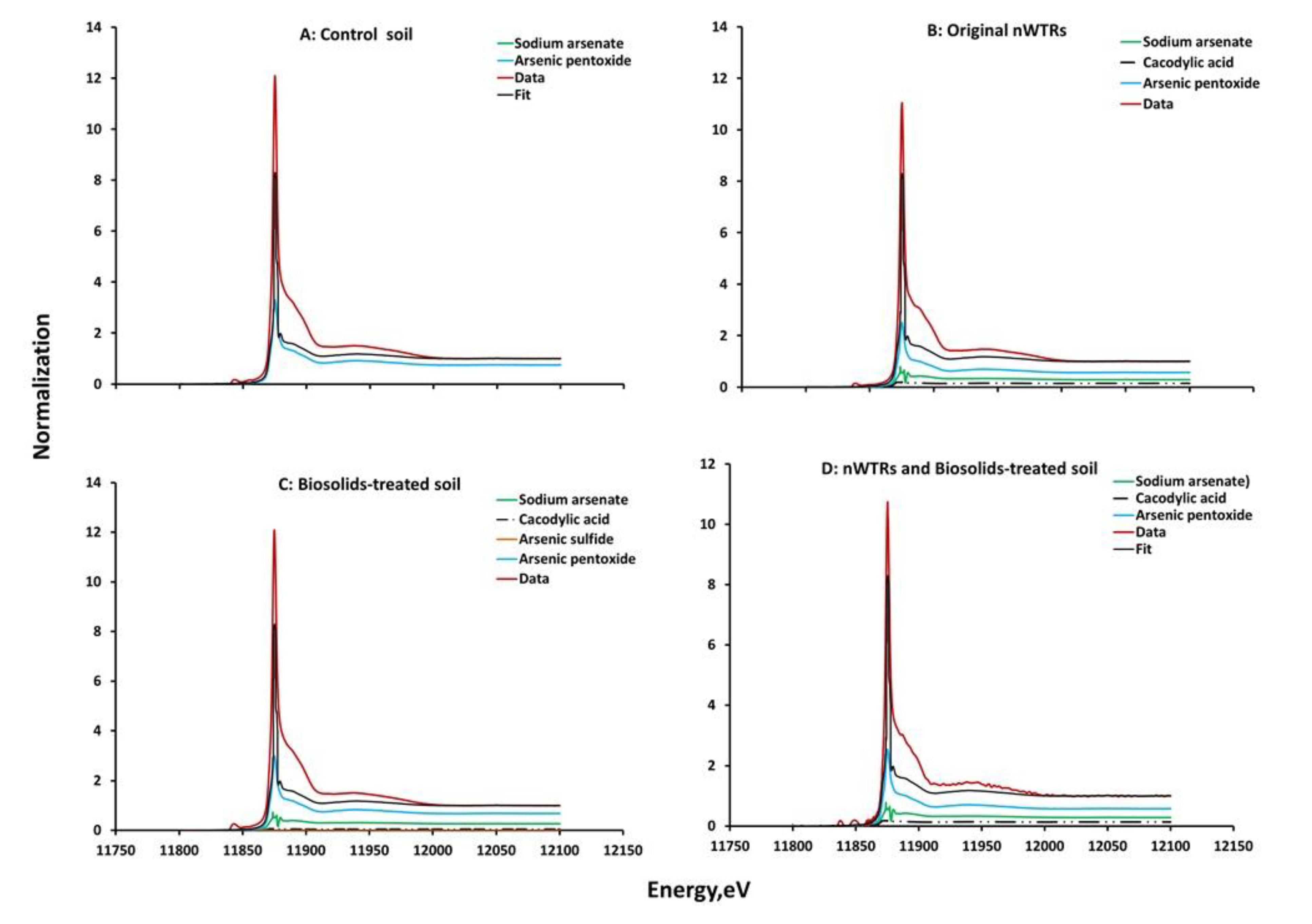
3.4. Effect of nWTRs on As Speciation in Biosolids-Amended Soil
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- SE EPA. Riskvärdering av Förorenad Mark–Etiska och Ekonomiska Perspektiv; Rapport 5539; Naturvårdsverket: Kumla, Sweden, 2006. [Google Scholar]
- Xu, Y.-H.; Nakajima, T.; Ohki, A. Adsorption and removal of arsenic (V) from drinking water by aluminum-loaded Shirasu-zeolite. J. Hazard. Mater. 2002, 92, 275–287. [Google Scholar] [CrossRef]
- Zhang, Y.; Yang, M.; Huang, X. Arsenic (V) removal with a Ce (IV)-doped iron oxide adsorbent. Chemosphere 2003, 51, 945–952. [Google Scholar] [CrossRef]
- Košutić, K.; Furač, L.; Sipos, L.; Kunst, B. Removal of arsenic and pesticides from drinking water by nanofiltration membranes. Sep. Purif. Technol. 2005, 42, 137–144. [Google Scholar] [CrossRef]
- Konjunktur Institutet. Remediation of Sites Contaminated by Arsenic; Brief Paper; National Institute of Economic Research: Stockholm, Sweden, 2008. [Google Scholar]
- Savage, N.; Diallo, M.S. Nanomaterials and water purification: Opportunities and challenges. J. Nanoparticle Res. 2005, 7, 331–342. [Google Scholar] [CrossRef]
- Schwarzenbach, R.P.; I Escher, B.; Fenner, K.; Hofstetter, T.B.; Johnson, C.A.; Von Gunten, U.; Wehrli, B. The challenge of micropollutants in aquatic systems. Science 2006, 313, 1072–1077. [Google Scholar] [CrossRef]
- Siddiqui, S.I.; Ravi, R.; Rathi, G.; Tara, N.; Ul-Islam, S.; Chaudhry, S.A. Decolorization of textile wastewater using composite materials. In Nanomaterials in the Wet Processing of Textiles; Wiley: New York NY, USA, 2018; pp. 187–218. [Google Scholar]
- Muñoz, M.J.L.; Arencibia, A.; Segura, Y.; Raez, J.M. Removal of As (III) from aque-ous solutions through simultaneous photocatalytic oxidation and adsorption byTiO2 and zero-valent iron. Catal. Today 2017, 280, 149–154. [Google Scholar] [CrossRef]
- Phanthasri, J.; Khamdahsag, P.; Jutaporn, P.; Sorachoti, K.; Wantala, K.; Tanboonchuy, V. Enhancement of arseniteremoval using manganese oxide coupled with iron (III) trimesic. Appl. Surf. Sci. 2018, 427, 545–552. [Google Scholar] [CrossRef]
- Lafferty, B.J.; Loeppert, R.H. Methyl arsenic adsorption and desorption behav-ior on iron oxides. Environ. Sci. Technol. 2005, 39, 2120–2127. [Google Scholar] [CrossRef] [PubMed]
- Porter, S.K.; Scheckel, K.; Impellitteri, C.A.; Ryan, J.A. Toxic metals in the environment: Thermodynamic considerations for possible immobilization strategies for Pb, Cd, As, and Hg. Crit. Rev. Environ. Sci. Technol. 2004, 34, 495–604. [Google Scholar] [CrossRef]
- Wang, S.; Mulligan, C.N. Natural attenuation processes for remediation of arsenic contaminated soils and groundwater. J. Hazard. Mater. 2006, 138, 459–470. [Google Scholar] [CrossRef]
- Henke, K.R.; Hutchison, A. Arsenic—Environmental Chemistry, Health Threats and Waste Treatment; Arsenic Chemistry Chapter 2; Wiley: Hoboken, NJ, USA, 2009; ISBN 978-0470-027585. [Google Scholar]
- Parker, D.R.; Chaney, R.L.; Norvell, W.A.; Loeppert, R.H.; Schwab, A.P.; Goldberg, S. Chemical equilibrium models: Applications to plant nutrition research. In Chemical Equilibrium and Reaction Models; the American Society of Agronomy and the Soil Science Society of America: San Antonio TX, USA, 2015; pp. 163–200. [Google Scholar] [CrossRef]
- Bennett, G.F. Environmental chemistry of arsenic. J. Hazard. Mater. 2002, 92, 213–215. [Google Scholar] [CrossRef]
- Al-Abed, S.R.; Jegadeesan, G.B.; Purandare, J.; Allen, D. Arsenic release from iron rich mineral processing waste: Influence of pH and redox potential. Chemosphere 2007, 66, 775–782. [Google Scholar] [CrossRef] [PubMed]
- Fendorf, S.; Nico, P.S.; Kocar, B.D.; Masue, Y.; Tufano, K.J. Arsenic chemistry in soils and sediments. In Developments in Soil Science; Elsevier: Amsterdam, The Netherlands, 2010; pp. 357–378. [Google Scholar]
- Garg, A.; Visht, S.; Sharma, P.K.; Kumar, N. Formulation, characterization and application on nanoparticle: A review. Der Pharm. Sin. 2011, 2, 17–26. [Google Scholar]
- Guo, D.; Xie, G.; Luo, J. Mechanical properties of NPs: Basics and applications. J. Phys. D Appl. Phys. 2014, 47, 013001–013026. [Google Scholar] [CrossRef]
- Prabhakar, R.; Samadder, S.R. Low cost and easy synthesis of aluminium oxideNPs for arsenite removal from groundwater: A complete batch study. J. Mol. Liq. 2018, 250, 192–201. [Google Scholar] [CrossRef]
- Elkhatib, E.A.; Mahdy, A.M.; Salama, K.A. Green synthesis of nanoparticles by milling residues of water treatment. Environ. Chem. Lett. 2015, 13, 333–339. [Google Scholar] [CrossRef]
- Mahdy, A.M.; Elkhatib, E.A.; Fathi, N.O.; Lin, Z.-Q. Effects of co-application of biosolids and water treatment residuals on corn growth and bioavailable phosphorus and aluminum in alkaline soils in Egypt. J. Environ. Qual. 2009, 38, 1501–1510. [Google Scholar] [CrossRef]
- Elkhatib, E.; Mahdy, A.; Sherif, F.; Hamadeen, H. Evaluation of a novel water treatment residual nanoparticles as a sorbent for arsenic removal. J. Nanomater. 2015, 2015, 1–10. [Google Scholar] [CrossRef]
- Caporale, A.G.; Punamiya, P.; Pigna, M.; Violante, A.; Sarkar, D. Effect of particle size of drinking-water treatment residuals on the sorption of arsenic in the presence of competing ions. J. Hazard. Mater. 2013, 260, 644–651. [Google Scholar] [CrossRef]
- Makris, K.C.; Harris, W.G. Time dependency and irreversibility of water desorption by drinking-water treatment residuals: Implications for sorption mechanisms. J. Colloid Interface Sci. 2006, 294, 151–154. [Google Scholar] [CrossRef]
- Tan, K.H. Soil Sampling, Preparation, and Analysis, 2nd ed.; Informa UK Limited: London, UK, 2005. [Google Scholar]
- Tessier, A.; Campbell, P.G.C.; Bisson, M. Sequential extraction procedure for the speciation of particulate trace metals. Anal. Chem. 1979, 51, 844–851. [Google Scholar] [CrossRef]
- Elkhatib, E.A.; Hern, J.L.; Staley, T.E. A Rapid Centrifugation Method for Obtaining Soil Solution. Soil Sci. Soc. Am. J. 1987, 51, 578–583. [Google Scholar] [CrossRef]
- Schecher, W.D.; McAvoy, D.C. MINEQL+, v 4.6: A Chemical Equilibrium Modeling System. Environmental Research Software. Available online: https://pcwin.com/Business___Finance/Applications/MINEQL_/index.htm (accessed on 13 April 2012).
- Webb, S.M. SIXPack a graphical user interface for XAS analysis using IFEFFIT. Phys. Scr. 2005, T115, 1011. [Google Scholar] [CrossRef]
- Costat Software Program. For the Design and Analysis of Agronomic Research Experiments, Cohort. Software, Costat 3-30; Costat: Berkeley, CA, USA, 2005.
- Saravanane, R.; Sundararajan, T.; Reddy, S.S. Efficiency of chemically modified low cost adsorbents for the removal of heavy metals from waste water: A comparative study. Indian J. Environ. Health 2002, 44, 78–87. [Google Scholar] [PubMed]
- Bohn, H.; McNeal, B.; O’connor, G. Soil Chemistry. Soil Sci. 1980, 129, 389. [Google Scholar] [CrossRef]
- Mahmoud, E.; El Baroudy, A.; Ali, N.; Sleem, M. Soil amendment with nanoresidues from water treatment increases P adsorption in saline soils. Environ. Chem. Lett. 2019, 18, 171–179. [Google Scholar] [CrossRef]
- Makris, K.C.; Harris, W.G.; O’Conno, G.A.; Obreza, T.A. Phosphorus immobilization in micropores of drinking-water treatment residuals: Implications for long-term stability. Environ. Sci. Technol. 2004, 38, 6590–6596. [Google Scholar] [CrossRef]
- Elkhatib, E.A.; Moharem, M.; Mahdy, A.; Mesalem, M. Sorption, release and forms of mercury in contaminated soils stabilized with water treatment residual nanoparticles. Land Degrad. Dev. 2016, 28, 752–761. [Google Scholar] [CrossRef]
- Moharem, M.L.; Elkhatib, E.A.; Elgammal, M.H. Reducing copper availability in contaminated soils using drinking water treatment residuals. Soil Sediment Contam. Int. J. 2013, 22, 595–613. [Google Scholar] [CrossRef]
- Elkhatib, E.A.; Moharem, M.L. Immobilization of copper, lead, and nickel in two arid soils amended with biosolids: Effect of drinking water treatment residuals. J. Soils Sediments 2015, 15, 1937–1946. [Google Scholar] [CrossRef]
- Moharem, M.; Elkhatib, E.A.; Mesalem, M. Remediation of chromium and mercury polluted calcareous soils using nanoparticles: Sorption—desorption kinetics, speciation and fractionation. Environ. Res. 2019, 170, 366–373. [Google Scholar] [CrossRef] [PubMed]
- Choi, N.C.; Cho, K.H.; Kim, M.S.; Park, S.J.; Lee, C.G. A Hybrid Ion-Exchange Fabric/Ceramic Membrane System to Remove As (V), Zn (II), and Turbidity from Wastewater. Appl. Sci. 2020, 10, 2414. [Google Scholar] [CrossRef]
- Hwang, S.; Her, Y.G.; Jun, S.M.; Song, J.-H.; Lee, G.; Kang, M. Characteristics of Arsenic Leached from Sediments: Agricultural Implications of Abandoned Mines. Appl. Sci. 2019, 9, 4628. [Google Scholar] [CrossRef]
- Wang, Y.; Jiao, J.J.; Zhu, S.; Li, Y. Arsenic K-edge X-ray absorption near-edge spectroscopy to determine oxidation states of arsenic of a coastal aquifer–aquitard system. Environ. Pollut. 2013, 179, 160–166. [Google Scholar] [CrossRef]

| Soil | nWTRs | Biosolids | |
|---|---|---|---|
| pH | 8.32 ± 0.06 | 7.66 ± 0.09 | 6.38 ± 0.05 |
| Electrical conductivity (EC) (dS/m) | 2.13 ± 0.04 | 1.12 ± 0.03 | 8.33 ± 0.17 |
| Organic Matter (g/kg) | 2.60 ± 0.18 | 48.00 ± 2.33 | 410.00 ± 3.77 |
| Cation Exchange Capacity (CEC) (cmol(+)/kg) | 21.00 ± 1.07 | 39.45 ± 1.38 | 77.77 ± 2.58 |
| Sand (g/kg) | 738.00 ± 3.70 | N/A † | N/A |
| Silt (g/kg) | 106.40 ± 1.90 | N/A | N/A |
| Clay (g/kg) | 155.60 ± 3.20 | N/A | N/A |
| Soil Texture | Sandy loam | N/A | N/A |
| CaCO3 (g/kg) | 326.20 ± 3.69 | N/A | N/A |
| KCl-Al (mg/kg) | 0.12 ± 0.03 | 18.12 ± 1.07 | 3.82 ± 0.14 |
| Olsen-P (mg/kg) | 15.30 ± 0.50 | 20.00 ± 2.11 | 44.40 ± 1.74 |
| Total As (mg/kg) | 1.23 ± 0.11 | 1.55 ± 0.13 | 6.22 ± 0.58 |
| Exchangeable As (mg/kg) | 0.07 ± 0.01 | 0.06 ± 0.01 | 0.16 ± 0.06 |
| Soluble As (mg/kg) | 0.04 ± 0.01 | 0.03 ± 0.01 | 0.09 ± 0.02 |
| Wavenumber Range, cm−1 | The FTIR Spectral of nWTRs/Biosolids-Treated Soil | |||
|---|---|---|---|---|
| Before As-Saturation | After As-Saturation | Difference | Functional Groups | |
| 3411–3200 | 3411 | 3412 | 1 | C-H methyl and methylene group |
| 3000–2921 | 2921 | 2921 | 0 | Bounded hydroxyl group (OH) |
| 1737–1625 | 1622 | 1621 | −1 | C-H stretching |
| 1508–1330 | 1508 | 1508 | 0 | Symmetric binding of CH3 |
| 1508–1330 | 1462 | 1462 | 0 | Carboxylic group |
| 1508–1330 | 1320 | 1320 | 0 | |
| 1330–1375 | 1374 | Absent | - | C-O stretching of COOH |
| 1290–1000 | 1246 | 1245 | −1 | O-H alcohol and aliphatic ether |
| 1290–1000 | 1053 | 1055 | 2 | C-O stretching of COOH |
| 800–600 | 780 | Absent | - | stretching vibration of NH2 |
| 800–600 | 608 | 609 | 1 | stretching vibration of NH2 |
| As Compounds | Control Soil | Biosolids (3%) | nWTRs (%) | ||
|---|---|---|---|---|---|
| 0.25 | 0.5 | 1 | |||
| AsO33− | 1.13 | 1.33 | 1.19 | 2.28 | 1.05 |
| HAsO32− | 1.22 | 2.23 | 1.27 | 2.27 | 0.57 |
| H3AsO3 | 94.11 | 80.02 | 36.04 | 19.59 | 13.98 |
| H2AsO3+ | 1.09 | 1.33 | 1.45 | 1.14 | 1.06 |
| H4AsO5+ | 1.12 | 2.2 | 1.21 | 1.57 | 1 |
| As(OH)5 (amorphous) | 45 | 56.04 | 59.45 | ||
| Cacodylic Acid | 1.33 | 9.66 | 10.58 | 12.22 | 17.55 |
| Arsenolite (As2O3) | 1.37 | 0.75 | 1.68 | 1.37 | |
| Claudetite (As2O3) | 1.42 | 0.85 | 1.1 | 1.3 | |
| di-arsenic pentoxide (As2O5) | 0.55 | 1.66 | 2.11 | 2.67 | |
| As Compounds | Control Soil | Original nWTRs | Biosolids-Treated Soil | Biosolids- and nWTR-Treated Soil |
|---|---|---|---|---|
| Sodium arsenate (HAsNa2O4) | 74.77 | 28.80 | 22.42 | 10.00 |
| Cacodylic acid [(CH3)2As2O2H] | 14.50 | 14.88 | 22.00 | |
| Arsenic pentoxide (As2 O5) | 25.23 | 56.70 | 61.48 | 68.00 |
| Arsenic sulfide (As2 S2) | 1.22 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mahdy, A.M.; Elkhatib, E.; Zhang, T.; Fathi, N.O.; Lin, Z.-Q. Nano-Scale Drinking Water Treatment Residuals Affect Arsenic Fractionation and Speciation in Biosolids-Amended Agricultural Soil. Appl. Sci. 2020, 10, 5633. https://doi.org/10.3390/app10165633
Mahdy AM, Elkhatib E, Zhang T, Fathi NO, Lin Z-Q. Nano-Scale Drinking Water Treatment Residuals Affect Arsenic Fractionation and Speciation in Biosolids-Amended Agricultural Soil. Applied Sciences. 2020; 10(16):5633. https://doi.org/10.3390/app10165633
Chicago/Turabian StyleMahdy, Ahmed M., Elsayed Elkhatib, Tiequan Zhang, Nieven O. Fathi, and Zhi-Qing Lin. 2020. "Nano-Scale Drinking Water Treatment Residuals Affect Arsenic Fractionation and Speciation in Biosolids-Amended Agricultural Soil" Applied Sciences 10, no. 16: 5633. https://doi.org/10.3390/app10165633
APA StyleMahdy, A. M., Elkhatib, E., Zhang, T., Fathi, N. O., & Lin, Z.-Q. (2020). Nano-Scale Drinking Water Treatment Residuals Affect Arsenic Fractionation and Speciation in Biosolids-Amended Agricultural Soil. Applied Sciences, 10(16), 5633. https://doi.org/10.3390/app10165633





