Biodeteriogens Characterization and Molecular Analyses of Diverse Funeral Accessories from XVII Century
Abstract
1. Introduction
2. Materials and Methods
2.1. Sampling Strategy, Microbiological Cultivation, and Microscopic Observation
2.2. DNA Identification of Isolated Microorganisms
2.3. Hydrolytic Extracellular Enzyme Assays
2.4. Total Microbial Community DNA Extraction and PCR Amplifications
2.5. Library Preparation for Microbial Communities’ Analysis
2.6. Pooled Library Preparation and Flow-Cell Processing
2.7. Sequencing Performance and Evaluation
2.8. DNA Analysis of Plant Sprig and Stiff Material
2.9. Illumina Sequencing of Plant Sprig and Stiff Material DNA
3. Results
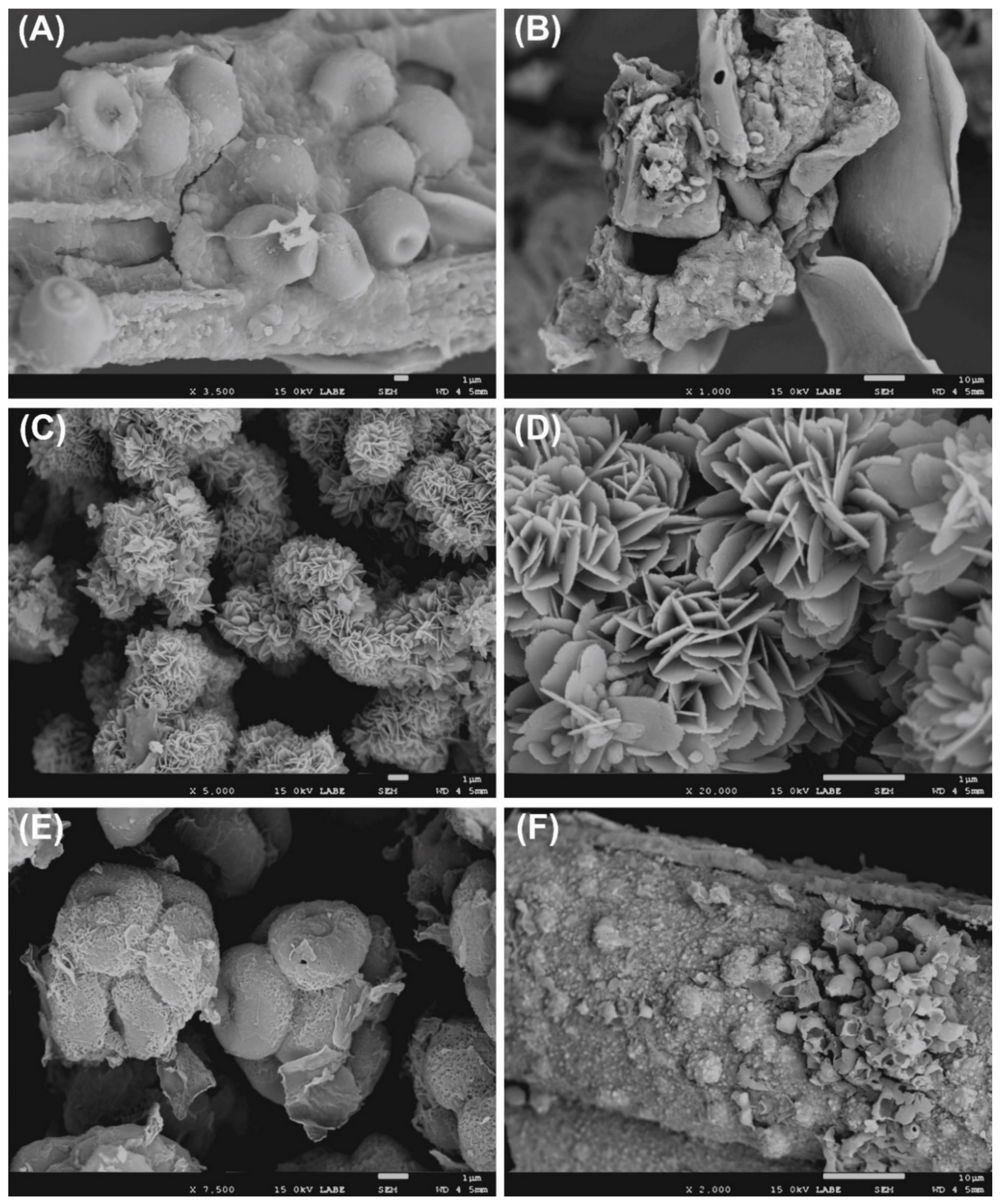
3.1. Microbial Isolates, Hydrolytic Properties, and SEM Observation
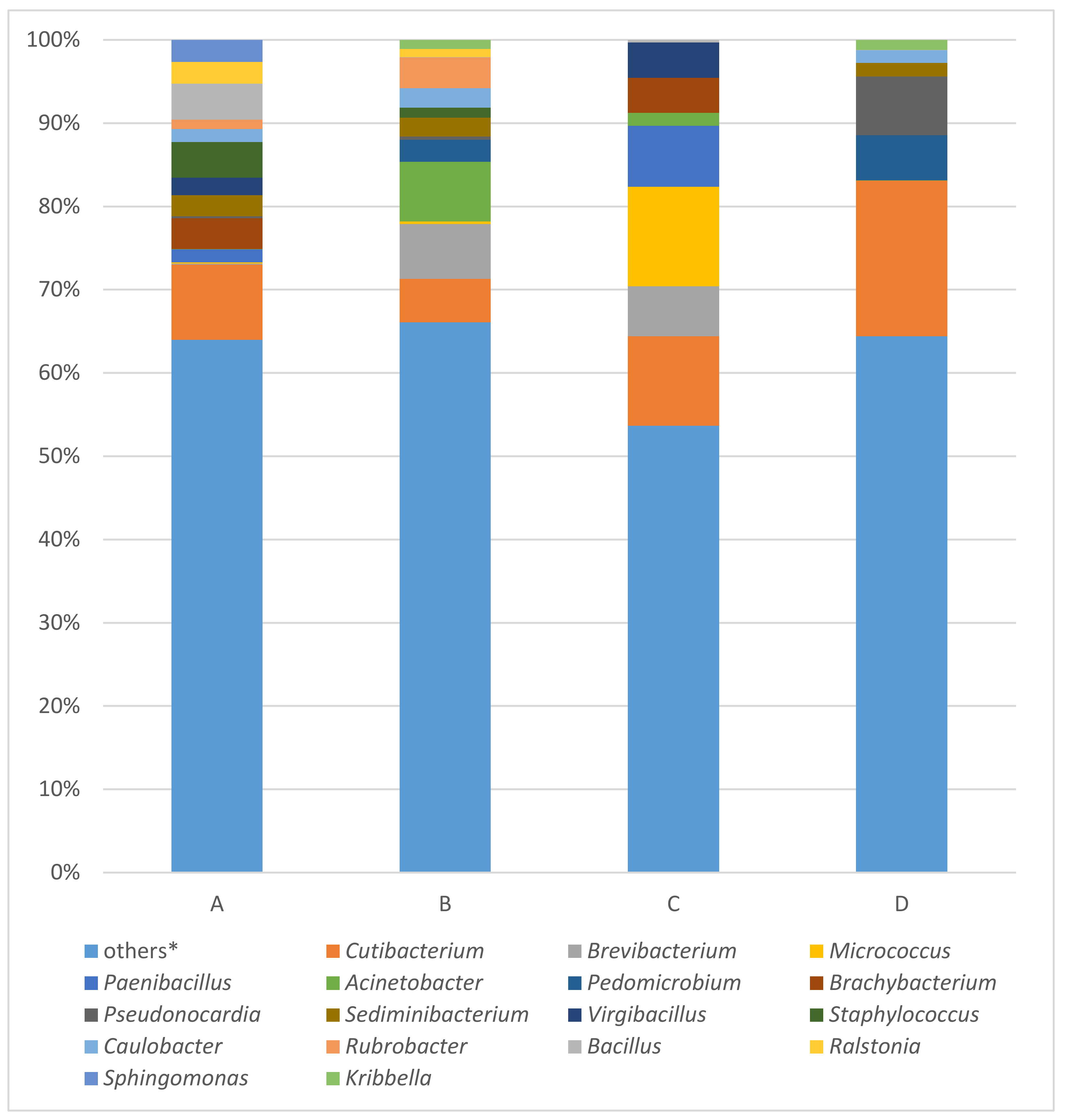
3.2. MinION Analysis of Bacterial 16S rRNA Gene
3.3. MinION Analysis of Fungal 28S rRNA Gene
3.4. MinION Analysis of Fungal ITS Fragment
3.5. DNA Analysis of Animal and Plant Samples
4. Discussion
4.1. Bacterial Community
4.2. Fungal Community
4.3. Microscopic Observation
4.4. Plant and Animal DNA Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Pangallo, D.; Kraková, L.; Chovanová, K.; Bučková, M.; Puškarová, A.; Šimonovičová, A. Disclosing a crypt: Microbial diversity and degradation activity of the microflora isolated from funeral clothes of Cardinal Peter Pázmány. Microbiol. Res. 2013, 168, 289–299. [Google Scholar] [CrossRef] [PubMed]
- Kraková, L.; Šoltys, K.; Puškárová, A.; Bučková, M.; Jeszeová, L.; Kucharík, M.; Budiš, J.; Orovčík, L.U.; Szemes, T.; Pangallo, D. The microbiomes of a XVIII century mummy from the castle of Krásna Hôrka (Slovakia) and its surrounding environment. Environ. Microbiol. 2018, 20, 3294–3308. [Google Scholar] [CrossRef] [PubMed]
- Sterflinger, K.; Piñar, G. Microbial deterioration of cultural heritage and works of art—tilting at windmills? Appl. Microbiol. Biotechnol. 2013, 97, 9637–9646. [Google Scholar] [CrossRef] [PubMed]
- Gutarowska, B.; Pietrzak, K.; Machnowski, W.; Milczarek, J.M. Historical textiles–A review of microbial deterioration analysis and disinfection methods. Text. Res. J. 2017, 87, 2388–2406. [Google Scholar] [CrossRef]
- Marvasi, M.; Cavalieri, D.; Mastromei, G.; Casaccia, A.; Perito, B. Omics technologies for an in-depth investigation of biodeterioration of cultural heritage. Int. Biodeterior. Biodegrad. 2019, 144, 104736. [Google Scholar] [CrossRef]
- Szostak-Kotowa, J. Biodeterioration of textiles. Int. Biodeterior. Biodegrad. 2004, 53, 165–170. [Google Scholar] [CrossRef]
- Forlani, G.; Seves, A.M.; Ciferri, O. A bacterial extracellular proteinase degrading silk fibroin. Int. Biodeterior. Biodegrad. 2000, 46, 271–275. [Google Scholar] [CrossRef]
- Brzozowska, I.; Bogdanowicz, A.; Szczęsny, P.; Zielenkiewicz, U.; Laudy, A. Evaluation of bacterial diversity on historical silk velvet textiles from the Museum of King John III’s Palace at Wilanów, Poland. Int. Biodeterior. Biodegrad. 2018, 131, 78–87. [Google Scholar] [CrossRef]
- Martins, C.; Pereira, C.S.; Plechkova, N.V.; Seddon, K.R.; Wang, J.; Whitfield, S.; Wong, W. Mycobiota of silk-faced ancient Mogao Grottoes manuscripts belonging to the Stein collection in the British library. Int. Biodeterior. Biodegrad. 2018, 134, 1–6. [Google Scholar] [CrossRef]
- MacHugh, D.E.; Larson, G.; Orlando, L. Taming the past: Ancient DNA and the study of animal domestication. Annu. Rev. Anim. Biosci. 2017, 5, 329–351. [Google Scholar] [CrossRef]
- Bieker, V.C.; Martin, M.D. Implications and future prospects for evolutionary analyses of DNA in historical herbarium collections. Bot. Lett. 2018, 165, 409–418. [Google Scholar] [CrossRef]
- Puškárová, A.; Bučková, M.; Habalová, B.; Kraková, L.; Maková, A.; Pangallo, D. Microbial communities affecting albumen photography heritage: A methodological survey. Sci. Rep. 2016, 6, 20810. [Google Scholar] [CrossRef] [PubMed]
- Grivalský, T.; Bučková, M.; Puškárová, A.; Kraková, L.; Pangallo, D. Water-related environments: A multistep procedure to assess the diversity and enzymatic properties of cultivable bacteria. World J. Microbiol. Biotechnol. 2016, 32, 42. [Google Scholar] [CrossRef] [PubMed]
- Lane, D.J. 16S/23S rRNA sequencing. In Nucleic Acid Techniques in Bacterial Systematics; Stackenbrandt, E., Goodfellow, M., Eds.; John Wiley & Sons: New York, NY, USA, 1991; pp. 115–148. [Google Scholar]
- White, T.J.; Bruns, T.; Lee, S.; Taylor, J. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In PCR Protocols: A Guide to Methods and Applications; Innis, M.A., Gelfand, D.H., Sninsky, J.J., White, T.J., Eds.; Academic Press: New York, NY, USA, 1990; pp. 315–321. [Google Scholar]
- Kurtzman, C.P.; Robnett, C.J. Identification and phylogeny of ascomycetous yeasts from analysis of nuclear large subunit (26S) ribosomal DNA partial sequences. Antonie Van Leeuwenhoek 1998, 73, 331–371. [Google Scholar] [CrossRef] [PubMed]
- Šoltys, K.; Planý, M.; Biocca, P.; Vianello, V.; Bučková, M.; Puškárová, A.; Sclocchi, M.C.; Colaizzi, P.; Bicchieri, M.; Pangallo, D.; et al. Lead soaps formation and biodiversity in a XVIII Century wax seal coloured with minium. Environ. Microbiol. 2020, 22, 1517–1534. [Google Scholar] [CrossRef] [PubMed]
- Taberlet, P.; Coissac, E.; Pompanon, F.; Gielly, L.; Miquel, C.; Valentini, A.; Vermat, T.; Corthier, G.; Brochmann, C.; Willerslev, E. Power and limitations of the chloroplast trn L (UAA) intron for plant DNA barcoding. Nucleic Acids Res. 2007, 35, e14. [Google Scholar] [CrossRef]
- Vilà, C.; Leonard, J.A.; Götherström, A.; Marklund, S.; Sandberg, K.; Lidén, K.; Wayne, R.K.; Ellegren, H. Widespread origins of domestic horse lineages. Science 2001, 291, 474–477. [Google Scholar] [CrossRef]
- Wawrzyk, A.; Gutarowska, B.; Rybitwa, D.; Pietrzak, K.; Machnowski, W.; Wrzosek, H.; Papis, A.; Walawska, A.; Otlewska, A.; Szulc, J.; et al. Vapourised hydrogen peroxide (VHP) and ethylene oxide (EtO) methods for disinfecting historical cotton textiles from the Auschwitz-Birkenau State Museum in Oświęcim, Poland. Int. Biodeterior. Biodegrad. 2008, 133, 42–51. [Google Scholar]
- Grady, E.N.; MacDonald, J.; Liu, L.; Richman, A.; Yuan, Z. Current knowledge and perspectives of Paenibacillus: A review. Microb. Cell Fact. 2016, 15, 203. [Google Scholar] [CrossRef]
- Piñar, G.; Ettenauer, J.; Sterflinger, K. La vie en rose: A review of rosy discoloration of subsurface monuments. Conserv. Subterr. Cult. Herit. 2014, 113–124. [Google Scholar]
- Pietrzak, K.; Puchalski, M.; Otlewska, A.; Wrzosek, H.; Guiamet, P.; Piotrowska, M.; Gutarowska, B. Microbial diversity of pre-Columbian archaeological textiles and the effect of silver nanoparticles misting disinfection. J. Cult. Herit. 2017, 23, 138–147. [Google Scholar] [CrossRef]
- Davis, C.P. Chapter 6 normal flora. In Medical Microbiology, 4th ed.; Baron, S., Ed.; University of Texas Medical Branch at Galveston: Galveston, TX, USA, 1996; ISBN -100-9631172-1-1. [Google Scholar]
- Scholz, C.F.; Kilian, M. The natural history of cutaneous propionibacteria, and reclassification of selected species within the genus Propionibacterium to the proposed novel genera Acidipropionibacterium gen. nov., Cutibacterium gen. nov. and Pseudopropionibacterium gen. nov. Int. J. Syst. Evol. Microbiol. 2016, 66, 4422–4432. [Google Scholar] [CrossRef] [PubMed]
- Dréno, B.; Pécastaings, S.; Corvec, S.; Veraldi, S.; Khammari, A.; Roques, C. Cutibacterium acnes (Propionibacterium acnes) and acne vulgaris: A brief look at the latest updates. J. Eur. Acad. Dermatol. Venereol. 2018, 2, 5–14. [Google Scholar] [CrossRef] [PubMed]
- Gharamti, A.A.; Kanafani, Z.A. Cutibacterium (formerly Propionibacterium) acnes infections associated with implantable devices. Expert Rev. Anti. Infect. Ther. 2017, 15, 1083–1094. [Google Scholar] [CrossRef]
- Hyde, E.R.; Haarmann, D.P.; Petrosino, J.F.; Lynne, A.M.; Bucheli, S.R. Initial insights into bacterial succession during human decomposition. Int. J. Legal. Med. 2014, 129, 661–671. [Google Scholar] [CrossRef]
- Philips, A.; Stolarek, I.; Kuczkowska, B.; Juras, A.; Handschuh, L.; Piontek, J.; Kozlowski, P.; Figlerowicz, M. Comprehensive analysis of microorganisms accompanying human archaeological remains. Gigascience 2017, 6, 1–13. [Google Scholar] [CrossRef]
- Nakaew, N.; Pathom-aree, W.; Lumyong, S. Generic diversity of rare actinomycetes from Thai cave soils and their possible use as new bioactive compounds. Actinomycetologica 2009, 23, 21–26. [Google Scholar] [CrossRef]
- De Leo, F.; Iero, A.; Zammit, G.; Urzı, C. Chemoorganotrophic bacteria isolated from biodeteriorated surfaces in cave and catacombs. Int. J. Speleol. 2012, 41, 125–136. [Google Scholar] [CrossRef]
- Diaz-Herraiz, M.; Jurado, V.; Cuezva, S.; Laiz, L.; Pallecchi, P.; Tiano, P.; Sanchez-Moral, S.; Saiz-Jimenez, C. The actinobacterial colonization of Etruscan paintings. Sci. Rep. 2013, 3, 1440. [Google Scholar] [CrossRef]
- Cirigliano, A.; Tomassetti, M.C.; Di Pietro, M.; Mura, F.; Maneschi, M.L.; Gentili, M.D.; Cardazzo, B.; Arrighi, C.; Mazzoni, C.; Negri, R.; et al. Calcite moonmilk of microbial origin in the Etruscan Tomba degli Scudi in Tarquinia, Italy. Sci. Rep. 2018, 8, 1–10. [Google Scholar] [CrossRef]
- Braun, B.; Richert, I.; Szewzyk, U. Detection of iron-depositing Pedomicrobium species in native biofilms from the Odertal National Park by a new, specific FISH probe. J. Microbiol. Methods 2009, 79, 37–43. [Google Scholar] [CrossRef]
- Zhu, Y.; Wang, H.; Li, X.; Hu, C.; Yang, M.; Qu, J. Characterization of biofilm and corrosion of cast iron pipes in drinking water distribution system with UV/Cl2 disinfection. Water Res. 2014, 60, 174–181. [Google Scholar] [PubMed]
- Lee, J.S.; Little, B.J. A mechanistic approach to understanding microbiologically influenced corrosion by metal-depositing bacteria. Corrosion 2019, 75, 6–11. [Google Scholar] [CrossRef]
- Männistö, M.K.; Häggblom, M.M. Characterization of psychrotolerant heterotrophic bacteria from Finnish Lapland. Syst. Appl. Microbiol. 2006, 29, 229–243. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.S.; Baik, K.S.; Park, S.C.; Rhee, M.S.; Oh, H.M.; Seong, C.N. Roseomonas frigidaquae sp. nov., isolated from a water-cooling system. Int. J. Syst. Evol. Microbiol. 2009, 59, 1630–1634. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Li, X.; Wang, H.; Zhang, Y.; Hu, C.; Yang, M. Characterization of the bacterial communities and iron corrosion scales in drinking groundwater distribution systems with chlorine/chloramine. Int. Biodeterior. Biodegrad. 2014, 96, 71–79. [Google Scholar] [CrossRef]
- Urzì, C.; De Leo, F.; Bruno, L.; Pangallo, D.; Krakova, L. New species description, biomineralization processes and biocleaning applications of Roman Catacombs-living bacteria. Conservation Subterr. Cult. Herit. 2014, 65–72. [Google Scholar]
- Heyrman, J.; Logan, N.A.; Busse, H.-J.; Balcaen, A.; Lebbe, L.; Rodriguez-Diaz, M.; Swings, J.; De Vos, P. Virgibacillus carmonensis sp. nov., Virgibacillus necropolis sp. nov. and Virgibacillus picturae sp. nov., three novel species isolated from deteriorated mural paintings, transfer of the species of the genus Salibacillus to Virgibacillus, as Virgibacillus marismortui comb. nov. and Virgibacillus salexigens comb. nov., and emended description of the genus Virgibacillus. Int. J. Syst. Evol. Microbiol. 2003, 53, 501–511. [Google Scholar]
- Kour, D.; Rana, K.L.; Kaur, T.; Singh, B.; Chauhan, V.S.; Kumar, A.; Rastegari, A.A.; Yadav, N.; Yadav, A.N.; Gupta, V.K. Extremophiles for hydrolytic enzymes productions: Biodiversity and potential biotechnological applications. Bioprocess. Biomol. Prod. 2019, 321–372. [Google Scholar]
- Batra, R.; Boekhout, T.; Guého, E.; Cabañes, F.J.; Dawson, T.L., Jr.; Gupta, A.K. Malassezia baillon, emerging clinical yeasts. FEMS Yeast Res. 2005, 5, 1101–1113. [Google Scholar] [CrossRef]
- Chouvenc, T.; Efstathion, C.A.; Elliott, M.L.; Su, N.-Y. Resource competition between two fungal parasites in subterranean termites. Naturwissenschaften 2012, 99, 949–958. [Google Scholar] [CrossRef]
- Martínez-Ramírez, J.A.; Strien, J.; Sanft, J.; Mall, G.; Walther, G.; Peters, F.T. Studies on drug metabolism by fungi colonizing decomposing human cadavers. Part I: DNA sequence-based identification of fungi isolated from postmortem material. Anal. Bioanal. Chem. 2013, 405, 8443–8450. [Google Scholar] [CrossRef]
- Šimonovičová, A.; Kraková, L.; Pangallo, D.; Majorošová, M.; Piecková, E.; Bodoriková, S.; Dörnhoferová, M. Fungi on mummified human remains and in the indoor air in the Kuffner family crypt in Sládkovičovo (Slovakia). Int. Biodeterior. Biodegrad. 2015, 99, 157–164. [Google Scholar] [CrossRef]
- Sterflinger, K. Fungi: Their role in deterioration of cultural heritage. Fungal Biol. Rev. 2010, 24, 47–55. [Google Scholar] [CrossRef]
- Strobel, G.; Ford, E.; Worapong, J.; Harper, J.K.; Arif, A.M.; Grant, D.M.; Fung, P.C.W.; Chau, R.M.W. Isopestacin, an isobenzofuranone from Pestalotiopsis microspora, possessing antifungal and antioxidant activities. Phytochemistry 2002, 60, 179–183. [Google Scholar] [CrossRef]
- Harper, J.K.; Arif, A.M.; Ford, E.J.; Strobel, G.A.; Porco, J.A., Jr.; Tomer, D.P.; Oneill, K.L.; Heider, E.M.; Grant, D.M. Pestacin: A 1,3-dihydro isobenzofuran from Pestalotiopsis microspora possessing antioxidant and antimycotic activities. Tetrahedron 2003, 59, 2471–2476. [Google Scholar] [CrossRef]
- Li, E.; Jiang, L.; Guo, L.; Zhang, H.; Che, Y. Pestalachlorides A–C, antifungal metabolites from the plant endophytic fungus Pestalotiopsis adusta. Bioorg. Med. Chem. 2008, 16, 7894–7899. [Google Scholar] [CrossRef]
- Piñar, G.; Poyntner, C.; Lopandic, K.; Tafer, H.; Sterflinger, K. Rapid diagnosis of biological colonization in cultural artefacts using the MinION nanopore sequencing technology. Int. Biodeterior. Biodegrad. 2020, 148, 104908. [Google Scholar] [CrossRef]
- Blanchette, R.A. A review of microbial deterioration found in archaeological wood from different environments. Int. Biodeterior. Biodegrad. 2000, 46, 189–204. [Google Scholar] [CrossRef]
- Brebbia, C.A. Structural Studies, Repairs and Maintenance of Heritage Architecture XI; Wessex Institute of Technology: Southampton, UK, 2009; ISBN 978-1-84564-196-2. [Google Scholar]
- Evidente, A.; Punzo, B.; Andolfi, A.; Cimmino, A.; Melck, D.; Luque, J. Lipophilic phytotoxins produced by Neofusicoccum parvum, a grapevine canker agent. Phytopathol. Mediterr. 2010, 49, 74–79. [Google Scholar]
- Williamson, B.; Tudzynski, B.; Tudzynsk, P.; Van Kan, J.A.L. Botrytis cinerea: The cause of grey mould disease. Mol. Plant Pathol. 2007, 8, 561–580. [Google Scholar] [CrossRef] [PubMed]
- Benito, S. The impact of Torulaspora delbrueckii yeast in winemaking. Appl. Microbiol. Biotechnol. 2018, 102, 3081–3094. [Google Scholar] [CrossRef] [PubMed]
- Zajc, J.; Kogej, T.; Galinski, E.A.; Ramos, J.; Gunde-Cimerman, N. Osmoadaptation strategy of the most halophilic fungus, Wallemia ichthyophaga, growing optimally at salinities above 15% NaCl. Appl. Environ. Microbiol. 2014, 80, 247–256. [Google Scholar] [CrossRef] [PubMed]
- Urbina, H.; Aime, M.C. A closer look at Sporidiobolales: Ubiquitous microbial community members of plant and food biospheres. Mycologia 2018, 110, 79–92. [Google Scholar] [CrossRef] [PubMed]
- Urzı, C.; De Leo, F. Sampling with adhesive tape strips: An easy and rapid method to monitor microbial colonization on monument surfaces. J. Microbiol. Methods 2001, 44, 1–11. [Google Scholar] [CrossRef]
- Hubka, V.; Kolařík, M.; Kubátová, A.; Peterson, S.W. Taxonomic revision of Eurotium and transfer of species to Aspergillus. Mycologia 2013, 105, 912–937. [Google Scholar] [CrossRef]
- Silva-Castro, G.A.; Uad, I.; Gonzalez-Martinez, A.; Rivadeneyra, A.; Gonzalez-Lopez, J.; Rivadeneyra, M.A. Bioprecipitation of calcium carbonate crystals by bacteria isolated from saline environments grown in culture media amended with seawater and real brine. BioMed Res. Int. 2015, 2015, 816102. [Google Scholar] [CrossRef]
- Dhami, N.K.; Mukherjee, A.; Watkin, E.L. Microbial diversity and mineralogical-mechanical properties of calcitic cave speleothems in natural and in vitro biomineralization conditions. Front. Microbiol. 2018, 9, 40. [Google Scholar] [CrossRef]
- Waugh, N. Corsets and Crinolines; Routledge: Abingdon-on-Thames, UK, 2017. [Google Scholar]
- Arnold, J.; Tiramani, J.; Costigliolo, L.; Passot, S.; Lucas, A.; Pietsch, J. Patterns of fashion 5: The Content, Cut, Construction and Context of Bodies, Stays, Hoops and Rumps c. 1595–1795; The School of Historical Dress: London, UK, 2018. [Google Scholar]
- Yang, H.X.; Chu, J.M.; Liu, X.S. Natural persistence of the coastal plant Glehnia littoralis along temperate sandy coasts. Sci. Rep. 2017, 7, 42784. [Google Scholar] [CrossRef]
- She, M.L.; Pu, F.T.; Pan, Z.H.; Watson, M.F.; Cannon, J.F.M.; Holmes-Smith, I.; Kljuykov, E.V.; Phillippe, L.R.; Pimenov, M.G. Apiaceae. In Flora of China Editorial Committee (ED), Flora of China; Missouri Botanical Garden Press: St. Louis, MO, USA, 2005; Volume 14, pp. 1–205. [Google Scholar]
- Sayed-Ahmad, B.; Talou, T.; Saad, Z.; Hijazi, A.; Merah, O. The apiaceae: Ethnomedicinal family as source for industrial uses. Ind. Crop. Prod. 2017, 109, 661–671. [Google Scholar] [CrossRef]
- Beneš, J.; Čulíková, V.; Kosňovská, J.; Frolík, J.; Matiášek, J. New plants at Prague Castle and Hradčany in the early modern period: A history of selected species. Interdiscip. Archaeol. 2012, 3, 103–114. [Google Scholar] [CrossRef]
- Tranberg, A. 10 burial customs in the northern Ostrobothnian region (Finland) from the late medieval period to the 20th Century. Plant remains in graves. In The Archaeology of Death in Post-Medieval Europe; De Gruyter: Berlin, Germany, 2015; pp. 189–203. [Google Scholar]
- Jarosińska, M.; Nowak, S.; Noryśkiewicz, A.M.; Badura, M. Plant identification and significance in funeral traditions exemplified by pillow filling from a child crypt burial in Byszewo (18th/19th centuries). Analecta Archaeol. Ressoviensia 2019, 14, 187–197. [Google Scholar] [CrossRef]


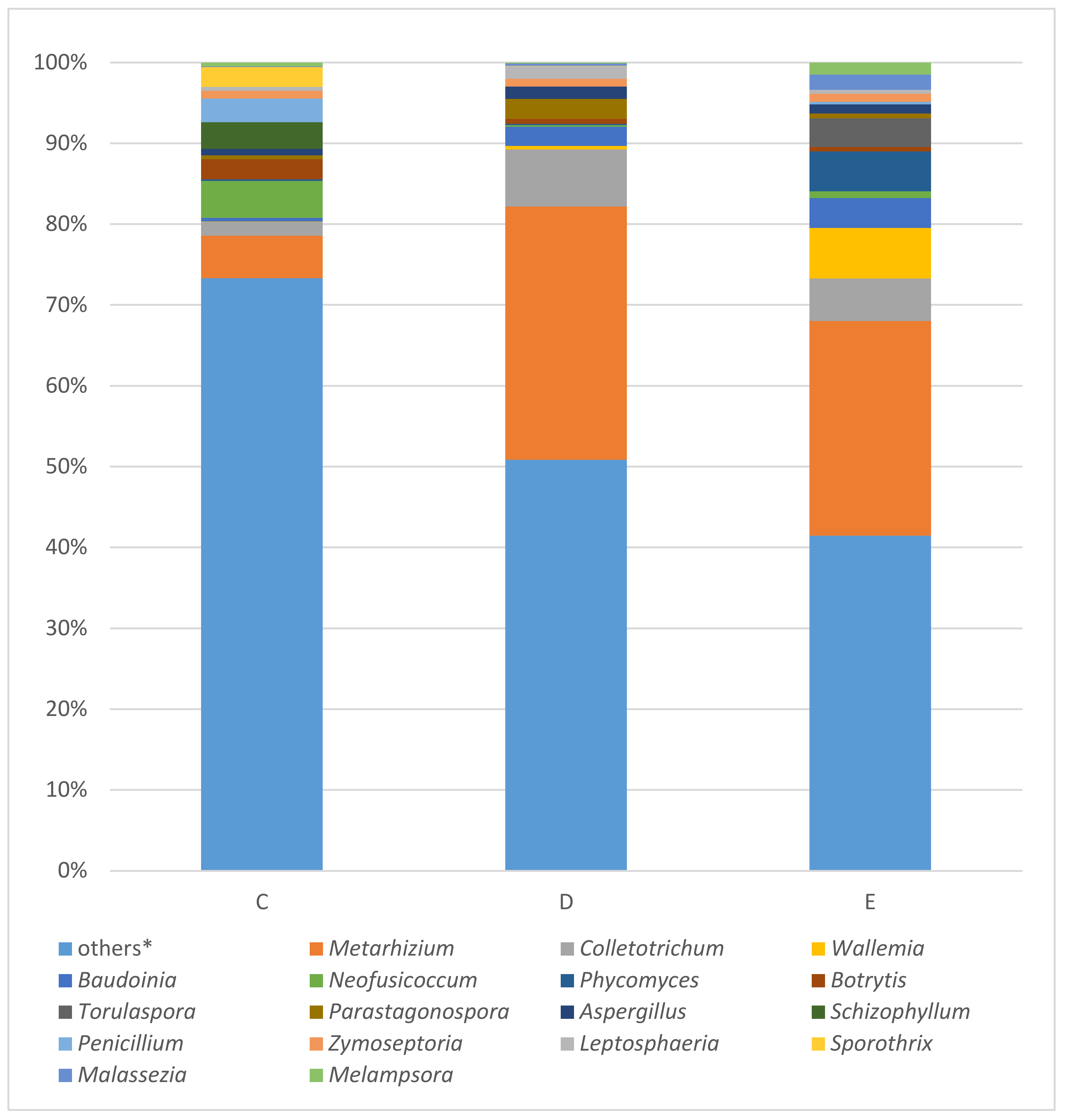
| Sample | Strain Code | Percent Identity | Keratinase Activity (mm) | Cellulase Activity (mm) | Proteinase 1 Activity (mm) | Proteinase 2 Activity (mm) | Fibroinase Activity (mm) |
|---|---|---|---|---|---|---|---|
| Bacteria | |||||||
| a, bodice | A1 | MT071578 Moraxella sp. 100% | 0 | 0 | 0 | 0 | 0 |
| A2 | CP024180 Moraxella osloensis 100% | 0 | 0 | 0 | 0 | 0 | |
| A3 | KY434635 Kocuria sp. 99.83% | 1 ± 0.3 | 1 ± 0.2 | 4 ± 0.6 | 2 ± 0.4 | 0 | |
| A4 | MT012185 Rhodococcus sp. 100% | 0 | 0 | 2 ± 0.3 | 0 | 4 ± 1 | |
| b, bodice | B1 | EF072311 Massilia sp. 99.22% | 7 ± 0.8 | 0 | 7 ± 1.2 | 4 ± 1.2 | 10 ± 1 |
| B2 | KY816357 Micrococcus yunnanensis 99.67% | 12 ± 0.5 | 2 ± 0.4 | 10 ± 1 | 15 ± 0.4 | 7 ± 0.6 | |
| d, bobbin lace cap | D1 | FJ999728 Paenibacillus illinoisensis 100% | 14 ± 0.2 | 0 | 5 ± 1 | 15 ± 1.5 | 20 ± 1 |
| D2 | KF815549 Sphingomonas sp. 100% | 5 ± 1 | 0 | 1 ± 0.6 | 4 ± 0.4 | 12 ± 1.3 | |
| D4 | MN396262 Roseomonas mucosa 99.61% | 2 ± 0.5 | 0 | 4 ± 0.8 | 1 ± 0.8 | 8 ± 0.6 | |
| e, coronet | E1 | HM480273 Staphylococcus sp. 99.85% | 0 | 0 | 1 ± 0.4 | 0 | 0 |
| E2 | KF424781 Paracoccus chinensis 99.14% | 0 | 0 | 0 | 0 | 0 | |
| E3 | MF804955 Paracoccus sp. 99.58% | 5 ± 0.7 | 0 | 3 ± 0.6 | 8 ± 0.8 | 2 ± 0.6 | |
| E4 | MN704104 Kocuria sp. 99.83% | 1 ± 0.6 | 1 ± 0.5 | 6 ± 1 | 0 | 9 ± 0.7 | |
| Fungi | |||||||
| b, bodice | B1-F | KY552625 Penicillium commune 99.37% | 4 ± 0.7 | 0 | 4 ± 1 | 4 ± 1.2 | 10 ± 1 |
| B2-F | KY105470 Sporidiobolus metaroseus 100% | 9 ± 0.4 | 2 ± 0.8 | 5 ± 1 | 10 ± 1 | 20 ± 1.5 | |
| c, bodice | C1-F | KP067214 Aspergillus versicolor 100% | 4 | 0 | 0 | 5 ± 0.6 | 10 ± 1 |
| C2-F | MN905799 Penicillium sp. 100% | 3 | 2 | 2 ± 0.7 | 5 ± 0.5 | 4 ± 0.5 | |
| C3-F | MT102669 Aspergillus sp. 100% | 0 | 0 | 0 | 0 | 12 ± 0.9 | |
| C4-F | MH393399 Penicillium granulatum 98.94% | 7 | 0 | 8 ± 1 | 5 ± 0.3 | 12 ± 1 | |
| C5-F | MH424607 Alternaria alternata 99.64% | 5 ± 0.3 | 2 ± 0.4 | 0 | 5 ± 0.4 | 9 ± 0.6 | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kisová, Z.; Planý, M.; Pavlović, J.; Bučková, M.; Puškárová, A.; Kraková, L.; Kapustová, M.; Pangallo, D.; Šoltys, K. Biodeteriogens Characterization and Molecular Analyses of Diverse Funeral Accessories from XVII Century. Appl. Sci. 2020, 10, 5451. https://doi.org/10.3390/app10165451
Kisová Z, Planý M, Pavlović J, Bučková M, Puškárová A, Kraková L, Kapustová M, Pangallo D, Šoltys K. Biodeteriogens Characterization and Molecular Analyses of Diverse Funeral Accessories from XVII Century. Applied Sciences. 2020; 10(16):5451. https://doi.org/10.3390/app10165451
Chicago/Turabian StyleKisová, Zuzana, Matej Planý, Jelena Pavlović, Mária Bučková, Andrea Puškárová, Lucia Kraková, Magdaléna Kapustová, Domenico Pangallo, and Katarína Šoltys. 2020. "Biodeteriogens Characterization and Molecular Analyses of Diverse Funeral Accessories from XVII Century" Applied Sciences 10, no. 16: 5451. https://doi.org/10.3390/app10165451
APA StyleKisová, Z., Planý, M., Pavlović, J., Bučková, M., Puškárová, A., Kraková, L., Kapustová, M., Pangallo, D., & Šoltys, K. (2020). Biodeteriogens Characterization and Molecular Analyses of Diverse Funeral Accessories from XVII Century. Applied Sciences, 10(16), 5451. https://doi.org/10.3390/app10165451






