Numerical 3D Modeling: Microwave Plasma Torch at Intermediate Pressure
Abstract
1. Introduction
2. Model Description
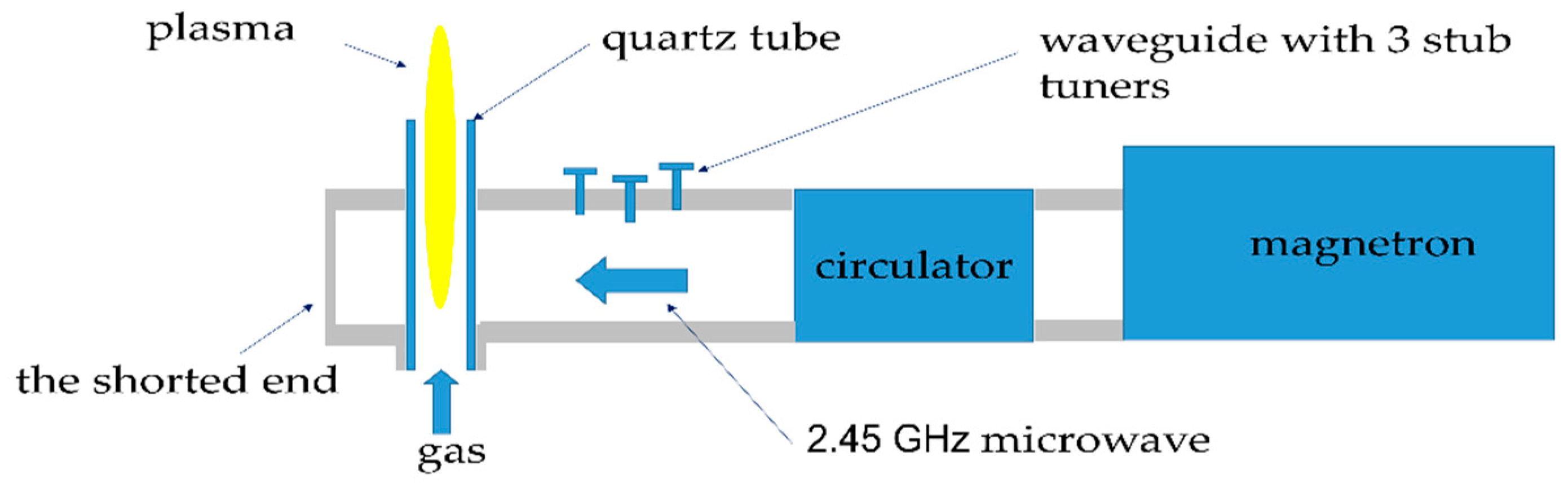
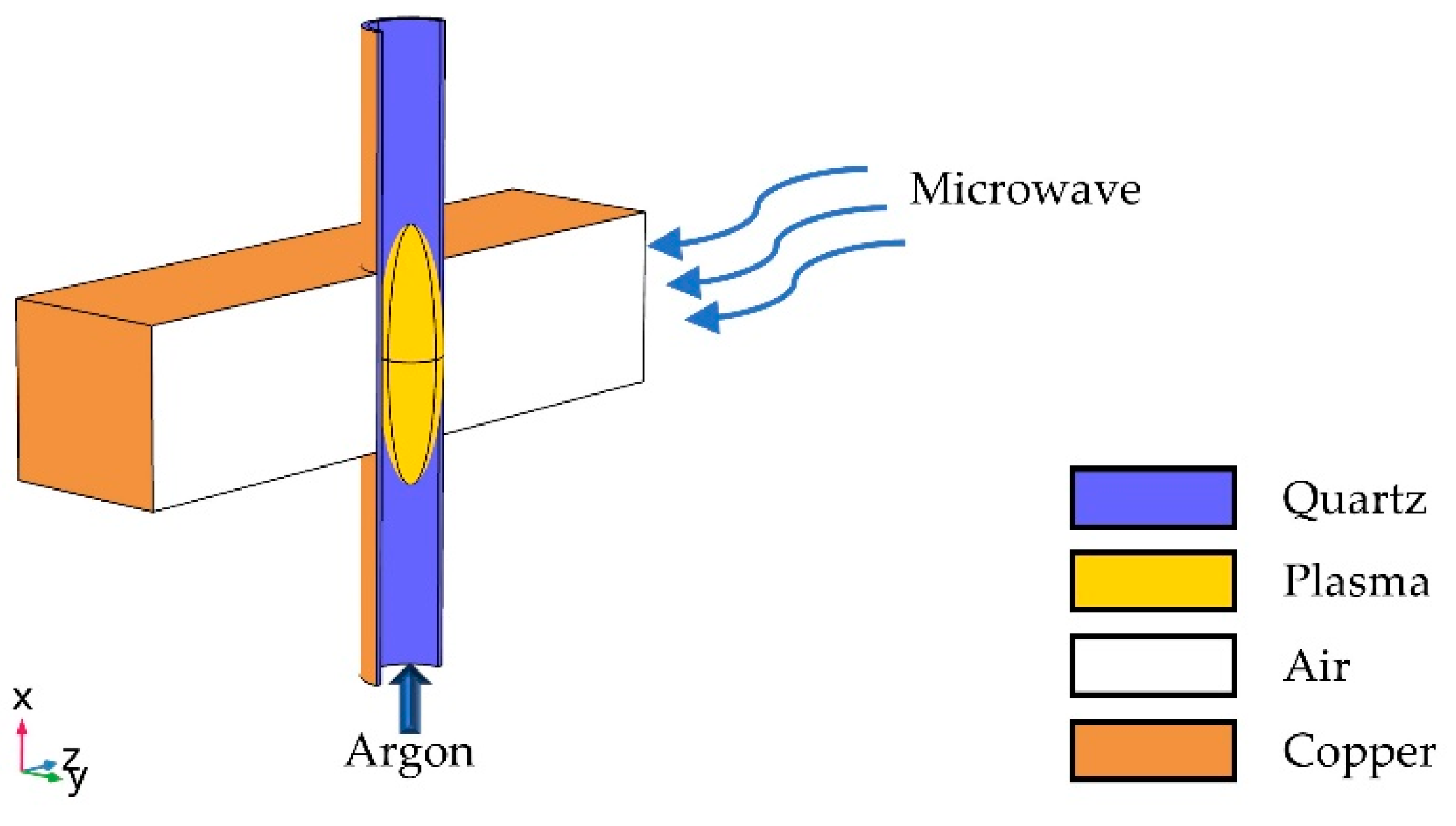
2.1. Microwave Plasma System
2.2. Plasma Chemistry
- (1)
- Elastic collision and inelastic collision: such reactions will lead to energy exchange in particles; the difference between them is that the elastic collision only leads to the transfer of kinetic energy between the colliding particles, but does not change internal energy.
- (2)
- Excitation and ionization: such reactions result in growth in the number of free electrons or a change in the energy level of an atom. The probability of their counter occurrence is related to the collision cross-section.
- (3)
- Charge transfer: this type of reaction results in the equivalent transfer of charge between particles. The kind of reaction mainly occurs in the collision process of ions and neutral particles.
- (4)
- Charge recombination: there are two forms—diffusion and recombination. Diffusion is the process by which the charged particle reaches the wall and electrode to disappear. Recombination is a process by which positive ions capture a free electron and combine with electrons or negative ions to form new neutral atoms.
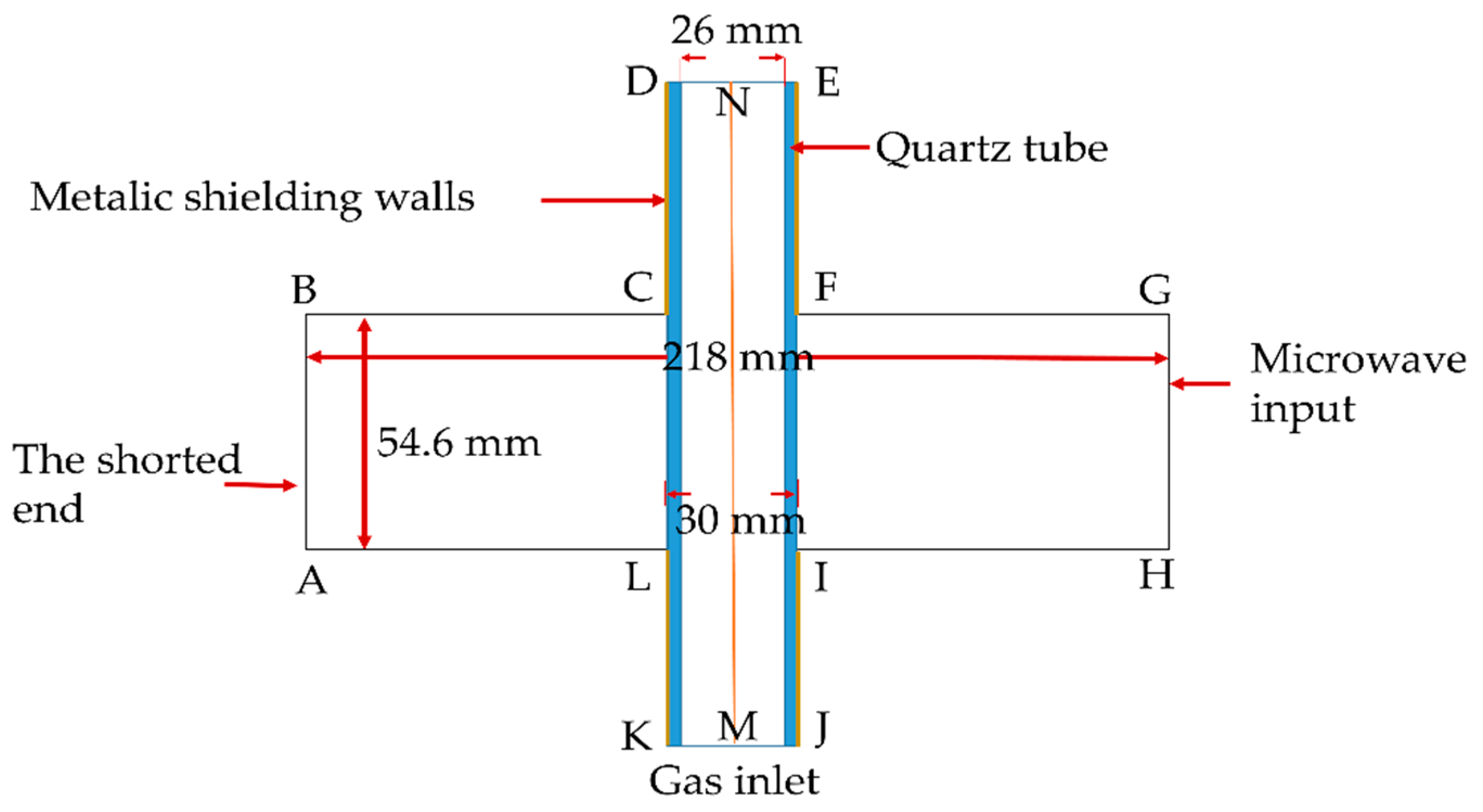
2.3. Boundary Condition
3. Governing Equations
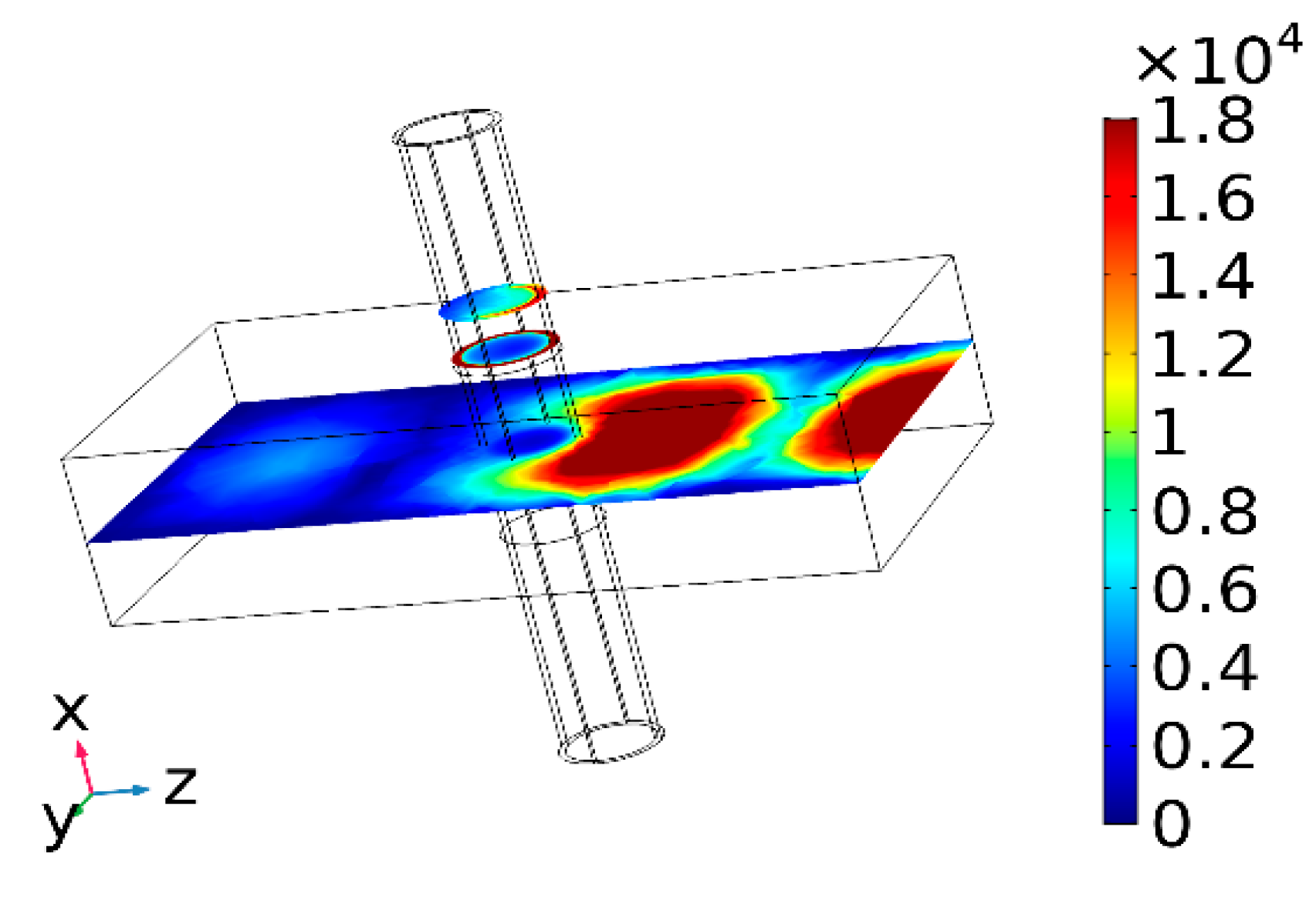
3.1. Wave Equation
3.2. Equations of the Plasma Model
3.3. Fluid Equation
3.4. Heat Transfer Equation
3.5. Ambipolar Diffusion
4. Results and Analysis
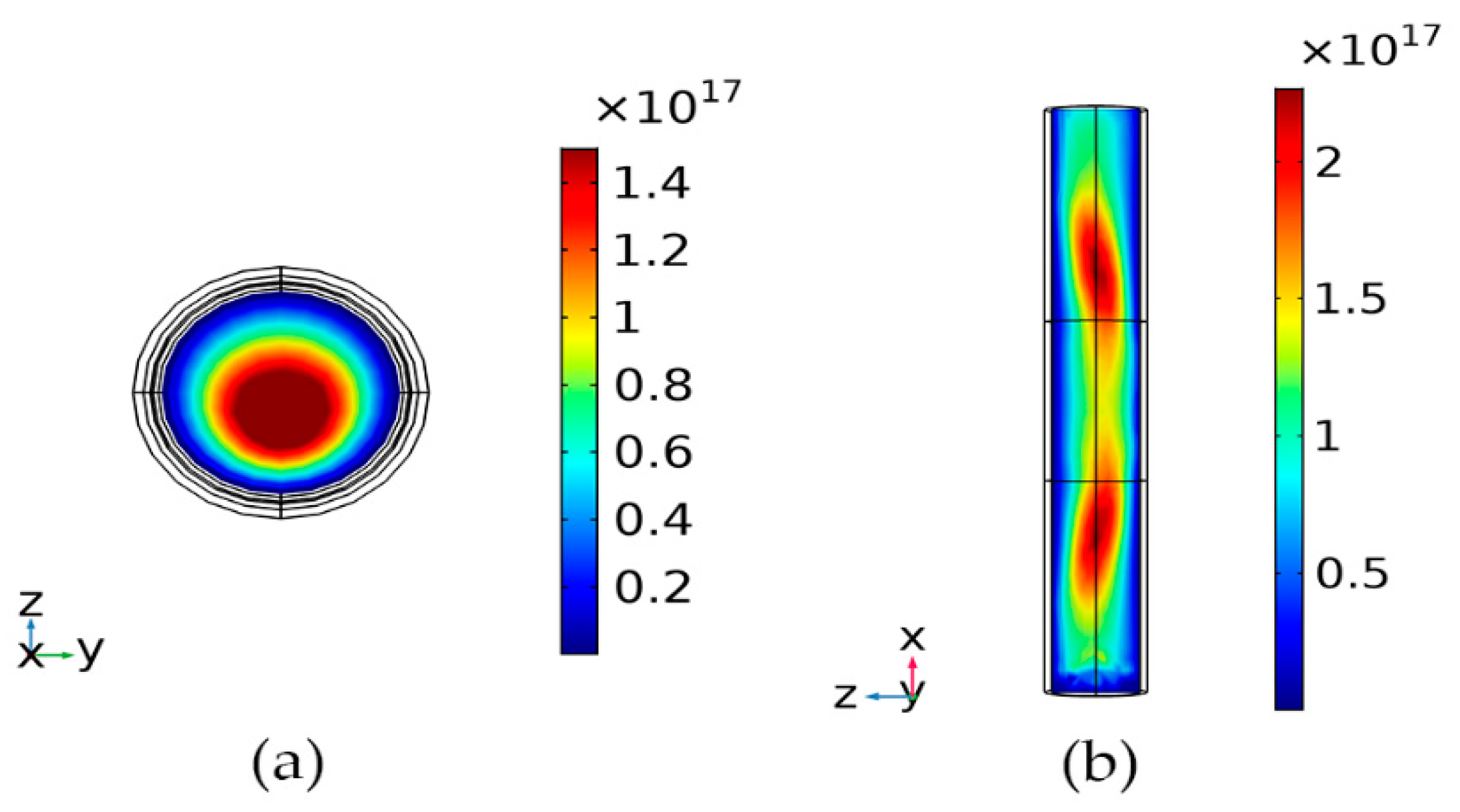
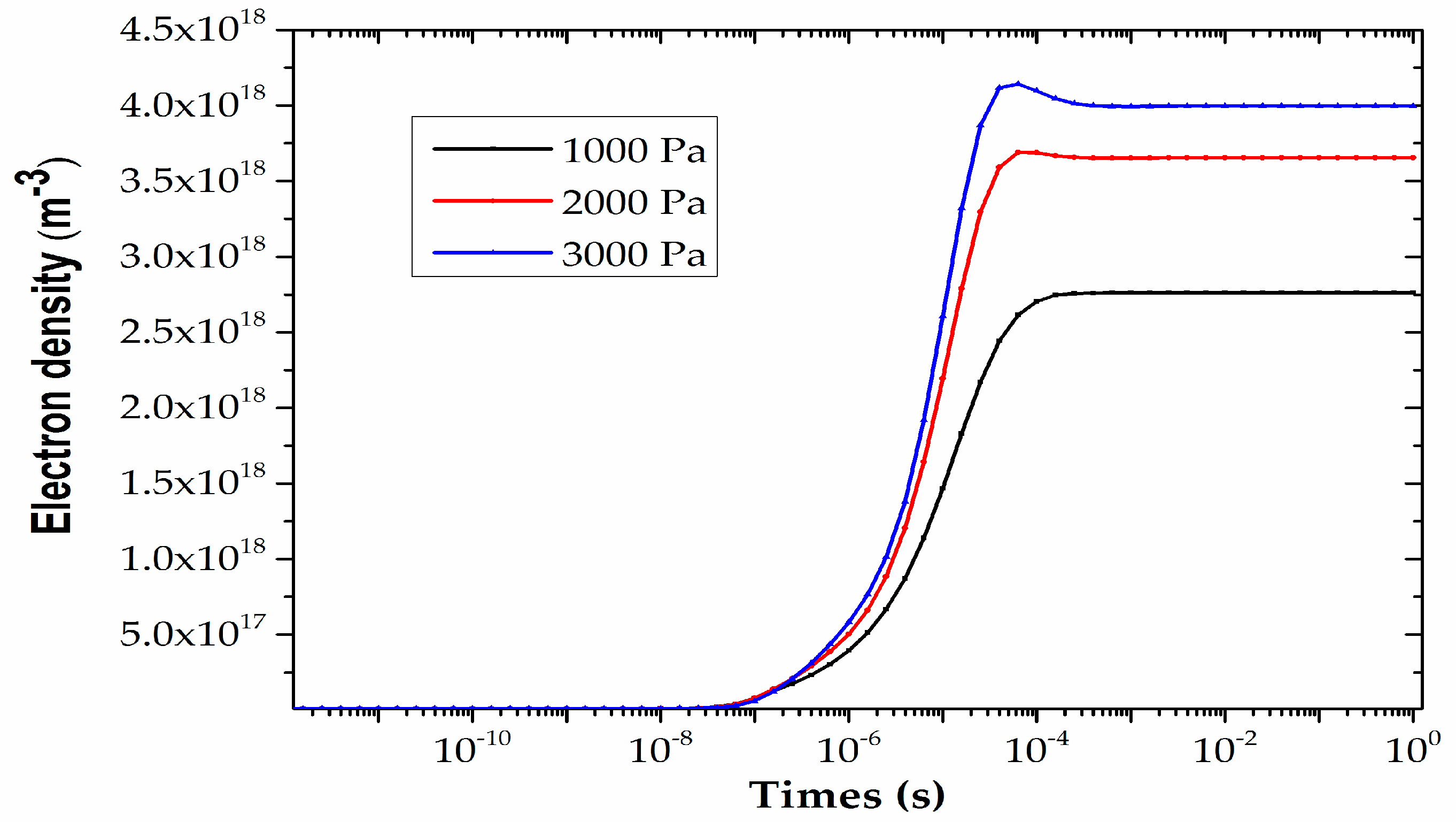
4.1. Analysis of Plasma Parameters at Different Times under the Same Pressure
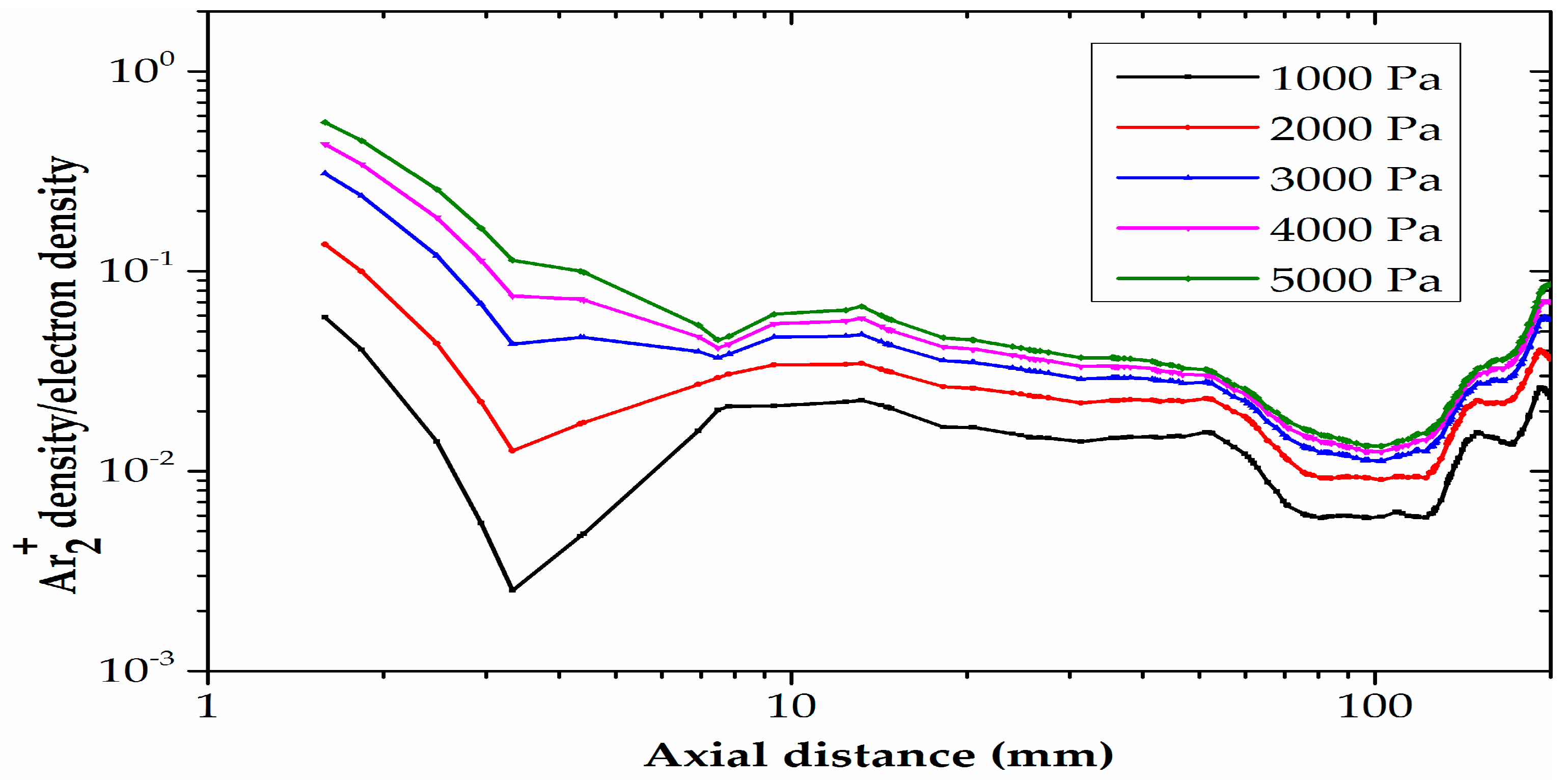
4.2. Analysis of the Effect of Different Pressures on Plasma Parameters
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hui, G.; Dongxue, H.; Li, X.; Ying, T.; Zhaobo, C.; Yimin, Z. Removal of low-concentration benzene in indoor air with plasma-MnO2 catalysis system. J. Electrost. 2015, 76, 216–221. [Google Scholar]
- Zhang, L.; Sha, X.; Zhang, L.; He, H.; Ma, Z.; Wang, L.; Wang, Y.; She, L. Synergistic catalytic removal NOX and the mechanism of plasma and hydrocarbon gas. AIP Adv. 2016, 6, 075015. [Google Scholar] [CrossRef]
- Ficek, M.; Sankaran, K.J.; Ryl, J.; Bogdanowicz, R.; Lin, I.; Haenen, K.; Darowicki, K. Ellipsometric investigation of nitrogen doped diamond thin films grown in microwave CH4/H2/N2 plasma enhanced chemical vapor deposition. Appl. Phys. Lett. 2016, 108, 241906. [Google Scholar] [CrossRef]
- Baranov, O.; Bazaka, K.; Kersten, H.; Keidar, M.; Cvelbar, U.; Xu, S.; Levchenko, I. Plasma under control: Advanced solutions and perspectives for plasma flux management in material treatment and nanosynthesis. Appl. Phys. Rev. 2017, 4, 041302. [Google Scholar] [CrossRef]
- Norberg, S.A.; Johnsen, E.; Kushner, M.J. Helium atmospheric pressure plasma jets touching dielectric and metal surfaces. J. Appl. Phys. 2015, 118, 013301. [Google Scholar] [CrossRef]
- Bai, B.; Li, X.; Xu, J.; Liu, Y. Reflections of electromagnetic waves obliquely incident on a multilayer stealth structure with plasma and radar absorbing material. IEEE Trans. Plasma Sci. 2015, 43, 2588–2597. [Google Scholar]
- Xu, J.; Bai, B.; Dong, C.; Dong, Y.; Zhu, Y.; Zhao, G. Evaluations of plasma stealth effectiveness based on the probability of radar detection. IEEE Trans. Plasma Sci. 2018, 45, 938–944. [Google Scholar] [CrossRef]
- Rincon, R.; Marinas, A.; Munoz, J.; Melero, C.; Calzada, M. Experimental research on ethanol-chemistry decomposition routes in a microwave plasma torch for hydrogen production. Chem. Eng. J. 2016, 284, 1117–1126. [Google Scholar] [CrossRef]
- Barankova, H.; Bardos, L. Atmospheric pressure plasma conversion of CO2 to solid deposits. Results Phys. 2015, 5, 257–258. [Google Scholar] [CrossRef]
- Qin, Y.; Niu, G.; Wang, X.; Luo, D.; Duan, Y. Status of CO2 conversion using microwave plasma. J. CO2 Util. 2018, 28, 283–291. [Google Scholar] [CrossRef]
- Javier, F.; Moreno, S.H.; Stankiewicz, A.I.; Stefanidis, G.D. On the improvement of chemical conversion in a surface-wave microwave plasma reactor for CO2 reduction with hydrogen (The Reverse Water-Gas Shift reaction). Int. J. Hydrogen Energy 2017, 42, 12943–12955. [Google Scholar]
- Bogaerts, A.; Berthelot, A.; Heijkers, S.; Kolev, S.; Snoeckx, R.; Sun, S.; Trenchev, G.; Van, L.K.; Wang, W. CO2 conversion by plasma technology: Insights from modeling the plasma chemistry and plasma reactor design. Plasma Sources Sci. Technol. 2017, 26, 063001. [Google Scholar] [CrossRef]
- Mohsenian, S.; Nagassou, D.; Elahi, R.; Yu, P.; Nallar, M.; Wong, H.; Trelles, J.P. Carbon dioxide conversion by solar-enhanced microwave plasma: Effect of specific power and argon/nitrogen carrier gases. J. CO2 Util. 2019, 35, 725–732. [Google Scholar] [CrossRef]
- Rutberg, P.G.; Kuznetsov, V.A.; Popov, V.E.; Popov, S.D.; Surov, A.V.; Subbotin, D.I.; Bratsev, A.N. Conversion of methane by CO2+ H2O+ CH4 plasma. Appl. Energy 2015, 148, 159–168. [Google Scholar] [CrossRef]
- Moreno, S.H.; Stankiewicz, A.I.; Stefanidis, G.D. A two-step modelling approach for plasma reactors–Experimental validation for CO2 dissociation in surface wave microwave plasma. React. Chem. Eng. 2019, 4, 1253–1269. [Google Scholar] [CrossRef]
- Stewig, C.; Schüttler, S.; Urbanietz, T.; Böke, M.; von Keudell, A. Excitation and dissociation of CO2 heavily diluted in noble gas atmospheric pressure plasma. J. Phys. D Appl. Phys. 2020, 13, 125205. [Google Scholar] [CrossRef]
- Fu, Y.; Krek, J.; Parsey, G.M.; Verboncoeur, J.P. Characterizing the dominant ions in low-temperature argon plasmas in the range of 1–800 Torr. Phys. Plasmas 2018, 25, 033505. [Google Scholar] [CrossRef]
- Arcese, E.; Rogier, F.; Boeuf, J. Plasma fluid modeling of microwave streamers: Approximations and accuracy. Phys. Plasmas 2017, 24, 113517. [Google Scholar] [CrossRef]
- Miotk, R.; Jasinski, M.; Mizeraczyk, J. Electromagnetic optimisation of a 2.45 GHz microwave plasma source operated at atmospheric pressure and designed for hydrogen production. Plasma Sources Sci. Technol. 2018, 27, 035011. [Google Scholar] [CrossRef]
- Gudmundsson, J.T.; Kawamura, E.; Lieberman, M.A. A benchmark study of a capacitively coupled oxygen discharge of the oopd1 particle-in-cell Monte Carlo code. Plasma Sources Sci. Technol. 2013, 22, 035011. [Google Scholar] [CrossRef]
- Lebedev, Y.A. Microwave discharges at low pressures and peculiarities of the processes in strongly non-uniform plasma. Plasma Sources Sci. Technol. 2015, 24, 053001. [Google Scholar] [CrossRef]
- Bernatskiy, A.; Kochetov, I.; Lagunov, V.; Ochkin, V. Electric fields and concentrations of charged and neutral hydrogen isotopic particles in the plasma of low pressure DC discharge. Phys. Plasmas 2019, 26, 083511. [Google Scholar] [CrossRef]
- Zhonghang, W.; Rongqing, L.; Masaaki, N.; Xijiang, C. The Characteristics of Columniform Surface Wave Plasma Excited Around a Quartz Rod by 2.45 GHz Microwaves. Plasma Sci. Technol. 2016, 18, 987. [Google Scholar]
- Xiao, W.; Huang, K.; Zhang, W.; Lin, Y. Modeling of argon plasma excited by microwave at atmospheric pressure in ridged waveguide. IEEE Trans. Plasma Sci. 2016, 44, 1075–1082. [Google Scholar] [CrossRef]
- Kabouzi, Y.; Graves, D.; Castanos-Martinez, E.; Moisan, M. Modeling of atmospheric-pressure plasma columns sustained by surface waves. Phys. Rev. E 2007, 75, 016402. [Google Scholar] [CrossRef] [PubMed]
- Kabouzi, Y.; Calzada, M.; Moisan, M.; Tran, K.; Trassy, C. Radial contraction of microwave-sustained plasma columns at atmospheric pressure. J. Appl. Phys. 2002, 91, 1008–1019. [Google Scholar] [CrossRef]
- Georgieva, V.; Berthelot, A.; Silva, T.; Kolev, S.; Graef, W.; Britun, N.; Chen, G.; Mullen, J.; Godfroid, T.; Mihailova, D. Understanding Microwave Surface-Wave Sustained Plasmas at Intermediate Pressure by 2D Modeling and Experiments. Plasma Process. Polym. 2017, 14, 1600185. [Google Scholar] [CrossRef]
- Bouherine, K.; Tibouche, A.; Ikhlef, N.; Leroy, O. 3-D numerical characterization of a microwave argon PECVD plasma reactor at low pressure. IEEE Trans. Plasma Sci. 2016, 44, 3409–3416. [Google Scholar] [CrossRef]
- Jimenez-Diaz, M.; Carbonem, E.A.D.; Dijk, J.; Mullen, J.J. A two-dimensional Plasimo multiphysics model for the plasma-electromagnetic interaction in surface wave discharge: The surfatron source. J. Phys. D Appl. Phys. 2012, 17, 335204. [Google Scholar] [CrossRef]
- Baeva, M.; Hempel, F.; Baierl, H.; Trautvetter, T.; Foest, R.; Loffhagen, D. Two-and three-dimensional simulation analysis of microwave excited plasma for deposition applications: Operation with argon at atmospheric pressure. J. Phys. D Appl. Phys. 2018, 51, 385202. [Google Scholar] [CrossRef]
- Bogdanov, T.; Tsonev, I.; Marinova, P.; Benova, E.; Rusanov, K.; Rusanova, M.; Atanassov, I.; Kozáková, Z.; Krčma, F. Microwave Plasma Torch Generated in Argon for Small Berries Surface Treatment. Appl. Sci. 2018, 8, 1870. [Google Scholar] [CrossRef]
- Lee, B.-J.; Jo, S.-I.; Jeong, G.-H. Synthesis of ZnO Nanomaterials Using Low-Cost Compressed Air as Microwave Plasma Gas at Atmospheric Pressure. Nanomaterials 2019, 9, 942. [Google Scholar] [CrossRef] [PubMed]
- Zhao, G.; Hua, W.; Guo, S.; Liu, Z. Three-dimensional simulation of microwave-induced helium plasma under atmospheric pressure. Phys. Plasmas 2016, 23, 073503. [Google Scholar] [CrossRef]
- Yang, Y.; Hua, W.; Guo, S. Numerical study on microwave-sustained argon discharge under atmospheric pressure. Phys. Plasmas 2014, 21, 040702. [Google Scholar] [CrossRef]
- Chen, G.; Georgieva, V.; Godfroid, T.; Snyders, R.; Delplancke-Ogletree, M. Plasma assisted catalytic decomposition of CO2. Appl. Catal. B Environ. 2016, 190, 115–124. [Google Scholar] [CrossRef]
- Jonkers, J.; Sande, M.; Sola, A.; Gamero, A.; Rodero, A.; Mullen, J. The role of molecular rare gas ions in plasmas operated at atmospheric pressure. Plasma Sources Sci. Technol. 2003, 12, 464. [Google Scholar] [CrossRef]
- Kaw, P.; Valeo, E.; Dawson, J.M. Interpretation of an experiment on the anomalous absorption of an electromagnetic wave in a plasma. Phys. Rev. Lett. 1970, 25, 430. [Google Scholar] [CrossRef]
- Baeva, M.; Bosel, A.; Ehlbeck, J.; Loffhagen, D. Modeling of microwave-induced plasma in argon at atmospheric pressure. Phys. Rev. E 2012, 85, 056404. [Google Scholar] [CrossRef]
- Naito, T.; Yamaura, S.; Fukuma, Y.; Sakai, O. Radiation characteristics of input power from surface wave sustained plasma antenna. Phys. Plasmas 2016, 23, 93504. [Google Scholar] [CrossRef]
- Zhang, W.; Wu, L.; Huang, K.; Tao, J. Propagating modes of the travelling wave in a microwave plasma torch with metallic enclosure. Phys. Plasmas 2019, 26, 042101. [Google Scholar] [CrossRef]
- Moisan, M.; Nowakowska, H. Contribution of surface-wave (SW) sustained plasma columns to the modeling of RF and microwave discharges with new insight into some of their features. A survey of other types of SW discharges. Plasma Sources Sci. Technol. 2018, 27, 073001. [Google Scholar] [CrossRef]
- Zhigang, L.; Zhongcai, Y.; Jiachun, W.; Jiaming, S. Simulation of propagation of the HPM in the low-pressure argon plasma. Plasma Sci. Technol. 2017, 20, 025401. [Google Scholar]
- Meindl, A.; Loehle, S.; Kistner, I.; Schulz, A.; Fasoulas, S. Two-Photon Induced Polarization Spectroscopy for Atomic Oxygen in Atmospheric Plasma and Xenon. In Proceedings of the AIAA Scitech 2019 Forum, San Diego, CA, USA, 7–11 January 2019. [Google Scholar]








| NO | Process | Reaction | Rate Coefficient (m3/s) | △E |
|---|---|---|---|---|
| 1 | Elastic scattering | |||
| 2 | Ground state excitation | 11.56 | ||
| 3 | Ground state ionization | 15.8 | ||
| 4 | Step-wise ionization | 4.24 | ||
| 5 | Superelastic collision | −11.56 | ||
| 6 | Metastable pooling | |||
| 7 | Two-body quenching | |||
| 8 | Three-body recombination | −15.76 | ||
| 9 | Dissociative recombination | |||
| 10 | Electron impact | |||
| 11 | Atomic ions conversion | |||
| 12 | Molecular ions dissociation atom impact | |||
| 13 | Diffusion |
| Physical Field | Plasma | ||||
|---|---|---|---|---|---|
| Boundary | |||||
| KJ | |||||
| DE | |||||
| DK, EJ | Surface Reactions | ||||
| Physical Field Boundary | Electromagnetic Wave | ||||
| GH | |||||
| BA, BC, FG, AL, IH, GH | |||||
| Physical Field Boundary | Laminar Flow | ||||
| KJ | |||||
| DE | |||||
| DK, EJ | |||||
| Physical Field Boundary | Heat Transfer in Fluids | ||||
| KJ | |||||
| DE | |||||
| DK, EJ | |||||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shen, Q.; Huang, R.; Xu, Z.; Hua, W. Numerical 3D Modeling: Microwave Plasma Torch at Intermediate Pressure. Appl. Sci. 2020, 10, 5393. https://doi.org/10.3390/app10155393
Shen Q, Huang R, Xu Z, Hua W. Numerical 3D Modeling: Microwave Plasma Torch at Intermediate Pressure. Applied Sciences. 2020; 10(15):5393. https://doi.org/10.3390/app10155393
Chicago/Turabian StyleShen, Qinghao, Run Huang, Zili Xu, and Wei Hua. 2020. "Numerical 3D Modeling: Microwave Plasma Torch at Intermediate Pressure" Applied Sciences 10, no. 15: 5393. https://doi.org/10.3390/app10155393
APA StyleShen, Q., Huang, R., Xu, Z., & Hua, W. (2020). Numerical 3D Modeling: Microwave Plasma Torch at Intermediate Pressure. Applied Sciences, 10(15), 5393. https://doi.org/10.3390/app10155393





