Selenium Deficiency—From Soil to Thyroid Cancer
Abstract
Featured Application
Abstract
1. Introduction
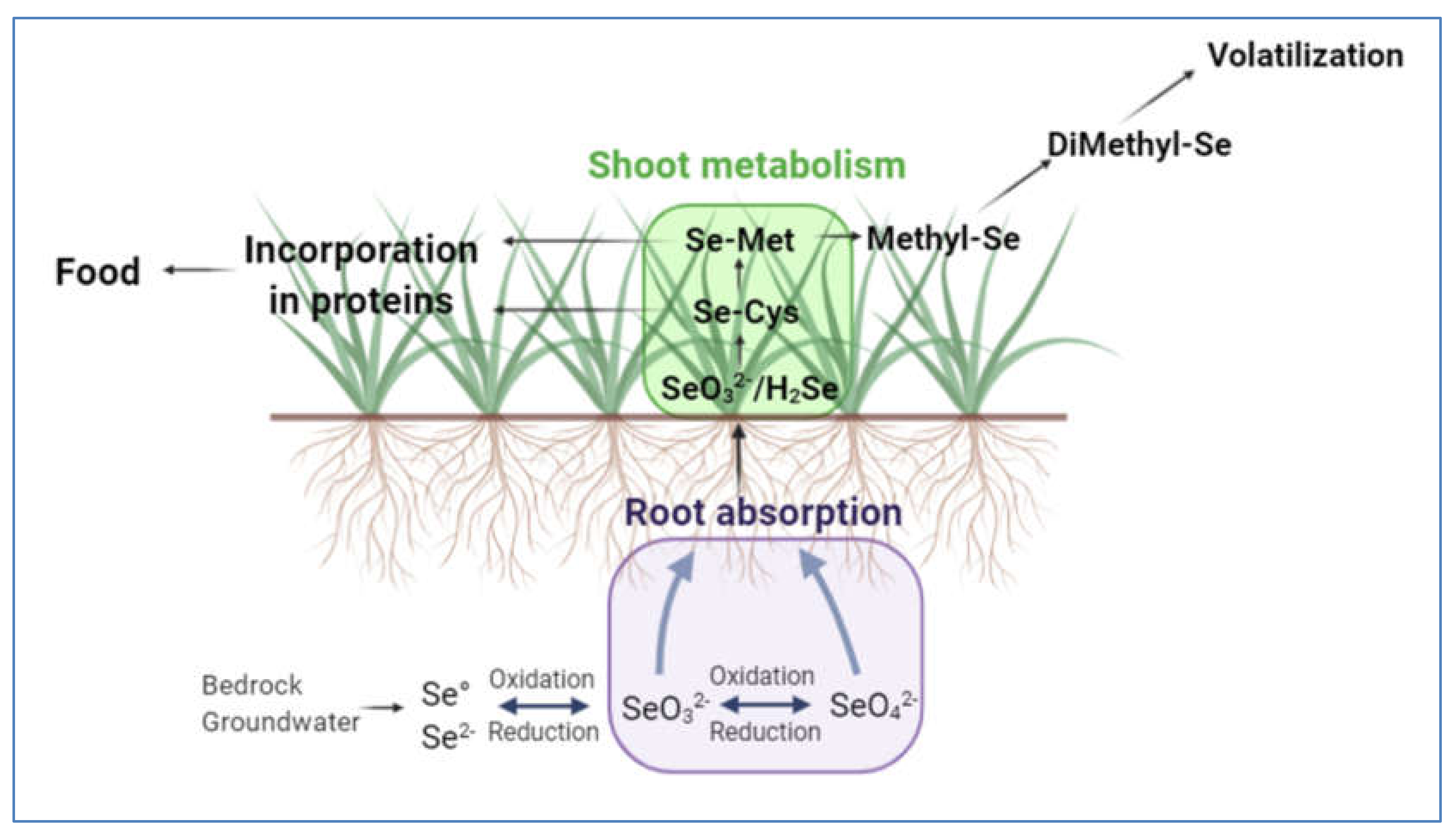
2. Selenium from Soil to Food Bowl
2.1. Selenium in Soil and Surface Waters
2.2. Transfer and Absorption of Se from Soil to Plant
2.3. Metabolism and Se Speciation in Plants
2.4. Selenium in Food and Dietary Intake
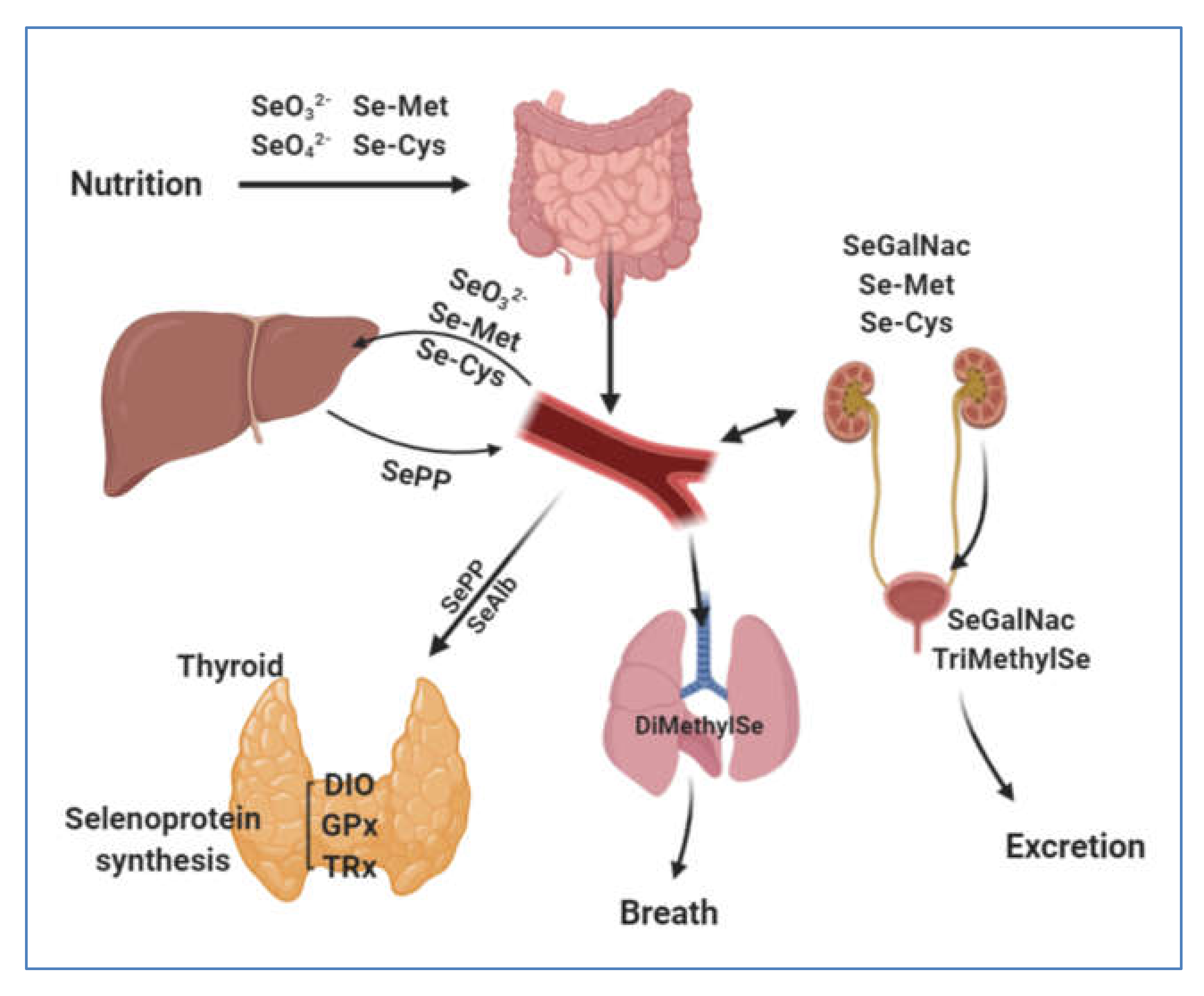
3. Selenium Human Uptake and Distribution
3.1. Gastrointestinal Uptake
3.2. Blood Transfer and Distribution of Se to Tissues via Selenoproteins P
4. Selenium in Thyroid Gland
4.1. Role of Selenoproteins in Thyroid Gland
4.2. Selenium and Thyroid Cancer
5. Conclusions
6. Future Perspectives
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Schrauzer, G.N.; Surai, P.F. Selenium in human and animal nutrition: Resolved and unresolved issues. A partly historical treatise in commemoration of the fiftieth anniversary of the discovery of the biological essentiality of selenium, dedicated to the memory of Klaus Schwarz (1914–1978) on the occasion of the thirtieth anniversary of his death. Crit. Rev. Biotechnol. 2009, 29, 2–9. [Google Scholar]
- Delbet, P. Tentatives de traitement de cancer par selenium. Bull. De l’Assoc. Francaise pour l’Etude de Cancer 1912, 5, 121–125. [Google Scholar]
- Nelson, A.A. Liver Tumors Following Cirrhosis Caused by Selenium in Rats. Cancer Res. 1943, 3, 230–236. [Google Scholar]
- Rayman, M.P. The importance of selenium to human health. Lancet 2000, 356, 233–241. [Google Scholar] [CrossRef]
- Rayman, M.P. Selenium and human health. Lancet 2012, 379, 1256–1268. [Google Scholar] [CrossRef]
- Kieliszek, M.; Błażejak, S. Current Knowledge on the Importance of Selenium in Food for Living Organisms: A Review. Molecules 2016, 21, 609. [Google Scholar] [CrossRef]
- Kato, T.; Read, R.; Rozga, J.; Burk, R.F. Evidence for intestinal release of absorbed selenium in a form with high hepatic extraction. Am. J. Physiol. Gastrointest. Liver Physiol. 1992, 262, G854–G858. [Google Scholar] [CrossRef]
- Duntas, L.H.; Benvenga, S. Selenium: An element for life. Endocrine 2015, 48, 756–775. [Google Scholar] [CrossRef] [PubMed]
- Köhrle, J. The trace element selenium and the thyroid gland. Biochimie 1999, 81, 527–533. [Google Scholar] [CrossRef]
- Mehl, S.; Sun, Q.; Görlich, C.L.; Hackler, J.; Kopp, J.F.; Renko, K.; Mittag, J.; Schwerdtle, T.; Schomburg, L. Cross-sectional analysis of trace element status in thyroid disease. J. Trace Elem. Med. Biol. 2020, 58, 126430. [Google Scholar] [CrossRef]
- Sakız, D.; Kaya, A.; Kulaksizoglu, M. Serum Selenium Levels in Euthyroid Nodular Thyroid Diseases. Biol. Trace Elem. Res. 2016, 174, 21–26. [Google Scholar] [CrossRef] [PubMed]
- Novotny, L.; Rauko, P.; Kombian, S.; Edafiogho, I. Selenium as a chemoprotective anti-cancer agent: Reality or wishful thinking? Neoplasma 2010, 57, 383–391. [Google Scholar] [CrossRef]
- Kristal, A.R.; Darke, A.K.; Morris, J.S.; Tangen, C.M.; Goodman, P.J.; Thompson, I.M.; Meyskens, F.L.; Goodman, G.E.; Minasian, L.M.; Parnes, H.L.; et al. Baseline Selenium Status and Effects of Selenium and Vitamin E Supplementation on Prostate Cancer Risk. JNCI J. Natl. Cancer Inst. 2014, 106. [Google Scholar] [CrossRef] [PubMed]
- Vinceti, M.; Filippini, T.; Del Giovane, C.; Dennert, G.; Zwahlen, M.; Brinkman, M.; Zeegers, M.P.; Horneber, M.; D’Amico, R.; Crespi, C.M. Selenium for preventing cancer. Cochrane Database Syst. Rev. 2018. [Google Scholar] [CrossRef]
- Rasmussen, L.B.; Schomburg, L.; Köhrle, J.; Pedersen, I.B.; Hollenbach, B.; Hög, A.; Ovesen, L.; Perrild, H.; Laurberg, P. Selenium status, thyroid volume, and multiple nodule formation in an area with mild iodine deficiency. Eur. J. Endocrinol. 2011, 164, 585–590. [Google Scholar] [CrossRef]
- Giray, B.; Arnaud, J.; Sayek, İ.; Favier, A.; Hıncal, F. Trace elements status in multinodular goiter. J. Trace Elem. Med. Biol. 2010, 24, 106–110. [Google Scholar] [CrossRef]
- Reich, H.J.; Hondal, R.J. Why Nature Chose Selenium. ACS Chem. Biol. 2016, 11, 821–841. [Google Scholar] [CrossRef]
- Hu, T.; Liang, Y.; Zhao, G.; Wu, W.; Li, H.; Guo, Y. Selenium Biofortification and Antioxidant Activity in Cordyceps militaris Supplied with Selenate, Selenite, or Selenomethionine. Biol. Trace Elem. Res. 2019, 187, 553–561. [Google Scholar] [CrossRef] [PubMed]
- Dumont, E.; Vanhaecke, F.; Cornelis, R. Selenium speciation from food source to metabolites: A critical review. Anal. Bioanal. Chem. 2006, 385, 1304–1323. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Martínez, A.; Charlet, L. Selenium environmental cycling and bioavailability: A structural chemist point of view. Rev. Env. Sci. Biotechnol. 2009, 8, 81–110. [Google Scholar] [CrossRef]
- Shahid, M.; Niazi, N.K.; Khalid, S.; Murtaza, B.; Bibi, I.; Rashid, M.I. A critical review of selenium biogeochemical behavior in soil-plant system with an inference to human health. Environ. Pollut. 2018, 234, 915–934. [Google Scholar]
- He, Y.; Xiang, Y.; Zhou, Y.; Yang, Y.; Zhang, J.; Huang, H.; Shang, C.; Luo, L.; Gao, J.; Tang, L. Selenium contamination, consequences and remediation techniques in water and soils: A review. Environ. Res. 2018, 164, 288–301. [Google Scholar] [CrossRef]
- Kieliszek, M. Selenium–Fascinating Microelement, Properties and Sources in Food. Molecules 2019, 24, 1298. [Google Scholar] [CrossRef] [PubMed]
- Wen, H.; Carignan, J. Reviews on atmospheric selenium: Emissions, speciation and fate. Atmos. Environ. 2007, 41, 7151–7165. [Google Scholar] [CrossRef]
- Söderlund, M.; Virkanen, J.; Holgersson, S.; Lehto, J. Sorption and speciation of selenium in boreal forest soil. J. Environ. Radioactiv. 2016, 164, 220–231. [Google Scholar] [CrossRef] [PubMed]
- Cao, S.; Duan, X.; Zhao, X.; Ma, J.; Dong, T.; Huang, N.; Sun, C.; He, B.; Wei, F. Health risks from the exposure of children to As, Se, Pb and other heavy metals near the largest coking plant in China. Sci. Total Environ. 2014, 472, 1001–1009. [Google Scholar] [CrossRef] [PubMed]
- Dennouni-Medjati, N.; Dali-Sahi, M.; Harek, H.; Harek, Y. Statut en sélénium de la population d’une région côtière de l’Ouest algérien à forte activité anthropique. Environ. Risque Sante 2019, 18, 8. [Google Scholar]
- Vriens, B.; Lenz, M.; Charlet, L.; Berg, M.; Winkel, L.H.E. Natural wetland emissions of methylated trace elements. Nat. Commun. 2014, 5, 3035. [Google Scholar] [CrossRef]
- Winkel, L.; Vriens, B.; Jones, G.; Schneider, L.; Pilon-Smits, E.; Bañuelos, G. Selenium Cycling Across Soil-Plant-Atmosphere Interfaces: A Critical Review. Nutrients 2015, 7, 4199–4239. [Google Scholar] [CrossRef]
- Zhu, Y.-G.; Pilon-Smits, E.A.H.; Zhao, F.-J.; Williams, P.N.; Meharg, A.A. Selenium in higher plants: Understanding mechanisms for biofortification and phytoremediation. Trends Plant Sci. 2009, 14, 436–442. [Google Scholar] [CrossRef]
- Manceau, A.; Charlet, L. The Mechanism of Selenate Adsorption on Goethite and Hydrous Ferric Oxide. J. Colloid Interface Sci. 1994, 168, 87–93. [Google Scholar] [CrossRef]
- Haug, A.; Graham, R.D.; Christophersen, O.A.; Lyons, G.H. How to use the world’s scarce selenium resources efficiently to increase the selenium concentration in food. Microb. Ecol. Health Dis. 2007, 19, 209–228. [Google Scholar] [PubMed]
- Lazard, M.; Dauplais, M.; Blanquet, S.; Plateau, P. Recent advances in the mechanism of selenoamino acids toxicity in eukaryotic cells. Biomol. Concepts 2017, 8, 93–104. [Google Scholar] [CrossRef] [PubMed]
- Sors, T.G.; Ellis, D.R.; Salt, D.E. Selenium uptake, translocation, assimilation and metabolic fate in plants. Photosynth. Res. 2005, 86, 373–389. [Google Scholar] [CrossRef] [PubMed]
- White, P.J. Selenium metabolism in plants. Biochim. Et Biophys. Acta (BBA) Gen. Subj. 2018, 1862, 2333–2342. [Google Scholar] [CrossRef]
- White, P.J. Selenium accumulation by plants. Ann. Bot. 2015, mcv180. [Google Scholar] [CrossRef]
- González-Morales, S.; Pérez-Labrada, F.; García-Enciso, E.; Leija-Martínez, P.; Medrano-Macías, J.; Dávila-Rangel, I.; Juárez-Maldonado, A.; Rivas-Martínez, E.; Benavides-Mendoza, A. Selenium and Sulfur to Produce Allium Functional Crops. Molecules 2017, 22, 558. [Google Scholar] [CrossRef]
- Zhang, L.; Hu, B.; Li, W.; Che, R.; Deng, K.; Li, H.; Yu, F.; Ling, H.; Li, Y.; Chu, C. OsPT2, a phosphate transporter, is involved in the active uptake of selenite in rice. New Phytol. 2014, 201, 1183–1191. [Google Scholar] [CrossRef]
- Zhao, X.Q.; Mitani, N.; Yamaji, N.; Shen, R.F.; Ma, J.F. Involvement of Silicon Influx Transporter OsNIP2;1 in Selenite Uptake in Rice. Plant Physiol. 2010, 153, 1871–1877. [Google Scholar] [CrossRef]
- Li, H.-F.; McGrath, S.P.; Zhao, F.-J. Selenium uptake, translocation and speciation in wheat supplied with selenate or selenite. New Phytol. 2008, 178, 92–102. [Google Scholar] [CrossRef]
- Wang, P.; Menzies, N.W.; Lombi, E.; McKenna, B.A.; James, S.; Tang, C.; Kopittke, P.M. Synchrotron-based X-ray absorption near-edge spectroscopy imaging for laterally resolved speciation of selenium in fresh roots and leaves of wheat and rice. EXBOTJ 2015, 66, 4795–4806. [Google Scholar] [CrossRef] [PubMed]
- Van Hoewyk, D. A tale of two toxicities: Malformed selenoproteins and oxidative stress both contribute to selenium stress in plants. Ann. Bot. 2013, 112, 965–972. [Google Scholar] [CrossRef] [PubMed]
- Grant, T.D.; Montes-Bayón, M.; LeDuc, D.; Fricke, M.W.; Terry, N.; Caruso, J.A. Identification and characterization of Se-methyl selenomethionine in Brassica juncea roots. J. Chromatogr. A 2004, 1026, 159–166. [Google Scholar] [CrossRef] [PubMed]
- Office of Dietary Supplements—Selenium. Available online: https://ods.od.nih.gov/factsheets/Selenium-HealthProfessional/ (accessed on 30 May 2020).
- Bañuelos, G.S.; Freeman, J.; Arroyo, I. Accumulation and speciation of selenium in biofortified vegetables grown under high boron and saline field conditions. Food Chem. X 2020, 5, 100073. [Google Scholar] [CrossRef] [PubMed]
- Fraczek, A. Selenium in medicine and treatment. J. Elementol. 2013, 18, 145–163. [Google Scholar] [CrossRef]
- Kieliszek, M.; Błażejak, S. Selenium: Significance, and outlook for supplementation. Nutrition 2013, 29, 713–718. [Google Scholar] [CrossRef] [PubMed]
- Fairweather-Tait, S.J.; Collings, R.; Hurst, R. Selenium bioavailability: Current knowledge and future research requirements. Am. J. Clin. Nutr. 2010, 91, 1484S–1491S. [Google Scholar] [CrossRef]
- Smrkolj, P.; Pograjc, L.; Hlastanribi, C.; Stibilj, V. Selenium content in selected Slovenian foodstuffs and estimated daily intakes of selenium. Food Chem. 2005, 90, 691–697. [Google Scholar] [CrossRef]
- Ariane, M.K.; Maristela, M.; Silmara, M.M.; Renata, H.S.; Karine, S.N.; Helyde, A.M.; Augusto, K.J. Properties of Brazil nuts: A review. Afr. J. Biotechnol. 2015, 14, 642–648. [Google Scholar] [CrossRef][Green Version]
- Hurst, R.; Collings, R.; Harvey, L.J.; King, M.; Hooper, L.; Bouwman, J.; Gurinovic, M.; Fairweather-Tait, S.J. EURRECA—Estimating Selenium Requirements for Deriving Dietary Reference Values. Crit. Rev. Food Sci. Nutr. 2013, 53, 1077–1096. [Google Scholar] [CrossRef]
- Kipp, A.P.; Strohm, D.; Brigelius-Flohé, R.; Schomburg, L.; Bechthold, A.; Leschik-Bonnet, E.; Heseker, H. Revised reference values for selenium intake. J. Trace Elem. Med. Biol. 2015, 32, 195–199. [Google Scholar] [CrossRef] [PubMed]
- Dietary Reference Intakes for Vitamin C, Vitamin E, Selenium, and Carotenoids; Institute of Medicine (U.S.), Panel on Dietary Antioxidants and Related Compounds, Eds.; National Academy Press: Washington, DC, USA, 2000; ISBN 978-0-309-06949-6. [Google Scholar]
- Referenzwerte für die Nährstoffzufuhr. Available online: https://www.dge.de/wissenschaft/referenzwerte/ (accessed on 13 June 2020).
- World Health Organization; Food and Agricultural Organisation. Vitamin and Mineral Requirements in Human Nutrition; World Health Organization: Geneva, Switzerland, 2004; ISBN 978-92-4-154612-6. [Google Scholar]
- Hazane-Puch, F.; Arnaud, J.; Trocmé, C.; Faure, P.; Laporte, F.; Champelovier, P. Sodium Selenite Decreased HDAC Activity, Cell Proliferation and Induced Apoptosis in Three Human Glioblastoma Cells. ACAMC 2016, 16, 490–500. [Google Scholar] [CrossRef] [PubMed]
- Vinceti, M.; Filippini, T.; Cilloni, S.; Bargellini, A.; Vergoni, A.V.; Tsatsakis, A.; Ferrante, M. Health risk assessment of environmental selenium: Emerging evidence and challenges. Mol. Med. Rep. 2017, 15, 3323–3335. [Google Scholar] [CrossRef] [PubMed]
- Mandrioli, J.; Michalke, B.; Solovyev, N.; Grill, P.; Violi, F.; Lunetta, C.; Conte, A.; Sansone, V.A.; Sabatelli, M.; Vinceti, M. Elevated Levels of Selenium Species in Cerebrospinal Fluid of Amyotrophic Lateral Sclerosis Patients with Disease-Associated Gene Mutations. Neurodegener. Dis. 2017, 17, 171–180. [Google Scholar] [CrossRef]
- Gammelgaard, B.; Jackson, M.I.; Gabel-Jensen, C. Surveying selenium speciation from soil to cell—Forms and transformations. Anal. Bioanal. Chem. 2011, 399, 1743–1763. [Google Scholar] [CrossRef] [PubMed]
- Schrauzer, G.N. Selenomethionine: A Review of Its Nutritional Significance, Metabolism and Toxicity. J. Nutr. 2000, 130, 1653–1656. [Google Scholar] [CrossRef]
- Nickel, A.; Kottra, G.; Schmidt, G.; Danier, J.; Hofmann, T.; Daniel, H. Characteristics of transport of selenoamino acids by epithelial amino acid transporters. Chem. Biol. Interact. 2009, 177, 234–241. [Google Scholar] [CrossRef]
- Vindry, C.; Ohlmann, T.; Chavatte, L. Translation regulation of mammalian selenoproteins. Biochim. Et Biophys. Acta (BBA) Gen. Subj. 2018, 1862, 2480–2492. [Google Scholar] [CrossRef]
- Esaki, N.; Karai, N.; Nakamura, T.; Tanaka, H.; Soda, K. Mechanism of reactions catalyzed by selenocysteine β-Lyase. Arch. Biochem. Biophys. 1985, 238, 418–423. [Google Scholar] [CrossRef]
- Cupp-Sutton, K.; Ashby, M. Biological Chemistry of Hydrogen Selenide. Antioxidants 2016, 5, 42. [Google Scholar] [CrossRef]
- Mangels, A.R.; Moser-Veillon, P.B.; Patterson, K.Y.; Veillon, C. Selenium utilization during human lactation by use of stable-isotope tracers. Am. J. Clin. Nutr. 1990, 52, 621–627. [Google Scholar] [CrossRef]
- Ha, H.Y.; Alfulaij, N.; Berry, M.J.; Seale, L.A. From Selenium Absorption to Selenoprotein Degradation. Biol. Trace Elem. Res. 2019, 192, 26–37. [Google Scholar] [CrossRef]
- Burk, R.F. Effects of Chemical Form of Selenium on Plasma Biomarkers in a High-Dose Human Supplementation Trial. Cancer Epidemiol. Biomark. Prev. 2006, 15, 804–810. [Google Scholar] [CrossRef]
- Xia, Y.; Hill, K.E.; Byrne, D.W.; Xu, J.; Burk, R.F. Effectiveness of selenium supplements in a low-selenium area of China. Am. J. Clin. Nutr. 2005, 81, 829–834. [Google Scholar] [CrossRef]
- Burk, R.F.; Hill, K.E. Selenoprotein P—Expression, functions, and roles in mammals. Biochim. Et Biophys. Acta (BBA) Gen. Subj. 2009, 1790, 1441–1447. [Google Scholar] [CrossRef]
- Burk, R.F.; Hill, K.E. SELENOPROTEIN P: An Extracellular Protein with Unique Physical Characteristics and a Role in Selenium Homeostasis. Annu. Rev. Nutr. 2005, 25, 215–235. [Google Scholar] [CrossRef]
- Méplan, C.; Nicol, F.; Burtle, B.T.; Crosley, L.K.; Arthur, J.R.; Mathers, J.C.; Hesketh, J.E. Relative Abundance of Selenoprotein P Isoforms in Human Plasma Depends on Genotype, Se Intake, and Cancer Status. Antioxid. Redox Signal. 2009, 11, 2631–2640. [Google Scholar] [CrossRef] [PubMed]
- Baclaocos, J.; Santesmasses, D.; Mariotti, M.; Bierła, K.; Vetick, M.B.; Lynch, S.; McAllen, R.; Mackrill, J.J.; Loughran, G.; Guigó, R.; et al. Processive Recoding and Metazoan Evolution of Selenoprotein P: Up to 132 UGAs in Molluscs. J. Mol. Biol. 2019, 431, 4381–4407. [Google Scholar] [CrossRef] [PubMed]
- Hill, K.E.; Zhou, J.; McMahan, W.J.; Motley, A.K.; Atkins, J.F.; Gesteland, R.F.; Burk, R.F. Deletion of Selenoprotein P Alters Distribution of Selenium in the Mouse. J. Biol. Chem. 2003, 278, 13640–13646. [Google Scholar] [CrossRef] [PubMed]
- Burk, R.F.; Hill, K.E. Regulation of Selenium Metabolism and Transport. Annu. Rev. Nutr. 2015, 35, 109–134. [Google Scholar] [CrossRef] [PubMed]
- Berry, M.J. Insights into the hierarchy of selenium incorporation. Nat. Genet. 2005, 37, 1162–1163. [Google Scholar] [CrossRef]
- Méplan, C.; Crosley, L.K.; Nicol, F.; Beckett, G.J.; Howie, A.F.; Hill, K.E.; Horgan, G.; Mathers, J.C.; Arthur, J.R.; Hesketh, J.E. Genetic polymorphisms in the human selenoprotein P gene determine the response of selenoprotein markers to selenium supplementation in a gender-specific manner (the SELGEN study). FASEB J. 2007, 21, 3063–3074. [Google Scholar] [CrossRef]
- Valea, A.; Georgescu, C.E. Selenoproteins in human body: Focus on thyroid pathophysiology. Hormones 2018, 17, 183–196. [Google Scholar] [CrossRef] [PubMed]
- Lacka, K.; Szeliga, A. Significance of selenium in thyroid physiology and pathology. Pol. Merkur. Lek. 2015, 38, 348–353. [Google Scholar]
- Köhrle, J. Selenium and the thyroid. Curr. Opin. Endocrinol. Diabetes Obes. 2015, 22, 392–401. [Google Scholar] [CrossRef] [PubMed]
- Orozco, A.; Valverde-R, C.; Olvera, A.; García-G, C. Iodothyronine deiodinases: A functional and evolutionary perspective. J. Endocrinol. 2012, 215, 207–219. [Google Scholar] [CrossRef] [PubMed]
- Krohn, K.; Maier, J.; Paschke, R. Mechanisms of Disease: Hydrogen peroxide, DNA damage and mutagenesis in the development of thyroid tumors. Nat. Rev. Endocrinol. 2007, 3, 713–720. [Google Scholar] [CrossRef] [PubMed]
- Schomburg, L. Selenium, selenoproteins and the thyroid gland: Interactions in health and disease. Nat. Rev. Endocrinol. 2012, 8, 160–171. [Google Scholar] [CrossRef]
- Guillin, O.M.; Vindry, C.; Ohlmann, T.; Chavatte, L. Selenium, Selenoproteins and Viral Infection. Nutrients 2019, 11, 2101. [Google Scholar] [CrossRef]
- Hu, S.; Rayman, M.P. Multiple Nutritional Factors and the Risk of Hashimoto’s Thyroiditis. Thyroid 2017, 27, 597–610. [Google Scholar] [CrossRef]
- Kurokawa, S.; Berry, M.J. Selenium. Role of the Essential Metalloid in Health. Met. Ions Life Sci. 2013, 13, 499–534. [Google Scholar] [PubMed]
- Dayan, C.M.; Panicker, V. Novel insights into thyroid hormones from the study of common genetic variation. Nat. Rev. Endocrinol. 2009, 5, 211–218. [Google Scholar] [CrossRef]
- Köhrle, J.; Gärtner, R. Selenium and thyroid. Best Pract. Res. Clin. Endocrinol. Metab. 2009, 23, 815–827. [Google Scholar] [CrossRef]
- Schmutzler, C.; Mentrup, B.; Schomburg, L.; Hoang-Vu, C.; Herzog, V.; Köhrle, J. Selenoproteins of the thyroid gland: Expression, localization and possible function of glutathione peroxidase 3. Biol. Chem. 2007, 388. [Google Scholar] [CrossRef]
- Brigelius-Flohé, R. Glutathione peroxidases and redox-regulated transcription factors. Biol. Chem. 2006, 387. [Google Scholar] [CrossRef]
- Schomburg, L. The other view: The trace element selenium as a micronutrient in thyroid disease, diabetes, and beyond. Hormones 2020, 19, 15–24. [Google Scholar] [CrossRef]
- Schomburg, L.; Schweizer, U. Hierarchical regulation of selenoprotein expression and sex-specific effects of selenium. Biochim. Et Biophys. Acta (BBA) Gen. Subj. 2009, 1790, 1453–1462. [Google Scholar] [CrossRef]
- Kipp, A.P. Selenium in colorectal and differentiated thyroid cancer. Hormones 2020, 19, 41–46. [Google Scholar] [CrossRef]
- Ferlay, J.; Soerjomataram, I.; Dikshit, R.; Eser, S.; Mathers, C.; Rebelo, M.; Parkin, D.M.; Forman, D.; Bray, F. Cancer incidence and mortality worldwide: Sources, methods and major patterns in GLOBOCAN 2012: Globocan 2012. Int. J. Cancer 2015, 136, E359–E386. [Google Scholar] [CrossRef] [PubMed]
- Schlumberger, M. Cancer papillaire et folliculaire de la thyroïde. Ann. D’endocrinologie 2007, 68, 120–128. [Google Scholar] [CrossRef] [PubMed]
- De Oliveira Maia, M.; Batista, B.A.M.; Sousa, M.P.; de Souza, L.M.; Maia, C.S.C. Selenium and thyroid cancer: A systematic review. Nutr. Cancer 2019, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Cabanillas, M.E.; McFadden, D.G.; Durante, C. Thyroid cancer. Lancet 2016, 388, 2783–2795. [Google Scholar] [CrossRef]
- Cai, X.; Wang, C.; Yu, W.; Fan, W.; Wang, S.; Shen, N.; Wu, P.; Li, X.; Wang, F. Selenium Exposure and Cancer Risk: An Updated Meta-analysis and Meta-regression. Sci. Rep. 2016, 6, 19213. [Google Scholar] [CrossRef] [PubMed]
- Jablonska, E.; Vinceti, M. Selenium and Human Health: Witnessing a Copernican Revolution? J. Environ. Sci. Healthpart C 2015, 33, 328–368. [Google Scholar] [CrossRef]
- Wallenberg, M.; Misra, S.; Björnstedt, M. Selenium Cytotoxicity in Cancer. Basic Clin. Pharm. Toxicol. 2014, 114, 377–386. [Google Scholar] [CrossRef]
- Murdolo, G.; Bartolini, D.; Tortoioli, C.; Piroddi, M.; Torquato, P.; Galli, F. Selenium and Cancer Stem Cells. In Advances in Cancer Research; Elsevier: Amsterdam, The Netherlands, 2017; Volume 136, pp. 235–257. ISBN 978-0-12-812016-3. [Google Scholar]
- Kucharzewski, M.; Braziewicz, J.; Majewska, U.; Gózdz, S. Concentration of Selenium in the Whole Blood and the Thyroid Tissue of Patients with Various Thyroid Diseases. BTER 2002, 88, 25–30. [Google Scholar] [CrossRef]
- Moncayo, R.; Kroiss, A.; Oberwinkler, M.; Karakolcu, F.; Starzinger, M.; Kapelari, K.; Talasz, H.; Moncayo, H. The role of selenium, vitamin C, and zinc in benign thyroid diseases and of selenium in malignant thyroid diseases: Low selenium levels are found in subacute and silent thyroiditis and in papillary and follicular carcinoma. BMC Endocr. Disord. 2008, 8, 2. [Google Scholar] [CrossRef]
- Baltaci, A.K.; Dundar, T.K.; Aksoy, F.; Mogulkoc, R. Changes in the Serum Levels of Trace Elements Before and After the Operation in Thyroid Cancer Patients. Biol. Trace Elem. Res. 2017, 175, 57–64. [Google Scholar] [CrossRef]
- Kucharzewski, M.; Braziewicz, J.; Majewska, U.; Góźdź, S. Copper, Zinc, and Selenium in Whole Blood and Thyroid Tissue of People with Various Thyroid Diseases. BTER 2003, 93, 9–18. [Google Scholar] [CrossRef]
- Jonklaas, J.; Danielsen, M.; Wang, H. A Pilot Study of Serum Selenium, Vitamin D, and Thyrotropin Concentrations in Patients with Thyroid Cancer. Thyroid 2013, 23, 1079–1086. [Google Scholar] [CrossRef]
- Ren, Y.; Kitahara, C.M.; de Gonzalez, A.B.; Clero, E.; Brindel, P.; Maillard, S.; Cote, S.; Dewailly, E.; Rachedi, F.; Boissin, J.-L.; et al. Lack of Association between Fingernail Selenium and Thyroid Cancer Risk: A Case-Control Study in French Polynesia. Asian Pac. J. Cancer Prev. 2014, 15, 5187–5194. [Google Scholar] [CrossRef] [PubMed]
- Przybylik-Mazurek, E.; Zagrodzki, P.; Kuźniarz-Rymarz, S.; Hubalewska-Dydejczyk, A. Thyroid Disorders—Assessments of Trace Elements, Clinical, and Laboratory Parameters. Biol. Trace Elem. Res. 2011, 141, 65–75. [Google Scholar] [CrossRef] [PubMed]
- Shen, F.; Cai, W.S.; Li, J.L.; Feng, Z.; Cao, J.; Xu, B. The Association Between Serum Levels of Selenium, Copper, and Magnesium with Thyroid Cancer: A Meta-analysis. Biol. Trace Elem. Res. 2015, 167, 225–235. [Google Scholar] [CrossRef] [PubMed]
- Chung, H.-K.; Nam, J.S.; Ahn, C.W.; Lee, Y.S.; Kim, K.R. Some Elements in Thyroid Tissue are Associated with More Advanced Stage of Thyroid Cancer in Korean Women. Biol. Trace Elem. Res. 2016, 171, 54–62. [Google Scholar] [CrossRef]
- Zhang, C.; Wu, H.B.; Cheng, M.X.; Wang, L.; Gao, C.B.; Huang, F. Association of exposure to multiple metals with papillary thyroid cancer risk in China. Environ. Sci. Pollut. Res. 2019, 26, 20560–20572. [Google Scholar] [CrossRef]
- Metere, A.; Frezzotti, F.; Graves, C.E.; Vergine, M.; De Luca, A.; Pietraforte, D.; Giacomelli, L. A possible role for selenoprotein glutathione peroxidase (GPx1) and thioredoxin reductases (TrxR1) in thyroid cancer: Our experience in thyroid surgery. Cancer Cell Int. 2018, 18, 7. [Google Scholar] [CrossRef]
- Zhao, H.; Li, J.; Li, X.; Han, C.; Zhang, Y.; Zheng, L.; Guo, M. Silencing GPX3 Expression Promotes Tumor Metastasis in Human Thyroid Cancer. CPPS 2015, 16, 316–321. [Google Scholar] [CrossRef]
- Hughes, D.; Kunická, T.; Schomburg, L.; Liška, V.; Swan, N.; Souček, P. Expression of Selenoprotein Genes and Association with Selenium Status in Colorectal Adenoma and Colorectal Cancer. Nutrients 2018, 10, 1812. [Google Scholar] [CrossRef]
- Kipp, A.P.; Müller, M.F.; Göken, E.M.; Deubel, S.; Brigelius-Flohé, R. The selenoproteins GPx2, TrxR2 and TrxR3 are regulated by Wnt signalling in the intestinal epithelium. Biochim. Et Biophys. Acta (BBA) Gen. Subj. 2012, 1820, 1588–1596. [Google Scholar] [CrossRef]
- Romitti, M.; Wajner, S.M.; Zennig, N.; Goemann, I.M.; Bueno, A.L.; Meyer, E.L.S.; Maia, A.L. Increased Type 3 Deiodinase Expression in Papillary Thyroid Carcinoma. Thyroid 2012, 22, 897–904. [Google Scholar] [CrossRef]
- Gupta, S.; Jaworska-Bieniek, K.; Lubinski, J.; Jakubowska, A. Can selenium be a modifier of cancer risk in CHEK2 mutation carriers? Mutagenesis 2013, 28, 625–629. [Google Scholar] [CrossRef][Green Version]
- Siołek, M.; Cybulski, C.; Gąsior-Perczak, D.; Kowalik, A.; Kozak-Klonowska, B.; Kowalska, A.; Chłopek, M.; Kluźniak, W.; Wokołorczyk, D.; Pałyga, I.; et al. CHEK 2 mutations and the risk of papillary thyroid cancer: Genetic association between thyroid and breast cancer. Int. J. Cancer 2015, 137, 548–552. [Google Scholar] [CrossRef] [PubMed]
- Weekley, C.M.; Jeong, G.; Tierney, M.E.; Hossain, F.; Maw, A.M.; Shanu, A.; Harris, H.H.; Witting, P.K. Selenite-mediated production of superoxide radical anions in A549 cancer cells is accompanied by a selective increase in SOD1 concentration, enhanced apoptosis and Se–Cu bonding. J. Biol. Inorg. Chem. 2014, 19, 813–828. [Google Scholar] [CrossRef] [PubMed]
- Kieliszek, M.; Lipinski, B.; Błażejak, S. Application of Sodium Selenite in the Prevention and Treatment of Cancers. Cells 2017, 6, 39. [Google Scholar] [CrossRef] [PubMed]
- Becker, N.-P.; Martitz, J.; Renko, K.; Stoedter, M.; Hybsier, S.; Cramer, T.; Schomburg, L. Hypoxia reduces and redirects selenoprotein biosynthesis. Metallomics 2014, 6, 1079–1086. [Google Scholar] [CrossRef]
- Dreher, I.; Jakobs, T.C.; Köhrle, J. Cloning and Characterization of the Human Selenoprotein P Promoter: Response of selenoprotein p expression to cytokines in liver cells. J. Biol. Chem. 1997, 272, 29364–29371. [Google Scholar] [CrossRef]
- Renko, K.; Hofmann, P.J.; Stoedter, M.; Hollenbach, B.; Behrends, T.; Köhrle, J.; Schweizer, U.; Schomburg, L. Down-regulation of the hepatic selenoprotein biosynthesis machinery impairs selenium metabolism during the acute phase response in mice. FASEB J. 2009, 23, 1758–1765. [Google Scholar] [CrossRef]
| Selenoproteines | Localization | Functions | References |
|---|---|---|---|
| Deiodinases (DIO) | [80] | ||
| DIO1 | Liver, kidney, thyroid gland, lung, eyes, pituitary, CNS | Conversion of T4 into T3and rT3 and T3 into rT3 or T2 | |
| DIO2 | Thyroid gland, pituitary gland, skeletal, heart muscles, brain, fat tissue, spinal cord, placenta | Conversion of T4 into T3 and of rT3 into T2 | |
| DIO3 | Gravid uterus, placenta, fetus liver, fetal and neonatal brain, skin | Conversion of T4 into T3 and of rT3 into T2 | |
| Gluthatione peroxidases (GPx) | [82,83] | ||
| GPx1 | Cytoplasm, ubiquitous | Cytosol Antioxidant | |
| GPx3 | Plasma and thyroid follicle | Plasma and extracellular antioxidant | |
| GPx4 | Mitochondrial membrane | Membrane antioxidant | |
| Thioredoxin reductase (TRx) | [82,84] | ||
| TRx1 | Principally cytosolic, ubiquitous | Inhibition of apoptosis, redox state of transcription factors | |
| TRx2 | Mitochondrial, ubiquitous | Reduce basal oxidative stress, | |
| TRx3 | Principally mitochondrial, ubiquitous | Regulation of apoptosis and signaling pathway | |
| Selenoprotein P (SePP) | Blood and thyroid | Transportation of selenium and storage, endothelial antioxidant | [85] |
| Analysis | Sample | Outcome | References |
|---|---|---|---|
| Serum Se and GPx3 concentration. | 25 patients with PTC 13 patients with FTC 20 Control | No significant differences in Se and GPx3 concentrations among groups. | [107] |
| Pre-surgery evaluation of serum Se and vit D3 at different stages of disease. | 35 patients with PTC 12 patients with FTC 17 patients with goiter | No significant differences among groups. | [105] |
| Fingernail Se level | 215 patients diagnosed with various thyroid cancer 331 controls | No significant association between fingernail Se levels in patients vs controls, at different thyroid cancer stage | [106] |
| Serum concentrations of Se, Cu, Mn | Meta-analysis on 1291 subjects performed in Norwegian, Austrian and Polish populations | Significantly lower levels of Se and Mn and higher level of Cu, in patients with thyroid carcinoma | [108] |
| Serum Zn and Se concentration before and after surgery or two weeks later, as well as in thyroid tissues. | 50 women and men with thyroid cancer | Lower serum Se and Zn concentrations before and after surgery but higher concentrations in thyroid tissue in the 2 groups | [103] |
| Pre-surgery Se, Cd, Zn serum concentration and correlation to cancer stage | 92 Korean women, of PTC | Se, Cd, Zn concentrations were significantly higher in cancer stages III and IV | [109] |
| Serum and urine concentration in eleven metals and in Se | 262 patients with PTC 262 controls | Se concentrations significantly lower in PTC. Urinary Se concentration negatively associated to PTC risk | [110] |
| GPx1 and TRx1 expression and analysis of free radicals tumor vs. healthy tissues | 20 samples of thyroid tumor 20 samples of healthy thyroid tissue | GPx1 and TRx1 in thyroid cancer tissue are lower in patients vs. controls Significant increase in production of free radicals in all thyroid tumor tissue samples vs. healthy tissue | [111] |
| Se, GPx3, SePP, Cu, Zn serum concentration | Patients with various thyroid pathologies including thyroid cancer (n = 323) 200 Controls | Significantly lower serum Se and Zn levels in patients vs. controls (particular the patients with thyroid malignancy). | [10] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kazi Tani, L.S.; Dennouni-Medjati, N.; Toubhans, B.; Charlet, L. Selenium Deficiency—From Soil to Thyroid Cancer. Appl. Sci. 2020, 10, 5368. https://doi.org/10.3390/app10155368
Kazi Tani LS, Dennouni-Medjati N, Toubhans B, Charlet L. Selenium Deficiency—From Soil to Thyroid Cancer. Applied Sciences. 2020; 10(15):5368. https://doi.org/10.3390/app10155368
Chicago/Turabian StyleKazi Tani, Latifa Sarra, Nouria Dennouni-Medjati, Benoit Toubhans, and Laurent Charlet. 2020. "Selenium Deficiency—From Soil to Thyroid Cancer" Applied Sciences 10, no. 15: 5368. https://doi.org/10.3390/app10155368
APA StyleKazi Tani, L. S., Dennouni-Medjati, N., Toubhans, B., & Charlet, L. (2020). Selenium Deficiency—From Soil to Thyroid Cancer. Applied Sciences, 10(15), 5368. https://doi.org/10.3390/app10155368





