Evaluation of the Effect of Freezing and Gamma Irradiation on Different Types of Tendon Allografts by DIC Assisted Tensile Testing
Abstract
Featured Application
Abstract
1. Introduction
2. Materials and Methods
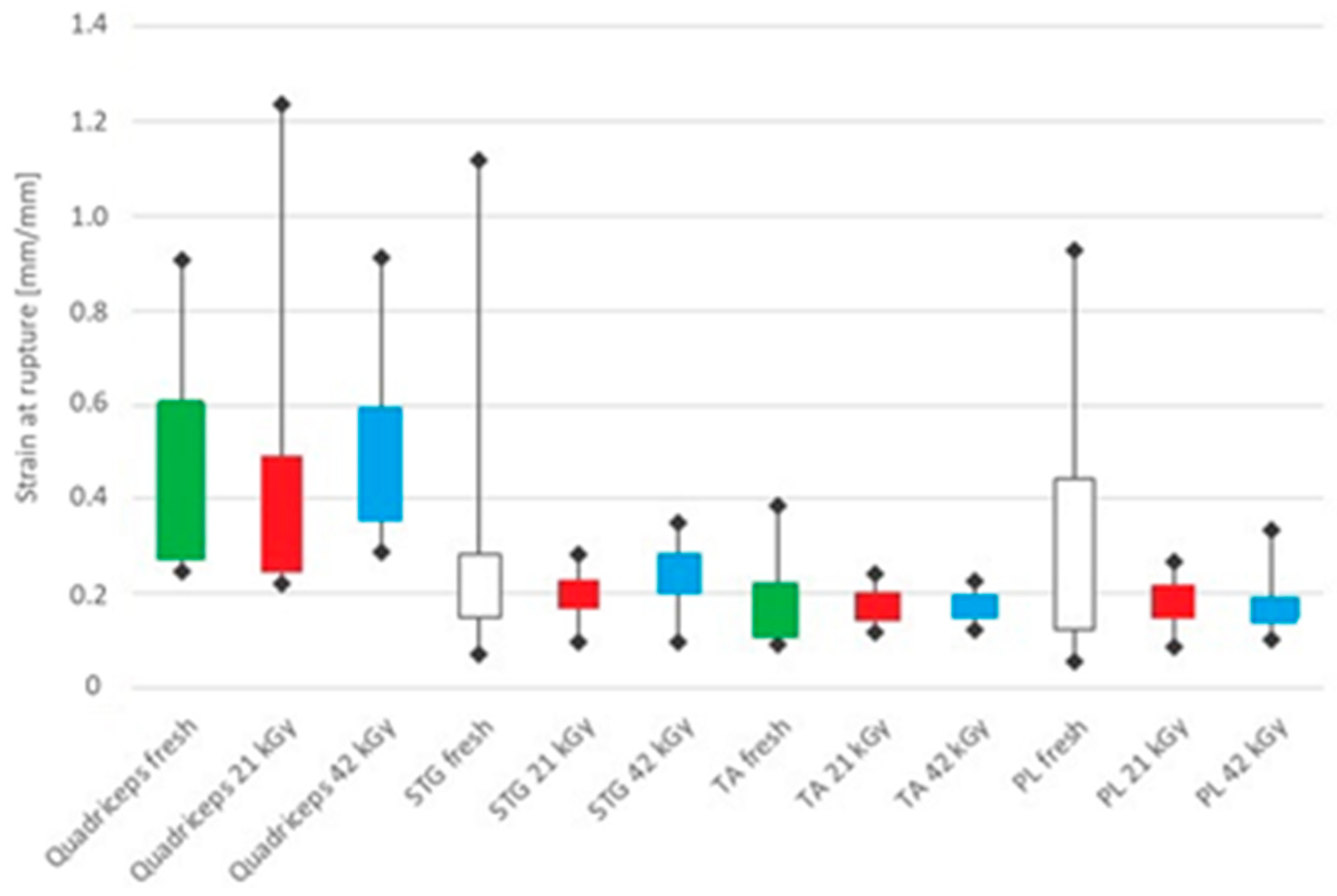
3. Results
4. Discussion
Author Contributions
Funding
Conflicts of Interest
References
- Jung, H.J.; Vangipuram, G.; Fisher, M.B.; Yang, G.; Hsu, S.; Bianchi, J.; Ronholdt, C.; Woo, S.L. The effects of multiple freeze-thaw cycles on the biomechanical properties of the human bone-patellar tendon-bone allograft. J. Orthop. Res. 2011, 8, 1193–1198. [Google Scholar] [CrossRef] [PubMed]
- Zaffagnini, S.; di Sarsina, T.R.; Bonanzinga, T.; Nitri, M.; Macchiarola, L.; Stefanelli, F.; Lucidi, G.; Grassi, A. Does donor age of nonirradiated achilles tendon allograft influence mid-term results of revision ACL reconstruction? Joints 2018, 6, 10–15. [Google Scholar] [CrossRef] [PubMed]
- Eagan, M.J.; McAllister, D.R. Biology of allograft incorporation. Clin. Sports Med. 2009, 28, 203–214. [Google Scholar] [CrossRef] [PubMed]
- Wilde, J.; Bedi, A.; Altchek, D.W. Graft selection for revision ACL reconstruction. In Revision ACL Reconstruction; Marx, R.G., Ed.; Springer: New York, NY, USA, 2014; pp. 75–86. [Google Scholar] [CrossRef]
- Buda, R.; Ruffilli, A.; Di Caprio, F.; Ferruzzi, A.; Faldini, C.; Cavallo, M.; Vannini, F.; Giannini, S. Allograft salvage procedure in multiple-revision anterior cruciate ligament reconstruction. Am. J. Sports Med. 2013, 41, 402–410. [Google Scholar] [CrossRef] [PubMed]
- Kainer, M.A.; Linden, J.V.; Whaley, D.N.; Holmes, H.T.; Jarvis, W.R.; Jernigan, D.B.; Archibald, L.K. Clostridium infections associated with musculoskeletal-tissue allografts. N. Engl. J. Med. 2004, 350, 2564–2571. [Google Scholar] [CrossRef] [PubMed]
- Scheffler, S.U.; Scherler, J.; Pruss, A.; von Versen, R.; Weiler, A. Biomechanical comparison of human bone-patellar tendon-bone grafts after sterilization with peracetic acid ethanol. Cell Tissue Bank. 2005, 6, 109–115. [Google Scholar] [CrossRef]
- Greaves, L.L.; Hecker, A.T.; Brown, C.H., Jr. The effect of donor age and low-dose gamma irradiation on the initial biomechanical properties of human tibialis tendon allografts. Am. J Sports Med. 2008, 36, 1358–1366. [Google Scholar] [CrossRef]
- Ng, K.W.; Wanivenhaus, F.; Chen, T.; Abrams, V.D.; Torzilli, P.A.; Warren, R.F.; Maher, S.A. Differential cross-linking and radio-protective effects of geninpin on mature bovine and patella tendons. Cell Tissue Bank. 2013, 14, 21–32. [Google Scholar] [CrossRef]
- Almqvist, K.F.; Jan, H.; Vercruysse, C.; Verbeeck, R.; Verdonk, R. The tibialis tendon as a valuable anterior cruciate ligament allograft substitute: Biomechanical properties. Knee Surg. Sports Traumatol. Arthrosc. 2007, 15, 1326–1330. [Google Scholar] [CrossRef]
- Hangody, G.; Szebényi, G.; Abonyi, B.; Kiss, R.; Hangody, L.; Pap, K. Does a different dose of gamma irradiation have the same effect on five different types of tendon allografts?—A biomechanical study. Int. Orthop. 2017, 41, 357–365. [Google Scholar] [CrossRef]
- Hangody, G.; Pánics, G.; Szebényi, G.; Kiss, R.; Hangody, L.; Pap, K. Pitfalls during biomechanical testing—Evaluation of different fixation methods for measuring tendons endurance properties. Acta Physiol. Int. 2016, 103, 86–93. [Google Scholar] [CrossRef] [PubMed]
- Grieb, T.A.; Forng, R.-Y.; Bogdansky, S.; Ronholdt, C.; Parks, B.; Drohan, W.N.; Burgess, W.H.; Lin, J. High-dose gamma irradiation for soft tissue allografts: High margin of safety with biomechanical integrity. J. Orthop. Res. 2006, 24, 1011–1018. [Google Scholar] [CrossRef] [PubMed]
- Vyas, D.; Rabuck, S.J.; Harner, C.D. Allograft anterior cruciate ligament reconstruction: Indications, techniques, and outcomes. J. Orthop. Sports Phys. Therap. 2012, 42, 196–207. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.; Li, T.; Wang, J.; Dong, J.; Gao, S. Medial collateral ligament reconstruction using bone-patellar tendon-bone allograft for chronic medial knee instability combined with multi-ligament injuries: A new technique. J. Orthop. Surg. Res. 2016, 11, 85–89. [Google Scholar] [CrossRef] [PubMed]
- Hoburg, A.; Keshlaf, S.; Schmidt, T.; Smith, M.; Gohs, U.; Perka, C.; Pruss, A.; Scheffler, S. High-dose electron beam sterilization of soft-tissue grafts maintains significantly improved biomechanical properties compared to standard gamma treatment. Cell Tissue Bank. 2015, 16, 219–226. [Google Scholar] [CrossRef]
- Pap, K. Dilemma of the orthopedic surgeon—What kind of graft should we use for ACL reconstruction? Orthop. Rheumatol. Open Access J. 2018, 10, 555786. [Google Scholar] [CrossRef]
- Chen, L.; Wu, Y.; Yu, J.; Jiao, Z.; Ao, Y.; Yu, C.; Wang, J.; Cui, G. Effect of repeated freezing-thawing on the Achilles tendon of rabbits. Knee Surg. Sports Traumatol. Arthrosc. 2011, 19, 1028–1034. [Google Scholar] [CrossRef]
- Giannini, S.; Buda, R.; Di Caprio, F.; Agati, P.; Bigi, A.; De Pasquale, V.; Ruggeri, A. Effects of freezing on the biomechanical and structural properties of human posterior tibial tendons. Int. Orthop. 2008, 32, 145–151. [Google Scholar] [CrossRef]
- Mabe, I.; Hunter, S. Quadriceps tendon allografts as an alternative to Achilles tendon allografts: A biomechanical comparison. Cell Tissue Bank. 2014, 15, 523–529. [Google Scholar] [CrossRef]
- Pearsall, A.W.; Hollis, M.J.; Russel, G.V., Jr.; Scheer, Z. A biomechanical comparison of three lower extremity tendons for ligamentous reconstruction about the knee. Arthroscopy 2003, 19, 1091–1096. [Google Scholar] [CrossRef]
- Di Matteo, B.; Loibl, M.; Andriolo, L.; Filardo, G.; Zellner, J.; Koch, M.; Angele, P. Biologic agents for anterior cruciate ligament healing: A systematic review. World J. Orthop. 2016, 7, 592–603. [Google Scholar] [CrossRef] [PubMed]
- Dong, S.; Huangfu, X.; Xie, G.; Zhang, Y.; Shen, P.; Li, X.; Qi, J.; Zhao, J. Decellularized versus fresh-frozen allografts in anterior cruciate ligament reconstruction: An in vitro study in a rabbit model. Am. J. Sports Med. 2015, 43, 1924–1934. [Google Scholar] [CrossRef] [PubMed]
- Conrad, B.P.; Rappé, M.; Horodyski, M.; Farmer, K.W.; Indelicato, P.A. The effect of sterilization on mechanical properties of soft tissue allografts. Cell Tissue Bank. 2013, 14, 359–366. [Google Scholar] [CrossRef] [PubMed]
- Nagura, T.; Matsumoto, H.; Kiriyama, Y.; Chaudhari, A.; Andriacchi, T.P. Tibiofemoral joint contact force in deep knee flexion and its consideration in knee osteoarthritis and joint replacement. J. Appl. Biomech. 2006, 22, 305–313. [Google Scholar] [CrossRef]
- Noyes, F.; Butler, D.; Grood, E.; Zernicke, R.F.; Hefzy, M.S. Biomechanical analysis of human ligament grafts used in knee-ligament repairs and reconstructions. J. Bone Jt. Surg. Am. 1985, 66, 344–352. [Google Scholar] [CrossRef]
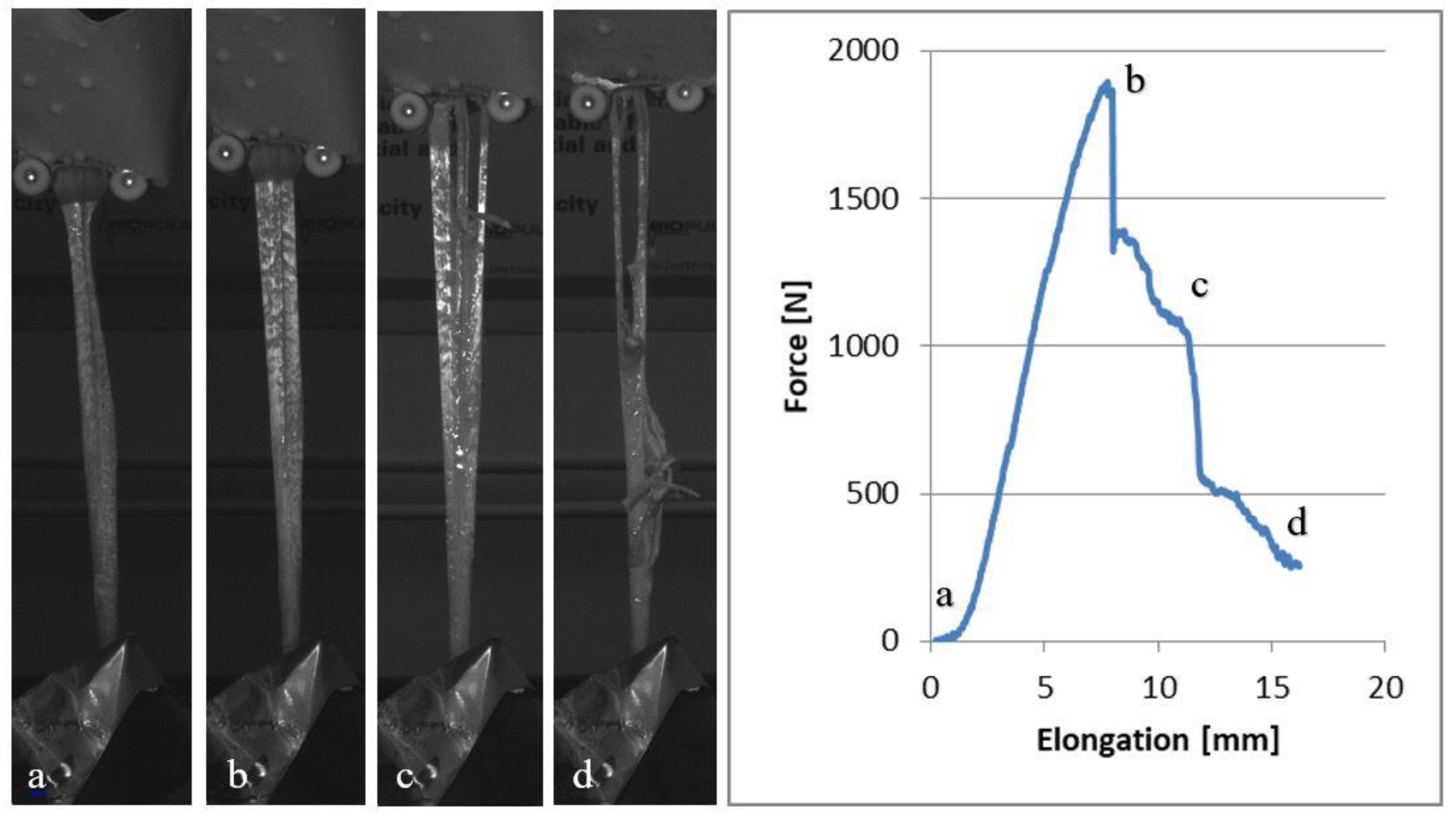
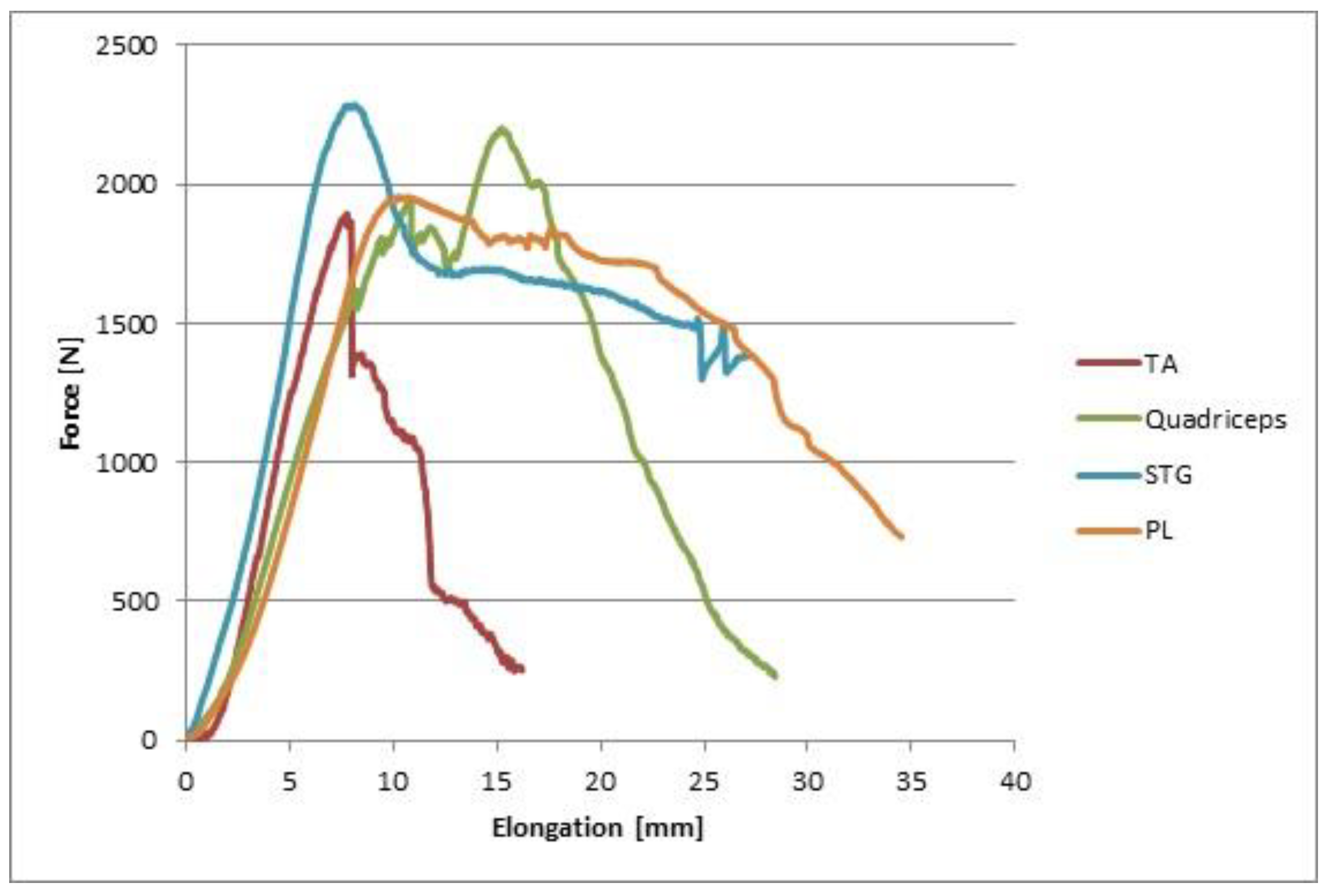
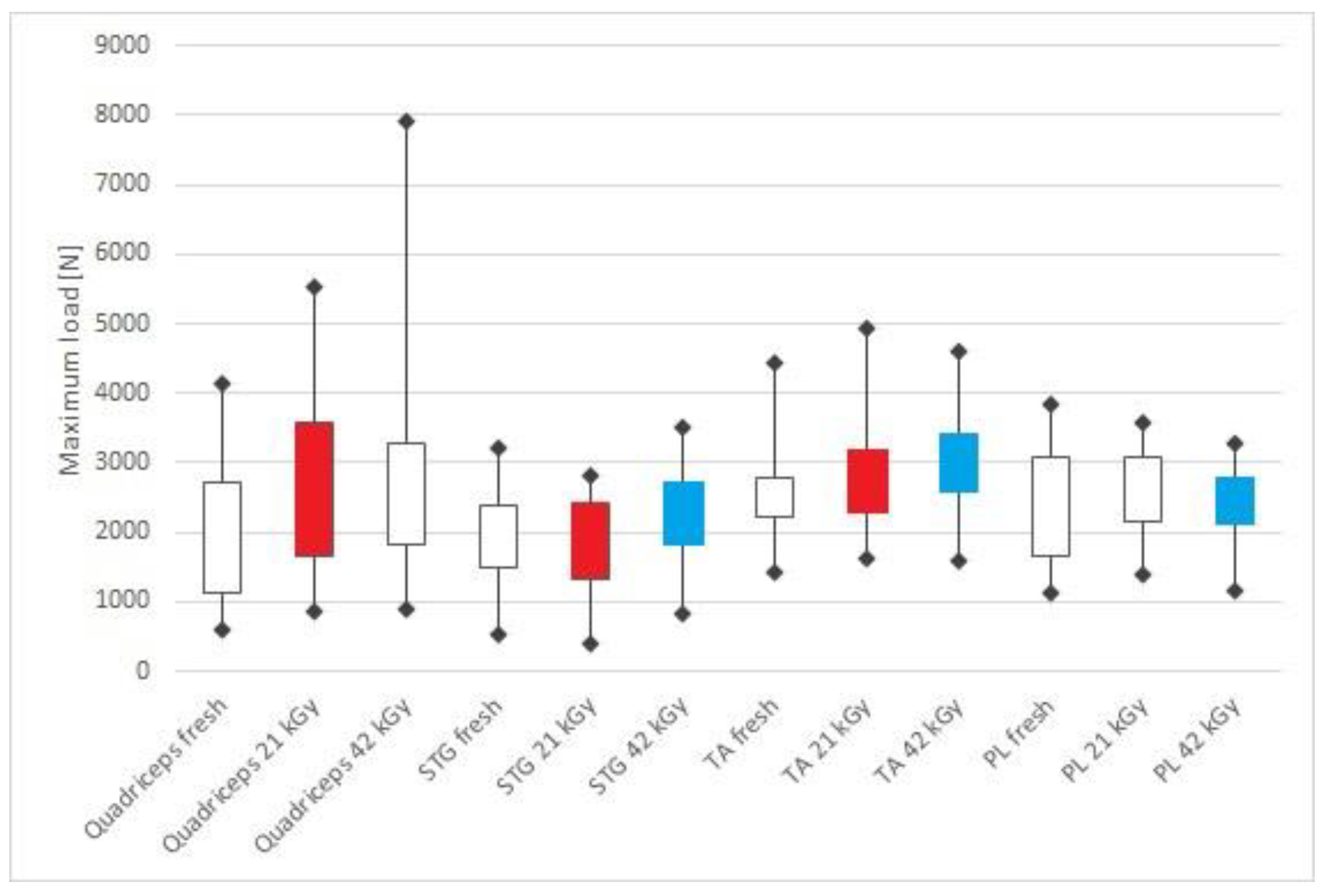
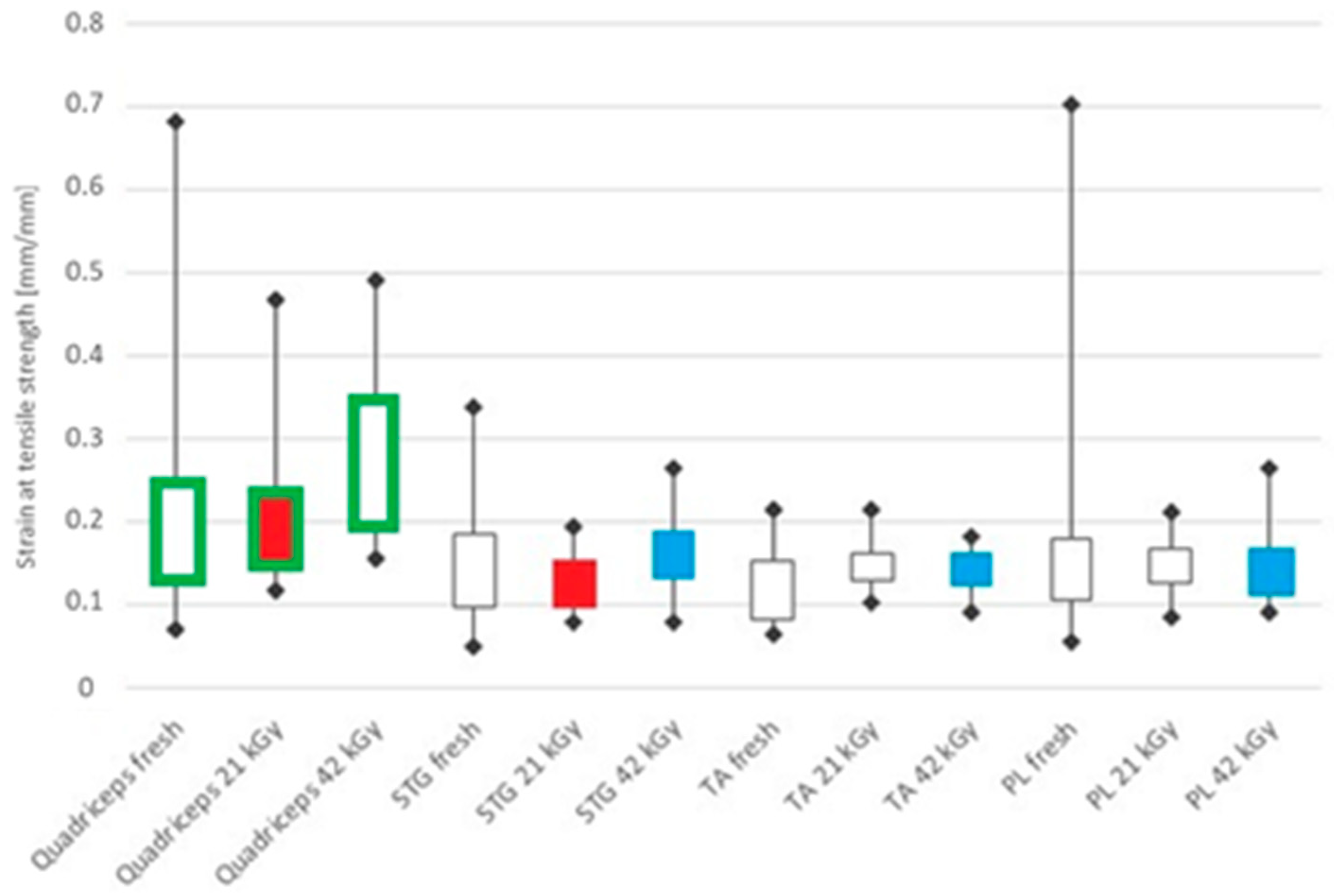

| Tendon Types and Mechanical Properties | Group A | Group B | Group C | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Median | 25% | 75% | Median | 25% | 75% | Median | 25% | 75% | |
| Quadriceps | |||||||||
| Tensile modulus (MPa) | 191.31 | 138.13 | 292.42 | 120.24 | 90.13 | 299.29 | 83.20 | 30.65 | 137.05 |
| Maximum load (N) | 1939.75 | 1121.97 | 2727.15 | 2803.68 | 1653.26 | 3591.97 | 2564.77 | 1835.38 | 3265.92 |
| Strain at maximum force (-) | 0.1540 | 0.1340 | 0.2485 | 0.1841 | 0.1467 | 0.2299 | 0.2729 | 0.1949 | 0.3472 |
| Fracture strain (-) | 0.3370 | 0.2766 | 0.6090 | 0.3231 | 0.2481 | 0.4885 | 0.4392 | 0.3574 | 0.5909 |
| Semitendinosus + gracilis (STG) | |||||||||
| Tensile modulus (MPa) | 186.46 | 142.82 | 229.26 | 210.28 | 137.08 | 244.11 | 222.66 | 128.59 | 255.73 |
| Maximum load (N) | 1922.96 | 1501.00 | 2374.84 | 2171.41 | 1330.37 | 2414.74 | 2357.61 | 1827.42 | 2670.83 |
| Strain at maximum force (-) | 0.1300 | 0.0980 | 0.1864 | 0.1346 | 0.0985 | 0.1540 | 0.1631 | 0.1315 | 0.1895 |
| Fracture strain (-) | 0.2460 | 0.1500 | 0.2850 | 0.1924 | 0.1685 | 0.2248 | 0.2220 | 0.1991 | 0.2815 |
| Tibialis anterior (TA) | |||||||||
| Tensile modulus (MPa) | 343.24 | 308.25 | 383.33 | 432.55 | 393.64 | 538.25 | 318.85 | 257.63 | 347.66 |
| Maximum load (N) | 2582.22 | 2236.38 | 2784.23 | 2552.10 | 2322.61 | 3176.63 | 3063.90 | 2603.71 | 3416.14 |
| Strain at maximum force (-) | 0.1041 | 0.0831 | 0.1543 | 0.1476 | 0.1285 | 0.1630 | 0.1482 | 0.1225 | 0.1618 |
| Fracture strain (-) | 0.1580 | 0.1099 | 0.2131 | 0.1656 | 0.1473 | 0.2021 | 0.1781 | 0.1611 | 0.1922 |
| Peroneus longus (PL) | |||||||||
| Tensile modulus (MPa) | 250.33 | 166.58 | 309.85 | 267.83 | 212.43 | 329.96 | 256.55 | 203.47 | 277.46 |
| Maximum load (N) | 2490.82 | 1657.51 | 3083.02 | 2398.30 | 2166.18 | 3092.63 | 2339.75 | 2111.68 | 2773.67 |
| Strain at maximum force (-) | 0.1402 | 0.1049 | 0.1790 | 0.1489 | 0.1269 | 0.1690 | 0.1420 | 0.1108 | 0.1675 |
| Fracture strain (-) | 0.1947 | 0.1266 | 0.4440 | 0.1827 | 0.1487 | 0.2186 | 0.1660 | 0.1417 | 0.1930 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Faragó, D.; Szebényi, G.; Temesi, T.; Mária Kiss, R.; Pap, K. Evaluation of the Effect of Freezing and Gamma Irradiation on Different Types of Tendon Allografts by DIC Assisted Tensile Testing. Appl. Sci. 2020, 10, 5369. https://doi.org/10.3390/app10155369
Faragó D, Szebényi G, Temesi T, Mária Kiss R, Pap K. Evaluation of the Effect of Freezing and Gamma Irradiation on Different Types of Tendon Allografts by DIC Assisted Tensile Testing. Applied Sciences. 2020; 10(15):5369. https://doi.org/10.3390/app10155369
Chicago/Turabian StyleFaragó, Dénes, Gábor Szebényi, Tamás Temesi, Rita Mária Kiss, and Károly Pap. 2020. "Evaluation of the Effect of Freezing and Gamma Irradiation on Different Types of Tendon Allografts by DIC Assisted Tensile Testing" Applied Sciences 10, no. 15: 5369. https://doi.org/10.3390/app10155369
APA StyleFaragó, D., Szebényi, G., Temesi, T., Mária Kiss, R., & Pap, K. (2020). Evaluation of the Effect of Freezing and Gamma Irradiation on Different Types of Tendon Allografts by DIC Assisted Tensile Testing. Applied Sciences, 10(15), 5369. https://doi.org/10.3390/app10155369





