Cortical Reorganization after Rehabilitation in a Patient with Conduction Aphasia Using High-Density EEG
Abstract
1. Introduction
2. Case Presentation
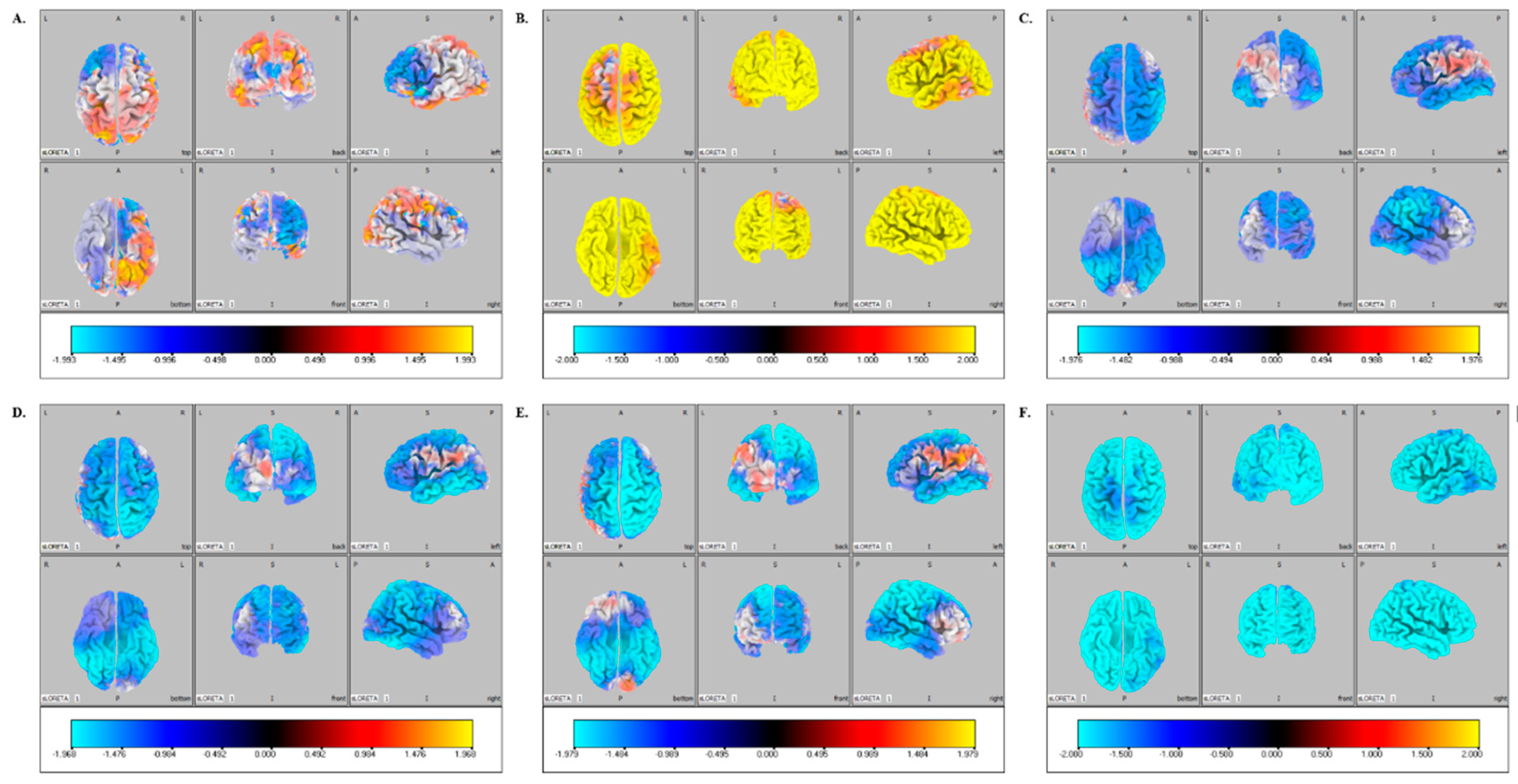
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Ardila, A. A Review of Conduction Aphasia. Curr. Neurol. Neurosci. Rep. 2010, 10, 499–503. [Google Scholar] [CrossRef] [PubMed]
- Acharya, A.B.; Maani, C.V. Conduction Aphasia; StatPearls Publishing: Treasure Island, FL, USA, 2020. [Google Scholar]
- Wernicke, C. Der aphasische symptomencomplex: Eine psychologische studie auf anatomischer basis. In Wernicke’s Works on Aphasia; Eggert, G.H., Ed.; Hague: Mouton, NY, USA, 1977; pp. 91–145. [Google Scholar]
- Geschwind, N. Disconnexion syndromes in animals and man. Brain 1965, 88, 585. [Google Scholar] [CrossRef] [PubMed]
- Hickok, G.; Erhard, P.; Kassubek, J.; Helms-Tillery, A.; Naeve-Velguth, S.; Strupp, J.P.; Strick, P.L.; Ugurbil, K. A functional magnetic resonance imaging study of the role of left posterior superior temporal gyrus in speech production: Implications for the explanation of conduction aphasia. Neurosci. Lett. 2000, 287, 156–160. [Google Scholar] [CrossRef]
- Phillips, O.R.; Clark, K.A.; Woods, R.P.; Subotnik, K.L.; Asarnow, R.F.; Nuechterlein, K.H.; Toga, A.W.; Narr, K.L. Topographical relationships between arcuate fasciculus connectivity and cortical thickness. Hum. Brain Mapp. 2010, 32, 1788–1801. [Google Scholar] [CrossRef]
- Nunnari, D.; Bonanno, L.; Bramanti, P.; Marino, S. Diffusion Tensor Imaging and Neuropsychologic Assessment in Aphasic Stroke. J. Stroke Cerebrovasc. Dis. 2014, 23, e477–e478. [Google Scholar] [CrossRef] [PubMed]
- Angrilli, A.; Elbert, T.; Cusumano, S.; Stegagno, L.; Rockstroh, B. Temporal dynamics of linguistic processes are reorganized in aphasics’ cortex: An EEG mapping study. NeuroImage 2003, 20, 657–666. [Google Scholar] [CrossRef]
- Breier, J.I.; Castillo, E.M.; Boake, C.; Billingsley, R.; Maher, L.; Francisco, G.; Papanicolaou, A.C. Spatiotemporal patterns of language-specific brain activity in patients with chronic aphasia after stroke using magnetoencephalography. NeuroImage 2004, 23, 1308–1316. [Google Scholar] [CrossRef]
- Spironelli, C.; Angrilli, A. Brain plasticity in aphasic patients: Intra- and inter-hemispheric reorganisation of the whole linguistic network probed by N150 and N350 components. Sci. Rep. 2015, 5, 12541. [Google Scholar] [CrossRef]
- Sarasso, S.; Määttä, S.; Ferrarelli, F.; Poryazova, R.; Tononi, G.; Small, S.L. Plastic changes following imitation-based speech and language therapy for aphasia: A high-density sleep EEG study. Neurorehabilit. Neural Repair 2014, 28, 129–138. [Google Scholar] [CrossRef]
- Qiu, W.H.; Wu, H.X.; Yang, Q.L.; Kang, Z.; Chen, Z.C.; Li, K.; Chen, S.Q. Evidence of cortical reorganization of language networks after stroke with subacute Broca’s aphasia: A blood oxygenation level dependent-functional magnetic resonance imaging study. Neural Regen. Res. 2017, 12, 109. [Google Scholar] [CrossRef]
- Northam, G.B.; Adler, S.; Msc, K.C.J.E.; Chong, W.K.; Cowan, F.M.; Baldeweg, T. Developmental conduction aphasia after neonatal stroke. Ann. Neurol. 2018, 83, 664–675. [Google Scholar] [CrossRef] [PubMed]
- Thompson, C.K.; Ouden, D.-B.D. Neuroimaging and recovery of language in aphasia. Curr. Neurol. NeuroSci. Rep. 2008, 8, 475–483. [Google Scholar] [CrossRef] [PubMed]
- Stefaniak, J.D.; Halai, A.D.; Ralph, M.A.L. The neural and neurocomputational bases of recovery from post-stroke aphasia. Nat. Rev. Neurol. 2019, 16, 43–55. [Google Scholar] [CrossRef] [PubMed]
- Demeurisse, G.; Capon, A. Brain Activation During a Linguistic Task in Conduction Aphasia. Cortex 1991, 27, 285–294. [Google Scholar] [CrossRef]
- Mattioli, F.; Ambrosi, C.; Mascaro, L.; Scarpazza, C.; Pasquali, P.; Frugoni, M.; Magoni, M.; Biagi, L.; Gasparotti, R. Early Aphasia Rehabilitation is Associated with Functional Reactivation of the Left Inferior Frontal Gyrus: A pilot study. Stroke 2014, 45, 545–552. [Google Scholar] [CrossRef]
- Mammone, N.; De Salvo, S.; Ieracitano, C.; Marino, S.; Cartella, E.; Bramanti, A.; Giorgianni, R.; Morabito, F.C. Compressibility of High-Density EEG Signals in Stroke Patients. Sensors 2018, 18, 4107. [Google Scholar] [CrossRef]
- Robertson, I.H.; Murre, J.M. Rehabilitation of brain damage: Brain plasticity and principles of guided recovery. Psychol. Bull. 1999, 125, 544. [Google Scholar] [CrossRef]
- Rossini, P.M.; Altamura, C.; Ferreri, F.; Melgari, J.; Tecchio, F.; Tombini, M.; Pasqualetti, P.; Vernieri, F. Neuroimaging experimental studies on brain plasticity in recovery from stroke. Eur. Med. 2007, 43, 241. [Google Scholar]
- Vigneau, M.; Beaucousin, V.; Hervé, P.; Duffau, H.; Crivello, F.; Houde, O.; Mazoyer, B.; Tzourio-Mazoyer, N. Meta-analyzing left hemisphere language areas: Phonology, semantics, and sentence processing. NeuroImage 2006, 30, 1414–1432. [Google Scholar] [CrossRef]
- Hickok, G.; Love-Geffen, T.; Klima, E.S. Role of the left hemisphere in sign language comprehension. Brain Lang. 2002, 82, 167–178. [Google Scholar] [CrossRef]
- Isaacs, E.; Christie, D.; Vargha-Khadem, F.; Mishkin, M. Effects of hemispheric side of injury, age at injury, and presence of seizure disorder on functional ear and hand asymmetries in hemiplegic children. NeuroPsychology 1996, 34, 127–137. [Google Scholar] [CrossRef]
- Lazar, R.M.; Marshall, R.S.; Pile-Spellman, J.; Duong, H.C.; Mohr, J.; Young, W.L.; Solomon, R.L.; Perera, G.M.; Delapaz, R.L. Interhemispheric transfer of language in patients with left frontal cerebral arteriovenous malformation. Neuropsychologia 2000, 38, 1325–1332. [Google Scholar] [CrossRef]
| AAT | Score T0 | Score T1 |
|---|---|---|
| Token test | 44 | 59 |
| Repetition | 36 | 45 |
| Written language | 42 | 53 |
| Naming | 42 | 59 |
| Comprehension | 56 | 56 |
| Test | Score | Cut Off |
|---|---|---|
| Raven-Coloured Matrices | 31 | 17.5 |
| Wisconsin Card Sorting Test global score | 54.1 | 90.50 |
| Wisconsin Card Sorting Test perseverative errors | 21 | 42.60 |
| Wisconsin Card Sorting Test no perseverative errors | 7.8 | 29.90 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Formica, C.; De Salvo, S.; Micchìa, K.; La Foresta, F.; Dattola, S.; Mammone, N.; Corallo, F.; Ciavola, A.; Arcadi, F.A.; Marino, S.; et al. Cortical Reorganization after Rehabilitation in a Patient with Conduction Aphasia Using High-Density EEG. Appl. Sci. 2020, 10, 5281. https://doi.org/10.3390/app10155281
Formica C, De Salvo S, Micchìa K, La Foresta F, Dattola S, Mammone N, Corallo F, Ciavola A, Arcadi FA, Marino S, et al. Cortical Reorganization after Rehabilitation in a Patient with Conduction Aphasia Using High-Density EEG. Applied Sciences. 2020; 10(15):5281. https://doi.org/10.3390/app10155281
Chicago/Turabian StyleFormica, Caterina, Simona De Salvo, Katia Micchìa, Fabio La Foresta, Serena Dattola, Nadia Mammone, Francesco Corallo, Adriana Ciavola, Francesca Antonia Arcadi, Silvia Marino, and et al. 2020. "Cortical Reorganization after Rehabilitation in a Patient with Conduction Aphasia Using High-Density EEG" Applied Sciences 10, no. 15: 5281. https://doi.org/10.3390/app10155281
APA StyleFormica, C., De Salvo, S., Micchìa, K., La Foresta, F., Dattola, S., Mammone, N., Corallo, F., Ciavola, A., Arcadi, F. A., Marino, S., Bramanti, A., & Bonanno, L. (2020). Cortical Reorganization after Rehabilitation in a Patient with Conduction Aphasia Using High-Density EEG. Applied Sciences, 10(15), 5281. https://doi.org/10.3390/app10155281









