1. Introduction
Pancreatic cancer remains one of the most difficult-to-treat cancers, and the overall survival of patients with an advanced stage is low [
1]. Pancreatic cancer is often diagnosed to be unresectable and the available therapeutic methods, including chemo- and radiotherapy, are not effective. Thus, alternative methods such as electroporation (EP)-based treatments could be considered here. EP is a physical method in which electrodes are placed within tissue, and short electrical impulses (from picosecond up to milliseconds) with an electric field intensity in the range of 1000 to 3000 V/cm are administered, which causes permeabilization of the cell membrane through lipid rearrangement [
2]. Consequently, new transport pathways termed “nanopores” for ions and other hydrophilic particles, including drugs, are created. Changes in the concentration of electrolytes such as potassium and sodium lead to rapid changes in cell membrane potential. Destabilization of the cell membrane causes disturbance in cell homeostasis, the relocation of calcium ions to inside of the cell, and death due to the depletion of energy sources [
3,
4]. Disturbances in calcium metabolism and homeostasis, mitochondrial damage, and depletion of ATP sources lead to a rapid necrotic type of cell death. However, overloading cells with a subthreshold concentration of calcium ions may induce the apoptotic pathway wherein a large number of damaged cells are removed by macrophages [
5,
6].
The changes induced in cells by electrical pulses can be reversible (Reversible Electroporation [RE]) or irreversible (Irreversible Electroporation [IRE]) depending on the voltage and number of pulses affecting the cell membrane. The effectiveness of this method for treating various types of cancers was proved by several in vivo and in vitro preclinical studies [
7,
8,
9,
10,
11]. The protocol of its application has also been developed and described, in combination with chemotherapy (CTH), in ESOPE (The European Standard Operating Procedures of Electrochemotherapy) for the treatment of cutaneous and subcutaneous lesions [
12,
13]. Electrotherapy is an ablation method with low thermal effect, and it is used for treating head and neck cancer, brain cancer, sarcoma, metastatic cancer, or primary cancer of liver, lungs, breast, pancreatic, and prostate cancer [
14,
15,
16,
17,
18,
19,
20,
21,
22,
23]. The combination of these two methods, established as electrochemotherapy (ECT), was applied the most to treat different solid tumors and with different chemotherapeutics in in vitro studies. The simultaneous conduction of EP and administration of a chemotherapeutic agent increase the cytotoxicity of a given drug against cancer cells and decrease the dose of a drug necessary for intravenous administration, which in turn decreases the drug toxicity affecting a patient. This leads to a higher “effectiveness’ of chemotherapeutics [
24,
25,
26,
27,
28,
29]. Some ECT research has been conducted on patients with pancreatic cancer who showed metastatic changes. The studies were conducted using bleomycin, cisplatin, and doxorubicin [
29,
30,
31,
32,
33,
34,
35,
36]. ECT using bleomycin was found to be most “effective”; this agent is 700 to 1000 times more toxic when administered simultaneously with IRE application [
24,
36,
37,
38]. The effectiveness of ECT using bleomycin was demonstrated only in an animal model with pancreatic cancer [
39] or melanoma [
26].
In studies, patients with nonresectable pancreatic cancer first received Folfrinox or Gemox CTH, and when the tumor was stabilized according to RECIST (i.e. response evaluation criteria in solid tumors) criteria, IRE alone was applied. Positive results were obtained, and the changes in the tumor mass were not visible in computer tomography but only in MRI [
40]. Another novel approach is the combination of EP with intratumoral (IT) administration of calcium ions. Calcium is an electrolyte involved in many cellular processes, including cell division, metabolism, and cell death, and its intracellular concentration is strictly regulated [
41,
42,
43,
44]. When the level of intracellular calcium increases, it induces a higher consumption of ATP. The production of ATP in mitochondria and its loss through the damaged cell membrane also decreases. Ultimately, this causes the death of cells due to ATP depletion, as demonstrated in in vitro and in vivo studies, which finally leads to tumor necrosis [
44,
45,
46,
47,
48].
The intratumoral administration of calcium ions causes the abscopal effect, which enables destroying not only the primary lesion itself but also distant metastasis [
39,
48,
49]. This effect is analogous to the abscopal effect, which occurs during radiotherapy. The low cost, availability, and low profile of adverse effects make the administration of calcium solutions very attractive. The effectiveness of calcium electroporation (CaEP) has been confirmed by Plaschke et al. [
50]. Moreover, IRE in combination with CTH stimulates the immune response. Tc lymphocytes (cytotoxic T cells) are stimulated, and the cancer cells undergo apoptosis. Probably, Tc cells that are responsible for the death of cancer cells are also involved in immunological memory. Several reports have shown that remission occurs not only locally but also systemically, i.e., metastases in distant areas that were not subjected to IRE are affected [
51,
52]. The advantages and simplicity of the IRE approach encourage the application of this method and further research on it. Thus, in this article, we report the first application of IRE supported by calcium ions in the treatment of three patients with unresectable pancreatic cancer.
2. Methods and Patient Representation
Our hypothesis is that calcium electroporation is a safe and sufficient method in locally advanced pancreatic cancer. Two first cases are presented in the current paper. The cases were individually discussed with an interdisciplinary team to ensure that all treating physicians agreed with the suggested therapeutic plan. All patients signed a consent form in accordance with the institutional guidelines (acc. Wroclaw Medical University, Poland). The study was approved by the Wroclaw Medical University ethical committee (No.: KB-330/2018).
The patients considered eligible for IRE/ECT had stage III pancreatic cancer (irresectable, locally advanced pancreatic cancer (LAPC) with no metastasis), which was histopathologically confirmed (adenocarcinoma). The inclusion criteria for nonresectable cases were more than 180° infiltration of the portal or mesenteric vein or infiltration of the mesenteric artery, celiac trunk, or hepatic artery. The exclusion criteria were severe cardiac failure with a pacemaker.
The performance status of the patients was assessed according to the WHO (World Health Organization) scale, additional comorbidities according to the Charlson Comorbidity Index, and pain level according to the visual analog scale (VAS) (before the procedure, immediately after it, and three months later). The level of Ca 19-9 before the procedure, after the procedure, and 3 months later and the amylase and lipase levels directly after the procedure were also measured. The decrease in body weight before the procedure and three months later was verified. The overall survival of the patients was noted. Patients were qualified for the procedure and then were monitored using MRI (if possible) and CT (computerized tomography). The IRE procedure was conducted using the open method, with general anesthesia, under the guidance of intraoperative ultrasound with assistance from a radiologist. Immediately after the procedure, surgical complications were assessed using the Clavien-Dindo scale. The IRE device, NanoKnife®, was provided by AngioDynamics (Queensbury, NY, USA). The IRE protocol was followed according to the manufacturer’s specifications and recommended parameters.
3. Results
3.1. Case 1: With Intratumoral Administration of Calcium Ions
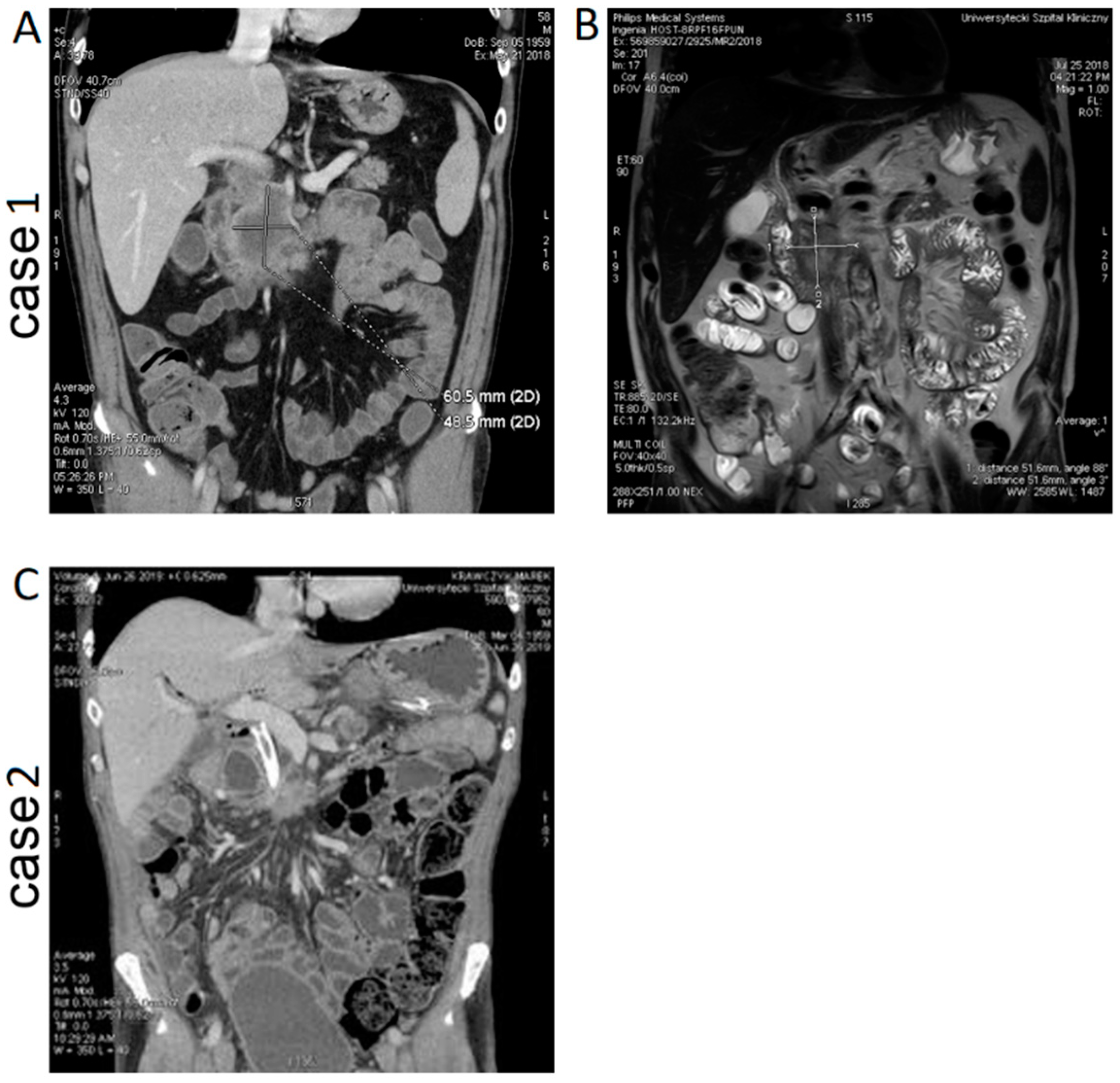
A 58-year-old patient with no significant medical history was qualified for an ablation procedure after stabilization during CTH of pancreatic cancer. The patient was primarily operated due to food intake disorder, and gastric bypass and biliary intestinal bypass were performed. After pancreatic cancer was confirmed by a histopathological examination, the patient was referred for CTH and received preoperatively 5 cycles of nab-paclitaxel + GCB with poor tolerance. Finally, after 3 months of CTH, the patient was qualified for IRE treatment. CT and MRI images of the patient are presented in
Figure 1A,B. Preoperative CT revealed a hypodense tumor mass at the head of the pancreas (61 × 49 mm) infiltrating the mesenteric and portal vein. The clinical parameters of the patient are shown in
Table 1. Analgesics (NSAIDs) i.e. nonsteroidal anti-inflammatory drugs were taken only on demand, and the VAS score was 2. The patient was qualified for the IRE procedure by the open method under the guidance of intraoperative ultrasound. The patient was recommended an option of intraprocedural administration of one dose of cisplatin and/or injecting the tumor with calcium solution. The patient agreed only for the injection of the tumor with calcium solution because of his poor CTH tolerance. The tumor was injected intraoperatively with calcium ion solution (10 mM), and after the injection, the ablation procedure was performed. IRE was performed intraoperatively with 4 needles according to the manufacturer’s instruction. The needles were placed around the tumor tissue to treat the adjacent regions with 6 series first and later for another 8 cycles. The surgical time was 2 h 50 min without the bypass procedure. During the procedure, it was found that after calcium administration, the tissue conductivity changed, which required a decrease in voltage, leading to stopping the procedure, so there was a need to decrease the voltage.
Postoperatively, the patient developed acute necrotizing pancreatitis with an increase in amylase level to 478 U/L. Symptoms of pancreatic fistula were found (amylase level in the drain fluid was 51,280 U/mL). Postoperatively, there was no increase in the Ca 19-9 level (28 U/mL). The VAS score of the pain level was 8. The patient was treated intensively according to the recommendations related to necrotic hemorrhagic pancreatitis, which was also confirmed by CT examination: in the head and the uncinate process of the pancreas, a hypodense lesion (4 cm) was found with features of lysis along with free fluid in the peritoneal cavity. The patient responded well to the conservative treatment and was discharged 10 days after the surgical procedure, with drain and drainage of around 100 mL of clear fluid. The complication was assessed at the level of III/IV in the Clavien-Dindo scale.
3.2. Case 2: With Intratumoral Administration Calcium Ions and Intravenous, Simultaneous CTH
A 60-year-old male without comorbidities was qualified to undergo ablation after stabilization of the size of the pancreatic tumor according to RECIST criteria. The patient had been operated on 9 months before admission and underwent a palliative, bypass procedure later with FOLFOX (folinic acid calcium folinate, fluorouracil, and oxaliplatin) chemotherapy. A CT image of the patient is presented in
Figure 1C. CT before ablative treatment revealed a pancreatic tumor of size 2 × 3 cm with infiltration of the mesenteric vein. The mesentery showed high density. There was no sign of metastatic disease. The patient experienced pain (VAS score was 6) and was treated with opioids and paresthesia of the hand and legs. Preoperative MRI was not performed because of the metal prosthesis of the common bile duct (SEMS, i.e. self-expandable metal stent).
The clinical parameters of the patient are shown in
Table 1.
The patient agreed to an intratumoral administration of calcium ions and intravenous, intraoperative administration of cisplatin. The open procedure under general anesthesia with an intraoperative US was performed. The metal prosthesis was removed before the ablative procedure. The operating time was 3 h 10 min. The procedure consisted of 11 sessions, one replacement of the needles, intratumoral administration of calcium ions after the ablative procedure, and intravenous cisplatin administration before the ablative procedure.
Postoperatively, the patient showed no severe complications. The amylase level was 61 U/L, and it decreased to 33 U/L. The VAS score after the procedure decreased to 4. Two weeks after the procedure, the patient developed pancreatic fistula type B with approximately 10–20 mL secretion per 24 h. However, this did not affect the further “treatment plan”. Necrosis of the tumor was apparent “immediately after” the CT scan. There were no signs of pancreatitis. Complications after the procedure were assessed at grade II in the Clavien-Dindo scale.
Table 2 shows the IRE conditions and pharmacological treatment applied during all sessions.
3.3. Three Months Observations and Overall Survival Post IRE
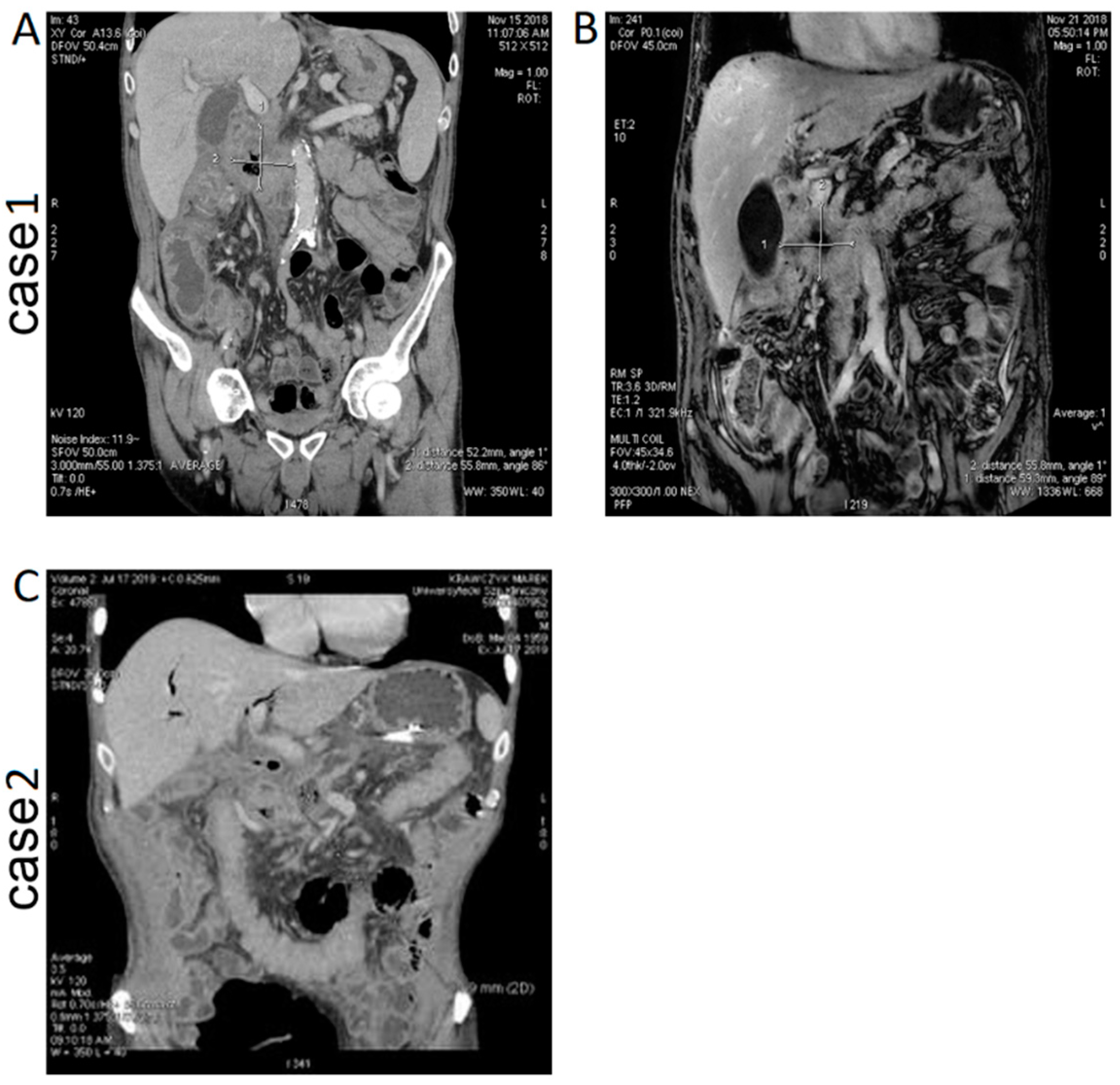
Case 1 (IRE with intratumoral administration of calcium ions). CT and MRI images of the patient are presented in
Figure 2A,B. In the follow-up CT examination, the size of the pancreatic tumor was found to be reduced, with features of internal lysis. The presence of air inside the tumor was related to a fistula between the pancreatic ducts and the tumor, and inflammation was also detected.
In the follow-up MRI (T1 images, contrast-dependent), a necrotic area in the tumor was also found. However, the patient was discharged in good condition and referred for further CTH. The patient was admitted for follow-up examination after the ablation procedure. After the procedure, he did not receive CTH due to persistent pancreatic fistula. The drain was removed, and the patient’s WHO performance status was I. He did not report impaired food intake; however, he did report appetite disorders, which was most likely related to acute pancreatitis, immediately after the procedure. A further decrease in body weight to 75 kg (8 kg since the procedure) occurred; however, at present, the patient does not report further weight loss. The patient periodically takes analgesics (NSAIDs), and the VAS level periodically equals to 1. The Ca 19-9 level was 408 U/L. The patient was discharged in good condition and referred for further CTH. Unfortunately, the patient did not respond well to the chemotherapy. The patient refused treatment after another 3 months and died after 9 months from diagnosis and 6 months from calcium electroporation.
Case 2 (IRE with intratumoral administration of calcium ions and intraoperative chemotherapy). CT image of the patient after the procedure is presented in
Figure 2C. In a short time after IRE-Ca administration, the patient continued with CTH (for 2 months). The pancreatic fistula had ceased to be active. Moreover, the patient gained 5 kg weight, and the VAS score was 1. The patient has stopped the intake of opioids, and his quality of life is currently significantly better.
After 21 months from diagnosis and 12 months from calcium electroporation and electrochemotherapy, the patient is well. He gained another 15 kg, has no fistula sign, and has no pain. He continued CTH, but to date has had a 2-month break. In CT, there is a stabilization of the tumor mass. He is still under observation and planning another procedure if there is any progression of the tumor.
Table 3 presents the comparison of clinical data between the cases.
4. Discussion
The use of electroablation for nonresectable pancreatic tumors is controversial, and the largest groups of patients include 200–300 individuals. Patients qualified for ablation are most often treated with CTH, which impedes the determination of the effectiveness of IRE alone. However, for patients with nonresectable pancreatic cancers, electroablation often remains the last treatment option, and at this stage of cancer, every attempt to extend life expectancy or, at least, improve the quality of life (for example, by decreasing pain) seems legitimate.
Here, we have presented two cases: the IRE method was combined with calcium chloride as a cytostatic drug. Irreversible and reversible electroporation occurs simultaneously during the procedure, and it is impossible to know the range of each. There are some simulation and predictive studies estimating the IRE range [
53,
54]. Thus, the application of chemotherapeutic agent seems reasonable. In the first case, changes in the tissue conductivity were observed with the intratumoral administration of calcium ions before the IRE procedure. This required an adjustment of the device and the application of lower voltage doses. Immediately after the procedure, the patient developed acute pancreatitis. The patient was hospitalized for a longer period of time, and the complication, namely pancreatic fistula, persisted up to one month after the procedure, delaying chemotherapeutic treatment according to the protocol. The patient lost weight and experienced significantly more severe pain related to the complication. Two months after the procedure, the patient was feeling fine; there was no impairment of food intake and no further weight loss. The patient qualified for systemic treatment, which was inefficient, and the patient died 9 months after diagnosis. In the second case, IRE was conducted with intraoperative chemotherapy and calcium electroporation, which was administered after the ablative procedure. The patient recovered well, developed pancreatic fistula with low secretion, which did not require any treatment, and his chemotherapy protocol was continued. After three months of observation, the patient did not feel any pain; hence, neurolysis during the ablative procedure might have occurred. The patient discontinued opioid treatment, gained weight, and is still alive 21 months after a pancreatic cancer diagnosis.
Currently, there are no standards for combining IRE or ECT with chemotherapeutic treatment protocols for pancreatic tumors. The present clinical data indicate a positive effect of IRE alone in pancreatic cancer [
52], including LAPC [
55]. Clinical trials in which pancreatic ductal adenocarcinoma (PDAC) was treated by IRE combined with anti-programmed cell death protein 1 (anti-PD1) have shown promising results [
56]. However, to date, our study is the first one in which the IRE method was combined with intratumoral administration of the calcium ions. Although the question of whether IRE alone should be applied as a palliative method has been responded to positively, the appropriate positioning of IRE application remains to be clarified. Studies on the use of calcium electroporation in skin cancers have shown no strong rationale for the application of IRE electrochemotherapy to treat pancreatic cancer, and the effect of the application of calcium ions in visceral tumors remains unknown. Based on the patient selection and the frequency of examination, it is necessary to conduct multicenter studies.
5. Conclusions
The cases presented in this study revealed that the IRE protocol can be applied for the treatment of LAPC. The IRE procedure with calcium ion administration was also effective (more extended tumor necrosis was visible), despite complications after the surgery. It is difficult to state whether the acute pancreatitis that developed in the first case and pancreatic fistula in the second case were caused by the IRE procedure alone or administration of calcium ions or too high concentration, and to what extent pancreatitis influenced the occurrence of necrosis. Therefore, the cause of pancreatitis and the reason for the larger necrosis visible in the imaging of the abdominal cavity are unknown. It remains unclear whether the administration of calcium ions is safe for the pancreas, which is a chimeric and delicate organ, and it requires studies on the animal model. However, this treatment option should be considered for chemoresistant patients. The determination of calcium ion concentration necessary for this procedure and the time of administration (administration after the IRE procedure seems to be safer) also requires further study. It seems that the administration of calcium ions may increase the effectiveness of the procedure itself.
In the first case, the general condition of the patient delayed the initiation of systemic treatment, which is a standard method of palliative pancreatic cancer treatment.
The implications of this procedure in terms of overall survival of the patient remain unknown because it is a case report. In the first case, overall survival was 9 months from diagnosis, but in the second case, it is already 21 months, and the patient is in very good condition (WHO 0). It is hard to say that this is due to calcium electroporation or electrochemotherapy (patient received an intravenous dose of cisplatin during the IRE procedure), but it seems promising for longer survival in palliative pancreatic cancer patients. Considering these two cases, it is certain that electroporation and systemic treatment should be used together as in the second case (the first one resigns twice from the CTH because of poor tolerance).
Summarizing, we conclude that IRE is a safe method for treating LAPC. The administration of calcium ions before the procedure changes tissue conductivity; therefore, calcium ions should be delivered after the IRE procedure rather than before it. Furthermore, the administration of calcium ions may increase the effectiveness of IRE for treating pancreatic cancer. However, further clinical studies are required to determine the influence and concentration of calcium ions in combination with the ablation procedures for treating pancreatic cancer to confirm the hypothesis about the safeties of this method, but calcium electroporation seems to be efficient in pancreatic cancer treatment.
Author Contributions
Conceptualization, J.R.-R. and J.K.; methodology, J.R.-R., M.P., M.G.; formal analysis and investigation, J.R.-R. and J.K.; resources, W.K.; data curation, M.P., M.G.; writing—original draft preparation, J.R.-R.; writing—review and editing, J.K., visualization, M.P., M.G.; supervision, J.R.-R. and J.K.; project administration, W.K.; funding acquisition, W.K. All authors have read and agreed to the published version of the manuscript.
Funding
This work was performed in the scope of the project of Polish National Science Centre from the project SONATA BIS 6 2016/22/E/NZ5/00671 (PI: Kulbacka, J.) and the funds of Medical University Hospital from Wroclaw SUB. A190.19.026 (PI: Kielan, W.).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Wilkowski, R.; Wolf, M.; Heinemann, V. Primary advanced unresectable pancreatic cancer. Recent Results Cancer Res. 2008, 177, 79–93. [Google Scholar]
- Glaser, R.W.; Leikin, S.L.; Chernomordik, L.V.; Pastushenko, V.F.; Sokirko, A.I. Reversible electrical breakdown of lipid bilayers: Formation and evolution of pores. Biochim. Biophys. Acta 1988, 940, 275–287. [Google Scholar] [CrossRef]
- Clausen, T.; Gissel, H. Role of Na,K pumps in restoring contractility following loss of cell membrane integrity in rat skeletal muscle. Acta Physiol. Scand. 2005, 183, 263–271. [Google Scholar] [CrossRef] [PubMed]
- Hojman, P.; Gissel, H.; Andre, F.M.; Cournil-Henrionnet, C.; Eriksen, J.; Gehl, J.; Mir, L.M. Physiologic effect of high and low-voltage pulse combinations for gene electrotransfer in muscle. Hum. Gene Ther. Methods 2008, 19, 1249–1260. [Google Scholar] [CrossRef]
- Dong, Z.; Saikumar, P.; Weinberg, J.M.; Venkatachalam, M.A. Calcium in cell injury and death. Annu. Rev. Pathol. Mech. Dis. 2006, 1, 405–434. [Google Scholar] [CrossRef] [PubMed]
- Gissel, H.; Lee, R.C.; Gehl, J. Electroporation and Cellular Physiology. In Clinical Aspects of Electroporation; Springer: New York, NY, USA, 2011; pp. 9–17. [Google Scholar]
- Miller, L.; Leor, J.; Rubinsky, B. Cancer cells ablation with irreversible electroporation. Technol. Cancer Res. Treat. 2005, 4, 699–705. [Google Scholar] [CrossRef] [PubMed]
- Neal, R.E.; Singh, R.; Hatcher, H.C.; Kock, N.D.; Torti, S.V.; Davalos, R.V. Treatment of breast cancer through the application of irreversible electroporation using a novel minimally invasive single needle electrode. Breast Cancer Res. Treat. 2010, 123, 295–301. [Google Scholar] [CrossRef] [PubMed]
- Campana, L.G.; Mocellin, S.; Basso, M.; Puccetti, O.; De Salvo, G.L.; Chiarion-Sileni, V.; Vecchiato, A.; Corti, L.; Rossi, C.R.; Nitti, D. Bleomycin-based electrochemotherapy: Clinical outcome from a single institution’s experience with 52 patients. Ann. Surg. Oncol. 2009, 16, 191–199. [Google Scholar] [CrossRef]
- Reinhold, U. Elektrochemotherapie von Hauttumoren. Hautarzt 2011, 62, 549–559. [Google Scholar] [CrossRef]
- Muñoz Madero, V.; Ortega Pérez, G. Electrochemotherapy for treatment of skin and soft tissue tumours. Update and definition of its role in multimodal therapy. Clin. Transl. Oncol. 2011, 13, 18–24. [Google Scholar] [CrossRef]
- Marty, M.; Sersa, G.; Garbay, J.R.; Gehl, J.; Collins, C.G.; Snoj, M.; Billard, V.; Geertsen, P.F.; Larkin, J.O.; Miklavcic, D.; et al. Electrochemotherapy—An easy, highly effective and safe treatment of cutaneous and subcutaneous metastases: Results of ESOPE (European Standard Operating Procedures of Electrochemotherapy) study. Eur. J. Cancer Suppl. 2006, 4, 3–13. [Google Scholar] [CrossRef]
- Gehl, J.; Sersa, G.; Matthiessen, L.W.; Muir, T.; Soden, D.; Occhini, A.; Quaglino, P.; Curatolo, P.; Campana, L.G.; Kunte, C.; et al. Updated standard operating procedures for electrochemotherapy of cutaneous tumours and skin metastases. Acta Oncol. 2018, 57, 874–882. [Google Scholar] [CrossRef] [PubMed]
- Garcia, P.A.; Neal, R.E.; Rossmeisl, J.H.; Davalos, R.V. Non-thermal irreversible electroporation for deep intracranial disorders. In Proceedings of the 2010 Annual International Conference of the IEEE Engineering in Medicine and Biology Society, EMBC’10, Buenos Aires, Argentina, 31 August–4 September 2010; Volume 2010, pp. 2743–2746. [Google Scholar]
- Neal, R.E.; Rossmeisl, J.H.; Garcia, P.A.; Lanz, O.I.; Henao-Guerrero, N.; Davalos, R.V. Successful treatment of a large soft tissue sarcoma with irreversible electroporation. J. Clin. Oncol. 2011, 29, e372–e377. [Google Scholar] [CrossRef] [PubMed]
- Frandsen, S.K.; Gehl, J. A Review on Differences in Effects on Normal and Malignant Cells and Tissues to Electroporation-Based Therapies: A Focus on Calcium Electroporation. Technol. Cancer Res. Treat. 2018, 17, 1533033818788077. [Google Scholar] [CrossRef]
- Kingham, T.P.; Karkar, A.M.; D’Angelica, M.I.; Allen, P.J.; Dematteo, R.P.; Getrajdman, G.I.; Sofocleous, C.T.; Solomon, S.B.; Jarnagin, W.R.; Fong, Y. Ablation of perivascular hepatic malignant tumors with irreversible electroporation. J. Am. Coll. Surg. 2012, 215, 379–387. [Google Scholar] [CrossRef] [PubMed]
- Niessen, C.; Thumann, S.; Beyer, L.; Pregler, B.; Kramer, J.; Lang, S.; Teufel, A.; Jung, E.M.; Stroszczynski, C.; Wiggermann, P. Percutaneous Irreversible Electroporation: Long-term survival analysis of 71 patients with inoperable malignant hepatic tumors. Sci. Rep. 2017, 7, 43687. [Google Scholar] [CrossRef]
- Curatolo, P.; Rotunno, R.; Miraglia, E.; Mancini, M.; Calvieri, S.; Giustini, S. Complete Remission of Merkel Cell Carcinoma Treated With Electrochemotherapy and Etoposide. G. Ital. Dermatol. Venereol. 2013, 148, 310–311. [Google Scholar]
- Plaschke, C.C.; Bertino, G.; McCaul, J.A.; Grau, J.J.; de Bree, R.; Sersa, G.; Occhini, A.; Groselj, A.; Langdon, C.; Heuveling, D.A.; et al. European Research on Electrochemotherapy in Head and Neck Cancer (EURECA) project: Results from the treatment of mucosal cancers. Eur. J. Cancer 2017, 87, 172–181. [Google Scholar] [CrossRef]
- Martin, R.C.G. Irreversible electroporation of locally advanced pancreatic neck/body adenocarcinoma. J. Gastrointest. Oncol. 2015, 6, 329–335. [Google Scholar]
- Martin, R.C.G.; Kwon, D.; Chalikonda, S.; Sellers, M.; Kotz, E.; Scoggins, C.; McMasters, K.M.; Watkins, K. Treatment of 200 locally advanced (Stage III) pancreatic adenocarcinoma patients with irreversible electroporation safety and efficacy. Ann. Surg. 2015, 262, 486–492. [Google Scholar] [CrossRef]
- Månsson, C.; Bergenfeldt, M.; Brahmstaedt, R.; Karlson, B.-M.; Nygren, P.; Nilsson, A. Safety and Preliminary Efficacy of Ultrasound-Guided Percutaneous Irreversible Electroporation for Treatment of Localized Pancreatic Cancer. Anticancer Res. 2014, 34, 289–293. [Google Scholar] [PubMed]
- Lambert, L.; Horejs, J.; Krska, Z.; Hoskovec, D.; Petruzelka, L.; Krechler, T.; Kriz, P.; Briza, J. Treatment of locally advanced pancreatic cancer by percutaneous and intraoperative irreversible electroporation: General hospital cancer center experience. Neoplasma 2016, 63, 269–273. [Google Scholar] [CrossRef]
- Orlowski, S.; Belehradek, J.; Paoletti, C.; Mir, L.M. Transient electropermeabilization of cells in culture. Increase of the cytotoxicity of anticancer drugs. Biochem. Pharmacol. 1988, 37, 4727–4733. [Google Scholar] [CrossRef]
- Jaroszeski, M.J.; Dang, V.; Pottinger, C.; Hickey, J.; Gilbert, R.; Heller, R. Toxicity of anticancer agents mediated by electroporation in vitro. Anticancer Drugs 2000, 11, 201–208. [Google Scholar] [CrossRef]
- Gehl, J.; Skovsgaard, T.; Mir, L.M. Enhancement of cytotoxicity by electropermeabilization: An improved method for screening drugs. Anticancer Drugs 1998, 9, 319–325. [Google Scholar] [CrossRef] [PubMed]
- Gehl, J. Electroporation: Theory and methods, perspectives for drug delivery, gene therapy and research. Acta Physiol. Scand. 2003, 177, 437–447. [Google Scholar] [CrossRef] [PubMed]
- Sersa, G.; Miklavcic, D.; Cemazar, M.; Rudolf, Z.; Pucihar, G.; Snoj, M. Electrochemotherapy in treatment of tumours. Eur. J. Surg. Oncol. 2008, 34, 232–240. [Google Scholar] [CrossRef]
- Kuriyama, S.; Matsumoto, M.; Mitoro, A.; Tsujinoue, H.; Nakatani, T.; Fukui, H.; Tsujii, T. Electrochemotherapy for colorectal cancer with commonly used chemotherapeutic agents in a mouse model. Dig. Dis. Sci. 2000, 45, 1568–1577. [Google Scholar] [CrossRef]
- Tafuto, S.; von Arx, C.; De Divitiis, C.; Tracey Maura, C.; Palaia, R.; Albino, V.; Fusco, R.; Membrini, M.; Petrillo, A.; Granata, V.; et al. Electrochemotherapy as a new approach on pancreatic cancer and on liver metastases. Int. J. Surg. 2015, 21, S78–S82. [Google Scholar] [CrossRef]
- Granata, V.; Fusco, R.; Piccirillo, M.; Palaia, R.; Petrillo, A.; Lastoria, S.; Izzo, F. Electrochemotherapy in locally advanced pancreatic cancer: Preliminary results. Int. J. Surg. 2015, 18, 230–236. [Google Scholar] [CrossRef]
- Michel, O.; Kulbacka, J.; Saczko, J.; Mdczynska, J.; Bbasiak, P.; Rossowska, J.; Rzechonek, A. Electroporation with Cisplatin against Metastatic Pancreatic Cancer: In Vitro Study on Human Primary Cell Culture. Biomed. Res. Int. 2018, 2018, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Saczko, J.; Pilat, J.; Choromanska, A.; Rembialkowska, N.; Bar, J.; Kaminska, I.; Zalewski, J.; Kulbacka, J. The effectiveness of chemotherapy and electrochemotherapy on ovarian cell lines in vitro. Neoplasma 2016, 63, 450–455. [Google Scholar] [CrossRef] [PubMed]
- Kulbacka, J.; Daczewska, M.; Dubińska-Magiera, M.; Choromańska, A.; Rembiałkowska, N.; Surowiak, P.; Kulbacki, M.; Kotulska, M.; Saczko, J. Doxorubicin delivery enhanced by electroporation to gastrointestinal adenocarcinoma cells with P-gp overexpression. Bioelectrochemistry 2014, 100, 96–104. [Google Scholar] [CrossRef] [PubMed]
- Miklavčič, D.; Mali, B.; Kos, B.; Heller, R.; Serša, G. Electrochemotherapy: From the drawing board into medical practice. Biomed. Eng. Online 2014, 13, 29. [Google Scholar] [CrossRef] [PubMed]
- Escoffre, J.-M.; Rols, M.-P. Electrochemotherapy: Progress and Prospects. Curr. Pharm. Des. 2012, 18, 3406–3415. [Google Scholar] [CrossRef] [PubMed]
- Mir, L.M.; Orlowski, S.; Belehradek, J.; Paoletti, C. Electrochemotherapy potentiation of antitumour effect of bleomycin by local electric pulses. Eur. J. Cancer Clin. Oncol. 1991, 27, 68–72. [Google Scholar] [CrossRef]
- Girelli, R.; Prejanò, S.; Cataldo, I.; Corbo, V.; Martini, L.; Scarpa, A.; Claudio, B. Feasibility and safety of electrochemotherapy (ECT) in the pancreas: A pre-clinical investigation. Radiol. Oncol. 2015, 49, 147–154. [Google Scholar] [CrossRef]
- Falk, H.; Lambaa, S.; Johannesen, H.H.; Wooler, G.; Venzo, A.; Gehl, J. Electrochemotherapy and calcium electroporation inducing a systemic immune response with local and distant remission of tumors in a patient with malignant melanoma—A case report. Acta Oncol. 2017, 56, 1126–1131. [Google Scholar] [CrossRef]
- Zhang, Z.; Li, W.; Procissi, D.; Tyler, P.; Omary, R.A.; Larson, A.C. Rapid dramatic alterations to the tumor microstructure in pancreatic cancer following irreversible electroporation ablation. Nanomedicine 2014, 9, 1181–1192. [Google Scholar] [CrossRef]
- Caracò, C.; Mozzillo, N.; Marone, U.; Simeone, E.; Benedetto, L.; Di Monta, G.; Di Cecilia, M.L.; Botti, G.; Ascierto, P.A. Long-lasting response to electrochemotherapy in melanoma patients with cutaneous metastasis. BMC Cancer 2013, 13, 564. [Google Scholar] [CrossRef]
- Carafoli, E.; Santella, L.; Branca, D.; Brini, M. Generation, control, and processing of cellular calcium signals. Crit. Rev. Biochem. Mol. Biol. 2001, 36, 107–260. [Google Scholar] [CrossRef] [PubMed]
- Berridge, M.J.; Bootman, M.D.; Roderick, H.L. Calcium signalling: Dynamics, homeostasis and remodelling. Nat. Rev. Mol. Cell Biol. 2003, 4, 517–529. [Google Scholar] [CrossRef] [PubMed]
- Kunte, C.; Letule, V.; Gehl, J.; Dahlstroem, K.; Curatolo, P.; Rotunno, R.; Muir, T.; Occhini, A.; Bertino, G.; Powell, B.; et al. Electrochemotherapy in the treatment of metastatic malignant melanoma: A prospective cohort study by InspECT. Br. J. Dermatol. 2017, 176, 1475–1485. [Google Scholar] [CrossRef] [PubMed]
- Frandsen, S.K.; Gehl, J. Effect of calcium electroporation in combination with metformin in vivo and correlation between viability and intracellular ATP level after calcium electroporation in vitro. PLoS ONE 2017, 12, e0181839. [Google Scholar] [CrossRef] [PubMed]
- Frandsen, S.K.; Gissel, H.; Hojman, P.; Tramm, T.; Eriksen, J.; Gehl, J. Direct therapeutic applications of calcium electroporation to effectively induce tumor necrosis. Cancer Res. 2012, 72, 1336–1341. [Google Scholar] [CrossRef]
- Hansen, E.L.; Sozer, E.B.; Romeo, S.; Frandsen, S.K.; Vernier, P.T.; Gehl, J. Dose-Dependent ATP depletion and cancer cell death following calcium electroporation, relative effect of calcium concentration and electric field strength. PLoS ONE 2015, 10, e0122973. [Google Scholar]
- Calvet, C.Y.; Famin, D.; André, F.M.; Mir, L.M. Electrochemotherapy with bleomycin induces hallmarks of immunogenic cell death in murine colon cancer cells. Oncoimmunology 2014, 3, e28131. [Google Scholar] [CrossRef]
- Plaschke, C.C.; Gehl, J.; Johannesen, H.H.; Fischer, B.M.; Kjaer, A.; Lomholt, A.F.; Wessel, I. Calcium electroporation for recurrent head and neck cancer: A clinical phase I study. Laryngoscope Investig. Otolaryngol. 2019, 4, 49–56. [Google Scholar] [CrossRef]
- Postow, M.A.; Callahan, M.K.; Barker, C.A.; Yamada, Y.; Yuan, J.; Kitano, S.; Mu, Z.; Rasalan, T.; Adamow, M.; Ritter, E.; et al. Immunologic correlates of the abscopal effect in a patient with melanoma. N. Engl. J. Med. 2012, 366, 925–931. [Google Scholar] [CrossRef]
- Tian, G.; Liu, X.; Zhao, Q.; Xu, D.; Jiang, T. Irreversible Electroporation in Patients With Pancreatic Cancer: How Important Is the New Weapon? Biomed. Res. Int. 2018, 2018, 1–12. [Google Scholar] [CrossRef]
- Scheltema, M.J.; O’Brien, T.J.; van den Bos, W.; de Bruin, D.M.; Davalos, R.V.; van den Geld, C.W.M.; Laguna, M.P.; Neal, R.E.; Varkarakis, I.M.; Skolarikos, A.; et al. Numerical simulation modeling of the irreversible electroporation treatment zone for focal therapy of prostate cancer, correlation with whole-mount pathology and T2-weighted MRI sequences. Ther. Adv. Urol. 2019, 11, 1756287219852305. [Google Scholar] [CrossRef] [PubMed]
- Županič, A.; Miklavčič, D. Optimization and Numerical Modeling in Irreversible Electroporation Treatment Planning. In Irreversible Electroporation; Rubinsky, B., Ed.; Springer: Berlin/Heidelberg, Germany, 2010; pp. 203–222. [Google Scholar]
- Tartaglia, E.; Fabozzi, M.; Rizzuto, A.; Settembre, A.; Abete, R.; Guerriero, L.; Favoriti, P.; Cuccurullo, D.; Corcione, F. Irreversible electroporation for locally advanced pancreatic cancer through a minimally invasive surgery supported by laparoscopic ultrasound. Int. J. Surg. Case Rep. 2018, 42, 290–294. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Wen, X.; Tian, L.; Li, T.; Xu, C.; Wen, X.; Melancon, M.P.; Gupta, S.; Shen, B.; Peng, W.; et al. Irreversible electroporation reverses resistance to immune checkpoint blockade in pancreatic cancer. Nat. Commun. 2019, 10, 1–14. [Google Scholar] [CrossRef] [PubMed]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).