X-ray Photoelectron Spectroscopy in Mineral Processing Studies
Abstract
Featured Application
Abstract
1. Introduction
2. Speciation of Surface Products
2.1. Oxidized Surfaces of Metal Sulfides
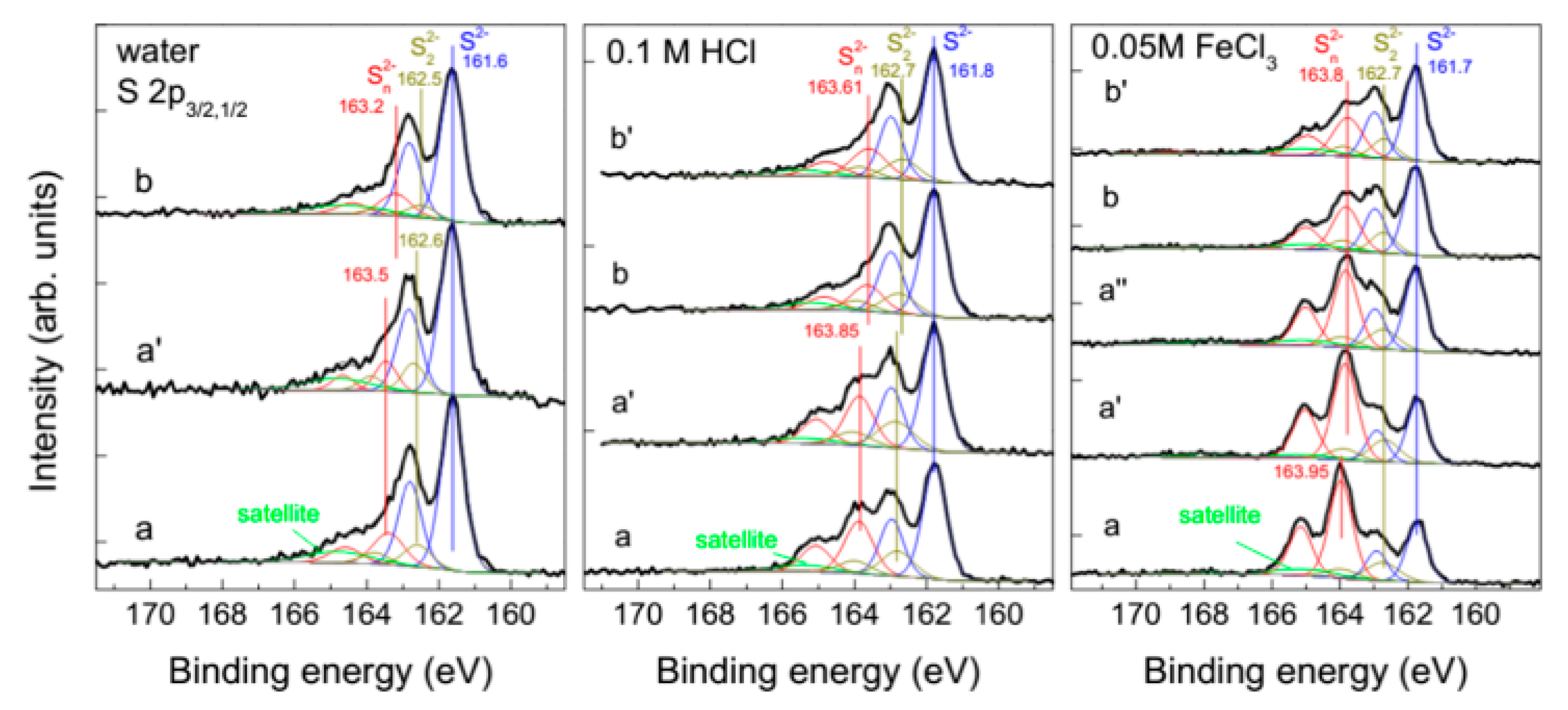
2.1.1. Detection of Elemental Sulfur, Sulfate, and Oxysulfur Species
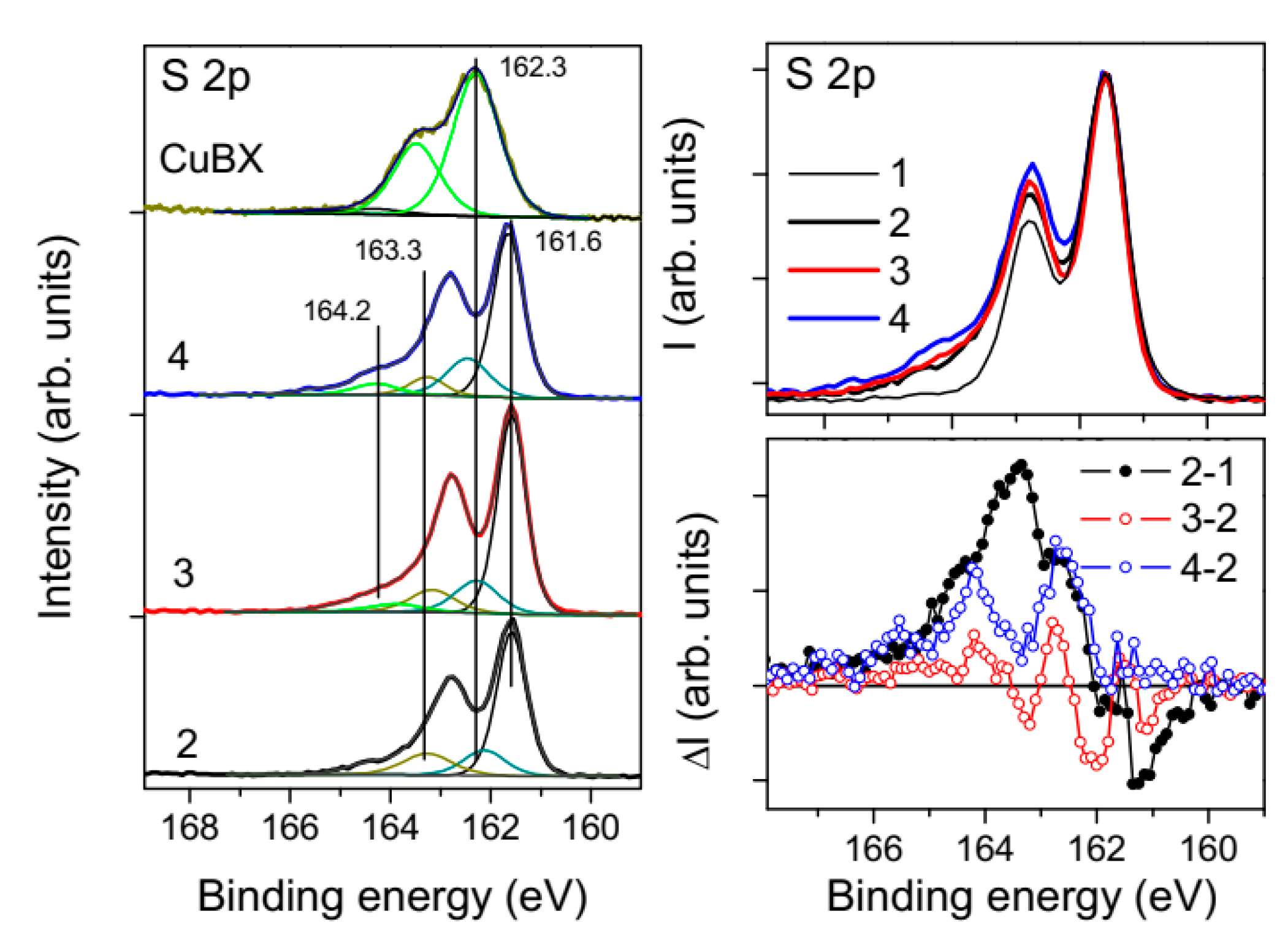
2.1.2. Metal-Deficient and Polysulfide Surfaces
2.2. Flotation Reagents
2.3. Quantitative Analysis of Mineral Surfaces
3. Spatial Resolution of Photoelectron Spectroscopy
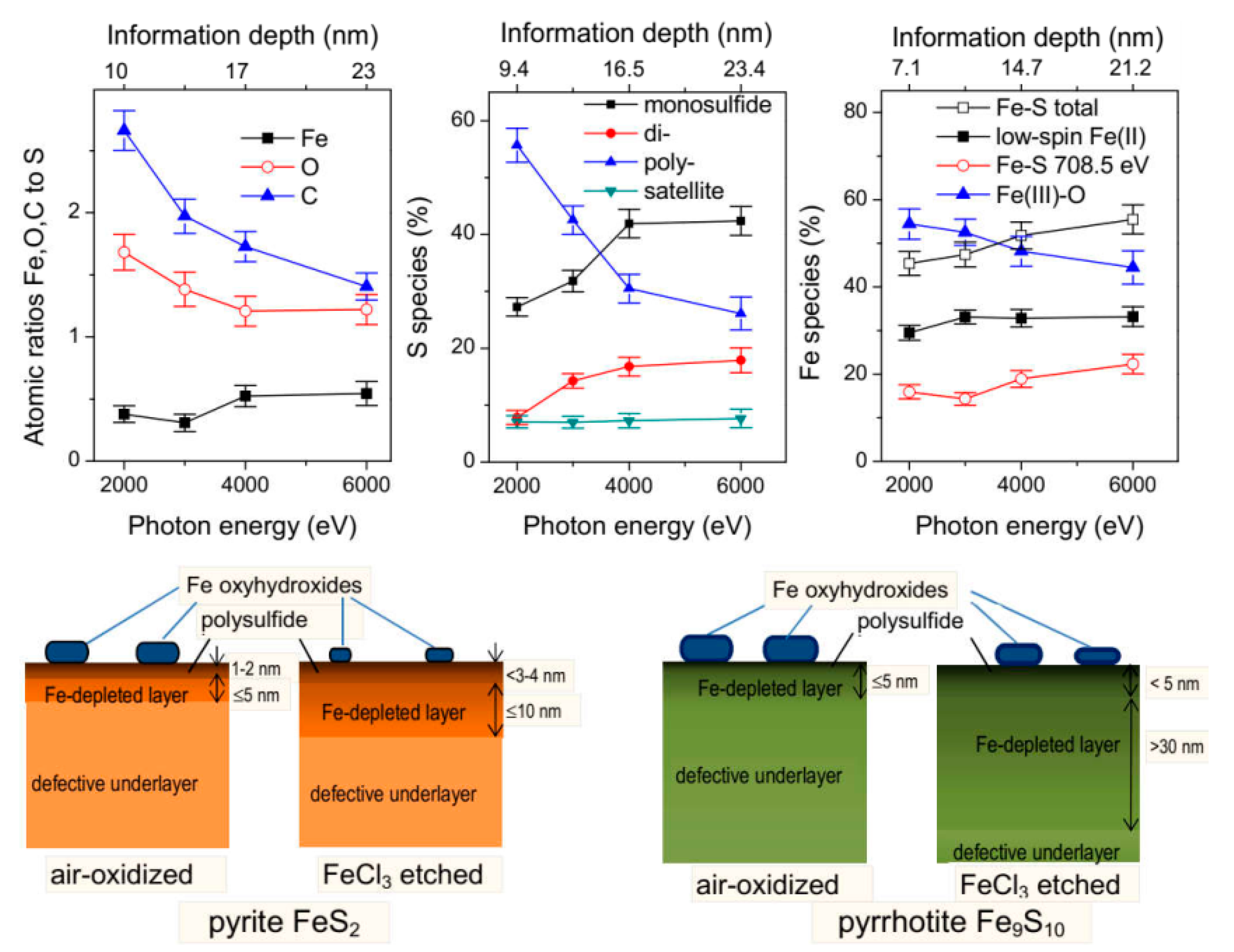
3.1. Ion Sputtering and Depth Profiling
3.2. Surface-Sensitive Techniques
3.3. Hard X-ray Photoelectron Spectroscopy
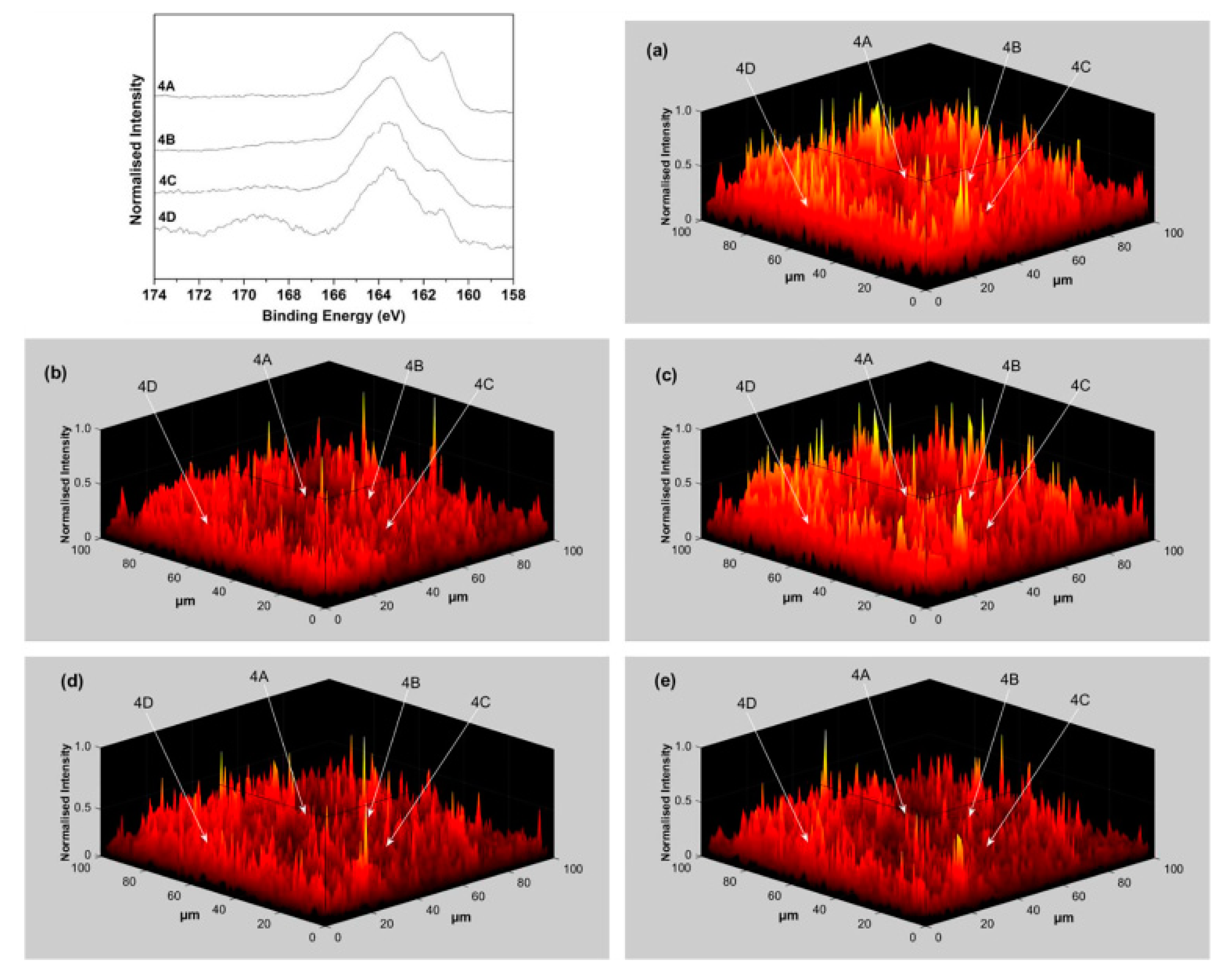
3.4. Lateral Resolution of Photoelectron Spectroscopy
4. Towards in Situ XPS
4.1. XPS Studies of Solid–Liquid Interfaces
4.1.1. Ambient Pressure XPS
4.1.2. Cryogenic XPS
4.2. Sampling and Exploring Natural Mineral Dispersions
5. Conclusions and Outlook
Funding
Conflicts of Interest
References
- Will’s Mineral Processing Technology. An Introduction to the Practical Aspects of Ore Treatment and Mineral Recovery, 7th ed.; Wills, B.A., Napier-Munn, T., Eds.; Elsevier Science & Technology Books: New York, NY, USA, 2006. [Google Scholar]
- Bulatovic, S.M. Handbook of Flotation Reagents: Chemistry, Theory and Practice. Volume 1: Flotation of Sulfide Ores, 1st ed.; Elsevier: Boston, MA, USA, 2007. [Google Scholar] [CrossRef]
- Free, M.L. Hydrometallurgy: Fundamentals and Applications; John Wiley & Sons: Hoboken, NJ, USA, 2013. [Google Scholar]
- Watling, H.R. Review of biohydrometallurgical metals extraction from polymetallic mineral resources. Minerals 2015, 5, 1–60. [Google Scholar] [CrossRef]
- Buckley, A.N.; Hope, G.A.; Woods, R. Metals from sulfide minerals: The role of adsorption of organic reagents in processing technologies. In Solid–Liquid Interfaces, Topics in Applied Physics; Wandelt, K., Thurgate, S., Eds.; Springer: Berlin/Heidelberg, Germany, 2003; Volume 85, pp. 61–96. [Google Scholar]
- Liu, G.; Yang, X.; Zhong, H. Molecular design of flotation collectors: A recent progress. Adv. Colloid Interface Sci. 2017, 246, 181–195. [Google Scholar] [CrossRef]
- Xing, Y.; Gui, X.; Pan, L.; Pinchasik, B.-E.; Cao, Y.; Liu, J.; Kappl, M.; Butt, H.-J. Recent experimental advances for understanding bubble-particle attachment in flotation. Adv. Colloid Interface Sci. 2017, 246, 105–132. [Google Scholar] [CrossRef] [PubMed]
- Krasowska, M.; Malysa, K.; Beattie, D.A. Recent advances in studies of bubble-solid interactions and wetting film stability. Curr. Opin. Colloid Interface Sci. 2019, 44, 48–58. [Google Scholar] [CrossRef]
- Chanturiya, V.A.; Kondratiev, S.A. Contemporary understanding and developments in the flotation theory of non-ferrous ores. Miner. Proc. Extr. Metall. Rev. 2019, 40, 390–401. [Google Scholar] [CrossRef]
- Buckley, A. Surface science and flotation surface and interface science. In Surface and Interface Science: Applications of Surface Science II, 1st ed.; Wandelt, K., Ed.; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2020; Volume 10, pp. 735–798. [Google Scholar] [CrossRef]
- Hackl, R.P.; Dreisinger, D.P.; Peters, E.; King, J.A. Passivation of chalcopyrite during oxidative leaching in sulfate media. Hydrometallurgy 1995, 39, 25–48. [Google Scholar] [CrossRef]
- Holmes, P.R.; Crundwell, F.K. Polysulfides do not cause passivation: Results from the dissolution of pyrite and implications for other sulfide minerals. Hydrometallurgy 2013, 139, 101–110. [Google Scholar] [CrossRef]
- Li, Y.; Kawashima, N.; Li, J.; Chandra, A.P.; Gerson, A.R. A review of the structure, and fundamental mechanisms and kinetics of the leaching of chalcopyrite. Adv. Colloid Interface Sci. 2013, 197, 1–32. [Google Scholar] [CrossRef]
- Zhao, H.; Zhang, Y.; Zhang, X.; Qian, L.; Sun, M.; Yang, Y.; Zhang, Y.; Wang, J.; Kim, H.; Qiu, G. The dissolution and passivation mechanism of chalcopyrite in bioleaching: An overview. Miner. Eng. 2019, 136, 140–154. [Google Scholar] [CrossRef]
- Van der Heide, P. X-ray Photoelectron Spectroscopy: An Introduction to Principles and Practices; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2012. [Google Scholar]
- Vickerman, J.; Gilmore, I. (Eds.) Surface Analysis: The Principal Techniques, 2nd ed.; John Wiley and Sons, Ltd.: Hoboken, NJ, USA, 2009. [Google Scholar]
- Smart, R.S.C. Surface layers in base metal sulphide flotation. Miner. Eng. 1991, 4, 891–909. [Google Scholar] [CrossRef]
- Smart, R.S.C.; Amarantidis, J.; Skinner, W.M.; Prestidge, C.A.; La Vanier, L.; Grano, S.R. Surface analytical studies of oxidation and collector adsorption in sulfide mineral flotation. In Solid—Liquid Interfaces. Topics in Applied Physics; Wandelt, K., Thurgate, S., Eds.; Springer: Berlin/Heidelberg, Germany, 2003; Volume 85, pp. 3–62. [Google Scholar] [CrossRef]
- Wincott, P.L.; Vaughan, D.J. Spectroscopic studies of sulfides. Rev. Mineral. Geochem. 2006, 61, 181–229. [Google Scholar] [CrossRef]
- Rosso, K.M.; Vaughan, D.J. Sulfide mineral surfaces. Rev. Mineral. Geochem. 2006, 61, 505–556. [Google Scholar] [CrossRef]
- Rosso, K.M.; Vaughan, D.J. Reactivity of sulfide mineral surfaces. Rev. Mineral. Geochem. 2006, 61, 557–607. [Google Scholar] [CrossRef]
- Goh, S.W.; Buckley, A.N.; Gong, B.; Woods, R.; Lamb, R.N.; Fan, L.J.; Yang, Y.W. Thiolate layers on metal sulfides characterised by XPS, ToF-SIMS and NEXAFS spectroscopy. Miner. Eng. 2008, 21, 1026–1037. [Google Scholar] [CrossRef]
- Smart, R.S.C.; Gerson, A.R.; Hart, B.R.; Beattie, D.A.; Young, C. Innovations in measurement of mineral structure and surface chemistry in flotation: Past, present, and future. In Mineral Processing and Extractive Metallurgy: 100 Years of Innovation; Society for Mining, Metallurgy & Exploration Inc.: Englewood, CO, USA, 2014; pp. 577–602. [Google Scholar]
- Qian, G.; Li, Y.; Gerson, A.R. Applications of surface analytical techniques in Earth Sciences. Surf. Sci. Rep. 2015, 70, 86–133. [Google Scholar] [CrossRef]
- Unger, W.E.S.; Wirth, T.; Hodoroaba, V.-D. Auger electron spectroscopy. In Characterization of Nanoparticles: Measurement Processes for Nanoparticles; Hodoroaba, V.-D., Unger, W.E.S., Shard, A.G., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 373–395. [Google Scholar] [CrossRef]
- Reniersa, F.; Tewell, C. New improvements in energy and spatial (x, y, z) resolution in AES and XPS applications. J. Electron. Spectrosc. Rel. Phenom. 2005, 142, 1–25. [Google Scholar] [CrossRef]
- Chehreh Chelgani, S.; Hart, B. TOF-SIMS studies of surface chemistry of minerals subjected to flotation separation—A review. Miner. Eng. 2014, 57, 1–11. [Google Scholar] [CrossRef]
- Zaera, F. Probing liquid/solid interfaces at the molecular level. In Surface and Interface Science: Liquid and Biological Interfaces, 1st ed.; Wandelt, K., Ed.; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2020; pp. 1–142. [Google Scholar] [CrossRef]
- Little, L.H.; Poling, G.W.; Leja, J. Infrared spectra of xanthate compounds: II. Assignment of vibrational frequencies. Can. J. Chem. 1961, 39, 745–754. [Google Scholar] [CrossRef]
- Leppinen, J.O. FT-IR and flotation investigation of the adsorption of ethyl xanthate on activated and non-activated sulfide minerals. Int. J. Miner. Process. 1990, 30, 245–263. [Google Scholar] [CrossRef]
- Beattie, D.A.; Larsson, M.L.; Holmgren, A.R. In situ total internal reflection Raman spectroscopy of surfactant adsorption at a mineral surface. Vibr. Spectrosc. 2006, 41, 198–204. [Google Scholar] [CrossRef]
- McQuillan, A.J. Probing solid-solution interfacial chemistry with ATR-IR spectroscopy of particle films. Adv. Mater. 2001, 13, 1034–1038. [Google Scholar] [CrossRef]
- Escudero, C.; Salmeron, M. From solid–vacuum to solid–gas and solid–liquid interfaces: In situ studies of structure and dynamics under relevant conditions. Surf. Sci. 2013, 607, 2–9. [Google Scholar] [CrossRef]
- Rickard, D.; Luther, G.W., III. Chemistry of iron sulfides. Chem. Rev. 2007, 107, 514–562. [Google Scholar] [CrossRef] [PubMed]
- Rimstidt, J.D.; Vaughan, D.J. Pyrite oxidation: A state of-the-art-assessment of the reaction mechanism. Geochim. Cosmochim. Acta 2003, 67, 873–880. [Google Scholar] [CrossRef]
- Murphy, R.; Strongin, D.R. Surface reactivity of pyrite and related sulfides. Surf. Sci. Rep. 2009, 64, 1–45. [Google Scholar] [CrossRef]
- Chandra, A.P.; Gerson, A.R. The mechanisms of pyrite oxidation and leaching: A fundamental perspective. Surf. Sci. Rep. 2010, 65, 93–315. [Google Scholar] [CrossRef]
- Wang, H. A review on process-related characteristics of pyrrhotite. Miner. Proc. Extr. Metall. Rev. 2008, 29, 1–41. [Google Scholar] [CrossRef]
- Hu, Y.; Wu, M.; Liu, R.; Sun, W. A review on the electrochemistry of galena flotation. Miner. Eng. 2020, 150, 106272. [Google Scholar] [CrossRef]
- Manocha, A.S.; Park, R.L. Flotation related ESCA studies on PbS surfaces. Appl. Surf. Sci. 1977, 1, 129–141. [Google Scholar] [CrossRef]
- Brion, D. Etude par spectroscopie de photoélectrons de la dégradation superficielle de FeS2, CuFeS2, ZnS et PbS á l’eau. Appl. Surf. Sci. 1980, 5, 133–152. [Google Scholar] [CrossRef]
- Evans, S.; Raftery, E. Electron spectroscopic studies of galena and its oxidation by microwave-generated oxygen species and by air. J. Chem. Soc. Faraday Trans. I 1982, 78, 3545–3560. [Google Scholar] [CrossRef]
- Luttrell, G.H.; Yoon, R.-H. Surface studies of the collectorless flotation of chalcopyrite. Colloids Surf. A 1984, 12, 239–254. [Google Scholar] [CrossRef]
- Buckley, A.N.; Woods, R. An X-ray photoelectron spectroscopic study of the oxidation of galena. Appl. Surf. Sci. 1984, 17, 401–414. [Google Scholar] [CrossRef]
- Buckley, A.N.; Woods, R. An X-ray photoelectron spectroscopic study of the oxidation of chalcopyrite. Austral. J. Chem. 1984, 37, 2403–2413. [Google Scholar] [CrossRef]
- Buckley, A.N.; Hamilton, I.C.; Woods, R. Investigation of the surface oxidation of bornite by linear potential sweep voltammetry and X-ray photoelectron spectroscopy. J. Appl. Electrochem. 1984, 14, 63–74. [Google Scholar] [CrossRef]
- Buckley, A.N.; Woods, R. X-ray photoelectron spectroscopy of oxidised pyrrhotite surfaces. II: Exposure to aqueous solutions. Appl. Surf. Sci. 1985, 20, 472–480. [Google Scholar] [CrossRef]
- Buckley, A.N.; Woods, R. X-ray photoelectron spectroscopy of oxidized pyrrhotite surfaces: I. Exposure to aire. Appl. Surf. Sci. 1985, 22, 280–287. [Google Scholar] [CrossRef]
- Buckley, A.N.; Woods, R. The surface oxidation of pyrite. Appl. Surf. Sci. 1987, 27, 437–452. [Google Scholar] [CrossRef]
- Buckley, A.N.; Woods, R.; Wouterlood, H.J. An XPS investigation of the surface of natural sphalerites under flotation-related conditions. Int. J. Miner. Process. 1989, 26, 29–49. [Google Scholar] [CrossRef]
- Buckley, A.N.; Wouterlood, H.J.; Woods, R. The surface composition of natural sphalerites under oxidative leaching conditions. Hydrometallurgy 1989, 22, 39–56. [Google Scholar] [CrossRef]
- Buckley, A.N.; Woods, R. Electrochemical and XPS studies of the surface oxidation of synthetic heazlewoodite (Ni3S2). J. Appl. Electrochem. 1991, 21, 575–582. [Google Scholar] [CrossRef]
- Buckley, A.N.; Woods, R. Surface composition of pentlandite under flotation-related conditions. Surf. Interface Anal. 1991, 17, 675–680. [Google Scholar] [CrossRef]
- Buckley, A.N.; Woods, R. Relaxation of the lead-deficient sulfide surface layer on oxidized galena. J. Appl. Electrochem. 1996, 26, 899–907. [Google Scholar] [CrossRef]
- Buckley, A.N.; Walker, G.W. The surface composition of arsenopyrite exposed to oxidizing environments. Appl. Surf. Sci. 1988, 35, 227–240. [Google Scholar] [CrossRef]
- Laajalehto, K.; Kartio, I.; Heinonen, M.; Laiho, T. Temperature controled photoelectron spectroscopic investigation of volatile species on PbS(100) surface. Jpn. J. Appl. Phys. 1999, 38, 265–268. [Google Scholar] [CrossRef]
- Mycroft, J.R.; Bancroft, G.M.; McIntyre, N.S.; Lorimer, J.W.; Hill, I.R. Detection of sulphur and polysulphides on electrochemically oxidized pyrite surfaces by X-ray photoelectron spectroscopy and Raman spectroscopy. J. Electroanal. Chem. Interface Electrochem. 1990, 292, 139–152. [Google Scholar] [CrossRef]
- Karthe, S.; Szargan, R.; Suoninen, E. Oxidation of pyrite surfaces: A photoelectron spectroscopic study. Appl. Surf. Sci. 1993, 72, 157–170. [Google Scholar] [CrossRef]
- Richardson, P.E.; O’Dell, C.S. Semiconducting characteristics of galena electrodes. Relationship to mineral flotation. J. Electrochem. Soc. 1985, 132, 1350–1356. [Google Scholar] [CrossRef]
- Richardson, P.E.; Yoon, R.H.; Woods, R.; Buckley, A.N. The photoelectrochemistry of galena. Int. J. Miner. Process. 1994, 41, 77–97. [Google Scholar] [CrossRef]
- Mikhlin, Y.; Romanchenko, A.; Shagaev, A. Scanning probe microscopy studies of PbS surfaces oxidized in air and etched in aqueous acid solutions. Appl. Surf. Sci. 2006, 252, 5645–5658. [Google Scholar] [CrossRef]
- Buckley, A.N.; Hamilton, I.C.; Woods, R. An investigation of the sulphur (− II)/sulphur (0) system on gold electrodes. J. Electroanal. Chem. Interfacial Electrochem. 1987, 216, 213–227. [Google Scholar] [CrossRef]
- Walker, G.W.; Richardson, P.E.; Buckley, A.N. Workshop on the flotation-related surface chemistry of sulfide minerals. Int. J. Miner. Process. 1989, 25, 153–158. [Google Scholar] [CrossRef]
- Termes, S.C.; Buckley, A.N.; Gillard, R.D. 2p electron binding energies for the sulfur atoms in metal polysulfides. Inorg. Chim. Acta 1987, 126, 79–82. [Google Scholar] [CrossRef]
- Smart, R.S.C.; Skinner, W.M.; Gerson, A.R. XPS of sulphide mineral surfaces: Metal-deficient, polysulphides, defects and elemental sulphur. Surf. Interface Anal. 1999, 28, 101–105. [Google Scholar] [CrossRef]
- Gerson, A.R.; Bredow, T. Interpretation of sulphur 2p XPS spectra in sulfide minerals by means of ab initio calculations. Surf. Interface Anal. 2000, 29, 145–150. [Google Scholar] [CrossRef]
- Fantauzzi, M.; Elsener, B.; Atzei, D.; Rigoldi, A.; Rossi, A. Exploiting XPS for the identification of sulfides and polysulfides. RSC Adv. 2015, 5, 75953–75963. [Google Scholar] [CrossRef]
- Nasluzov, V.; Shor, A.; Romanchenko, A.; Tomashevich, Y.; Mikhlin, Y. DFT+U and low-temperature XPS studies of Fe-depleted chalcopyrite (CuFeS2) surfaces: A focus on polysulfide species. J. Phys. Chem. C 2019, 123, 21031–21041. [Google Scholar] [CrossRef]
- Parker, G.K.; Woods, R.; Hope, G.A. Raman investigation of chalcopyrite oxidation. Colloids Surf. A Physicochem. Eng. Asp. 2008, 318, 160–168. [Google Scholar] [CrossRef]
- Parker, G.K.; Hope, G.A.; Woods, R. Gold-enhanced Raman observation of chalcopyrite leaching. Colloids Surf. A Physicochem. Eng. Asp. 2008, 325, 132–140. [Google Scholar] [CrossRef]
- Smart, R.S.C.; Jasieniak, M.; Prince, K.E.; Skinner, W.M. SIMS studies of oxidation mechanisms and polysulfide formation in reacted sulfide surfaces. Miner. Eng. 2000, 13, 857–870. [Google Scholar] [CrossRef]
- Klauber, C.; Parker, A.; Van Bronswijk, W.; Watling, H.R. Sulphur speciation of leached chalcopyrite surfaces as determined by X-ray photoelectron spectroscopy. Int. J. Miner. Process. 2001, 62, 65–94. [Google Scholar] [CrossRef]
- Parker, A.; Klauber, C.; Kougianos, A.; Watling, H.R.; Van Bronswijk, W. An X-ray photoelectron spectroscopy study of the mechanism of oxidative dissolution of chalcopyrite. Hydrometallurgy 2003, 71, 265–276. [Google Scholar] [CrossRef]
- Klauber, C. A critical review of the surface chemistry of acidic ferric sulphate dissolution of chalcopyrite with regards to hindered dissolution. Int. J. Miner. Process. 2008, 86, 1–17. [Google Scholar] [CrossRef]
- Jones, C.F.; LeCount, S.; Smart, R.; White, T.J. Compositional and structural alteration of pyrrhotite surfaces in solution: XPS and XRD studies. Appl. Surf. Sci. 1992, 55, 65–85. [Google Scholar] [CrossRef]
- Pratt, A.R.; Muir, I.J.; Nesbitt, H.W. X-ray photoelectron and auger electron spectroscopic studies of pyrrhotite, and mechanism of air oxidation. Geochim. Cosmochim. Acta 1994, 58, 827–841. [Google Scholar] [CrossRef]
- Pratt, A.R.; Nesbitt, H.W.; Muir, I.J. Generation of acids from mine waste: Oxidative leaching of pyrrhotite in dilute H2SO4 solutions (pH 3). Geochim. Cosmochim. Acta 1994, 58, 5147–5159. [Google Scholar] [CrossRef]
- Mycroft, J.R.; Nesbitt, H.W.; Pratt, A.R. X-ray photoelectron and auger electron spectroscopy of air-oxidized pyrrhotite: Distribution of oxidized species with depth. Geochim. Cosmochim. Acta 1995, 59, 721–733. [Google Scholar] [CrossRef]
- Pratt, A.R.; Nesbitt, H.W. Pyrrhotite leaching in acid mixtures of HCl and H2SO4. Am. J. Sci. 1997, 297, 807–820. [Google Scholar] [CrossRef]
- Mikhlin, Y.L.; Tomashevich, Y.V.; Pashkov, G.L.; Okotrub, A.V.; Asanov, I.P.; Mazalov, L.N. Electronic structure of non-equilibrium iron-deficient layer at hexagonal pyrrhotite. Appl. Surf. Sci. 1998, 125, 73–84. [Google Scholar] [CrossRef]
- Mikhlin, Y.; Varnek, V.; Asanov, I.; Tomashevich, Y.; Okotrub, A.; Livshits, A.; Selyutin, G.; Pashkov, G. Reactivity of pyrrhotite (Fe9S10) surfaces: Spectroscopic studies. Phys. Chem. Chem. Phys. 2000, 2, 4393–4398. [Google Scholar] [CrossRef]
- Mikhlin, Y. Reactivity of pyrrhotite surfaces: An electrochemical study. Phys. Chem. Chem. Phys. 2000, 2, 5672–5677. [Google Scholar] [CrossRef]
- Mikhlin, Y.L.; Kuklinskiy, A.V.; Pavlenko, N.I.; Varnek, V.A.; Asanov, I.P.; Okotrub, A.V.; Selyutin, G.E.; Solovyev, L.A. Spectroscopic and XRD studies of the air degradation of acid-reacted pyrrhotites. Geochim. Cosmochim. Acta 2002, 66, 4077–4087. [Google Scholar] [CrossRef]
- Thomas, J.E.; Jones, C.F.; Skinner, W.M.; Smart, R.; White, T.J. The role of surface sulphur species in the inhibition of pyrrhotite dissolution in acid conditions. Geochim. Cosmochim. Acta 1998, 62, 1555–1565. [Google Scholar] [CrossRef]
- Thomas, J.; Smart, R.; Skinner, W. Kinetic factors for oxidative and non-oxidative dissolution of iron sulfides. Miner. Eng. 2000, 13, 1149–1159. [Google Scholar] [CrossRef]
- Thomas, J.E.; Skinner, W.M.; Smart, R.S.C. A mechanism to explain sudden changes in rates and products for pyrrhotite dissolution in acid solution. Geochim. Cosmochim. Acta 2001, 65, 1–12. [Google Scholar] [CrossRef]
- Mikhlin, Y.L.; Tomashevich, Y.V.; Asanov, I.P.; Okotrub, A.V.; Varnek, V.A.; Vyalikh, D.V. Spectroscopic and electrochemical characterization of the surface layers of chalcopyrite (CuFeS2) reacted in acidic solutions. Appl. Surf. Sci. 2004, 225, 395–409. [Google Scholar] [CrossRef]
- Harmer, S.L.; Thomas, J.E.; Fornasiero, D.; Gerson, A.R. The evolution of surface layers formed during chalcopyrite leaching. Geochim. Cosmochim. Acta 2006, 70, 4392–4402. [Google Scholar] [CrossRef]
- Mikhlin, Y.; Tomashevich, Y.; Vorobyev, S.; Saikova, S.; Romanchenko, A.; Félix, R. Hard X-ray photoelectron and X-ray absorption spectroscopy characterization of oxidized surfaces of iron sulfides. Appl. Surf. Sci. 2016, 387, 796–804. [Google Scholar] [CrossRef]
- Mikhlin, Y.; Nasluzov, V.; Romanchenko, A.; Tomashevich, Y.; Shor, A.; Félix, R. Layered structure of the near-surface region of oxidized chalcopyrite (CuFeS2): Hard X-ray photoelectron spectroscopy, X-ray absorption spectroscopy and DFT+U studies. Phys. Chem. Chem. Phys. 2017, 19, 2749–2759. [Google Scholar] [CrossRef]
- Nowak, P.; Laajalehto, K.; Kartio, I. A flotation related X-ray photoelectron spectroscopy study of the oxidation of galena surface. Colloids Surf. A Physicochem. Eng. Asp. 2000, 161, 447–460. [Google Scholar] [CrossRef]
- Buckley, A.N.; Goh, S.W.; Lamb, R.N.; Woods, R. Interaction of thiol collectors with pre-oxidised sulfide minerals. Int. J. Miner. Process. 2003, 72, 163–174. [Google Scholar] [CrossRef]
- Niu, X.; Chen, J.; Li, Y.; Xia, L.; Li, L.; Sun, H.; Ruan, R. Correlation of surface oxidation with xanthate adsorption and pyrite flotation. Appl. Surf. Sci. 2019, 495, 143411. [Google Scholar] [CrossRef]
- Moimane, T.; Plackowski, C.; Peng, Y. The critical degree of mineral surface oxidation in copper sulphide flotation. Miner. Eng. 2020, 145, 106075. [Google Scholar] [CrossRef]
- Shchukarev, A.V.; Kravets, I.M.; Buckley, A.N.; Woods, R. Submonolayer adsorption of alkyl xanthates on galena. Int. J. Miner. Process. 1994, 41, 99–114. [Google Scholar] [CrossRef]
- Mielczarski, J.A.; Mielczarski, E.; Cases, J.M. Influence of chain length on adsorption of xanthates on chalcopyrite. Int. J. Miner. Process. 1998, 52, 215–231. [Google Scholar] [CrossRef]
- Piantadosi, C.; Smart, R.S.C. Statistical comparison of hydrophobic and hydrophilic species on galena and pyrite particles in flotation concentrates and tails from TOF-SIMS evidence. Int. J. Miner. Process. 2002, 64, 43–54. [Google Scholar] [CrossRef]
- Kartio, I.; Laajalehto, K.; Suoninen, E.; Karthe, S.; Szargan, R. Technique for XPS measurements of volatile adsorbed layers: Application to studies of sulphide flotation. Surf. Interface Anal. 1992, 18, 807–810. [Google Scholar] [CrossRef]
- Szargan, R.; Karthe, S.; Suoninen, E. XPS studies of xanthate adsorption on pyrite. Appl. Surf. Sci. 1992, 55, 227–232. [Google Scholar] [CrossRef]
- Deng, M.J.; Karpuzov, D.; Liu, Q.X.; Xu, Z.H. Cryo-XPS study of xanthate adsorption on pyrite. Suf. Interface Anal. 2013, 45, 805–810. [Google Scholar] [CrossRef]
- Mikhlin, Y.L.; Karacharov, A.A.; Likhatski, M.N. Effect of adsorption of butyl xanthate on galena, PbS, and HOPG surfaces as studied by atomic force microscopy and spectroscopy and XPS. Int. J. Miner. Process. 2015, 144, 81–89. [Google Scholar] [CrossRef]
- Mikhlin, Y.; Karacharov, A.; Tomashevich, Y.; Shchukarev, A. Cryogenic XPS study of fast-frozen sulfide minerals: Flotation-related adsorption of n-butyl xanthate and beyond. J. Electron Spectrosc. Rel. Phenom. 2016, 206, 65–73. [Google Scholar] [CrossRef]
- Mikhlin, Y.; Karacharov, A.; Tomashevich, Y.; Shchukarev, A. Interaction of sphalerite with potassium n-butyl xanthate and copper sulfate solutions studied by XPS of fast-frozen samples and zeta-potential measurement. Vacuum 2016, 125, 98–105. [Google Scholar] [CrossRef]
- Mikhlin, Y.; Vorobyev, S.; Saikova, S.; Tomashevich, Y.; Fetisova, O.; Kozlova, S.; Zharkov, S. Preparation and characterization of colloidal copper xanthate nanoparticles. New J. Chem. 2016, 40, 3059–3065. [Google Scholar] [CrossRef]
- Vorobyev, S.; Saikova, S.; Novikova, S.; Fetisova, O.; Zharkov, S.; Krylov, A.; Likhatski, M.; Mikhlin, Y. Colloidal and immobilized nanoparticles of lead xanthates. ACS Omega 2019, 4, 11472–11480. [Google Scholar] [CrossRef]
- Firkala, T.; Kuschewski, F.; Nörenberg, T.; Klopf, J.M.; Pashkin, A.; Foerstendorf, H.; Rudolph, M.; Kehr, S.C.; Eng, L.M. Near-field optical examination of potassium n-butyl xanthate/chalcopyrite flotation products. Minerals 2018, 8, 118. [Google Scholar] [CrossRef]
- Nefedov, V.I.; Salyn, Y.V.; Solozhenkin, P.M.; Pulatov, G.Y. X-ray photoelectron study of surface compounds formed during flotation of minerals. Surf. Interface Anal. 1980, 2, 170–172. [Google Scholar] [CrossRef]
- Prestidge, C.A.; Skinner, W.M.; Ralston, J.; Smart, R.S.C. Copper II activation and cyanide deactivation of zinc sulphide under mildly alkaline conditions. Appl. Surf. Sci. 1997, 108, 333–344. [Google Scholar] [CrossRef]
- Finkelstein, N.P. The activation of sulphide minerals for flotation: A review. Int. J. Miner. Process. 1997, 52, 81–120. [Google Scholar] [CrossRef]
- Kartio, I.J.; Basilio, C.I.; Yoon, R.H. An XPS study of sphalerite activation by copper. Langmuir 1998, 14, 5274–5278. [Google Scholar] [CrossRef]
- Gerson, A.R.; Lange, A.G.; Prince, K.E.; Smart, R.S.C. The mechanism of copper activation of sphalerite. Appl. Surf. Sci. 1999, 137, 207–223. [Google Scholar] [CrossRef]
- Pattrick, R.A.D.; England, K.E.R.; Charnock, J.M.; Mosselmans, J.F.W. Copper activation of sphalerite and its reaction with xanthate in relation to flotation: An X-ray absorption spectroscopy (reflection extended X-ray absorption fine structure) investigation. Int. J. Miner. Process. 1999, 55, 247–265. [Google Scholar] [CrossRef]
- Weisener, C.; Gerson, A. An investigation of the Cu (II) adsorption mechanism on pyrite by ARXPS and SIMS. Miner. Eng. 2000, 13, 1329–1340. [Google Scholar] [CrossRef]
- Harmer, S.L.; Mierczynska-Vasilev, A.; Beattie, D.A.; Shapter, J.G. The effect of bulk iron concentration and heterogeneities on the copper activation of sphalerite. Miner. Eng. 2008, 21, 1005–1012. [Google Scholar] [CrossRef]
- Simpson, D.J.; Bredow, T.; Chandra, A.P.; Cavallaro, G.P.; Gerson, A.R. The effect of iron and copper impurities on the wettability of sphalerite (110) surface. J. Comput. Chem. 2011, 32, 2022–2030. [Google Scholar] [CrossRef] [PubMed]
- Chandra, A.P.; Gerson, A.R. A review of the fundamental studies of the copper activation mechanisms for selective flotation of the sulfide minerals, sphalerite and pyrite. Adv. Colloid Interface Sci. 2009, 145, 97–110. [Google Scholar] [CrossRef] [PubMed]
- Chandra, A.P.; Puskar, L.; Simpson, D.J.; Gerson, A.R. Copper and xanthate adsorption onto pyrite surfaces: Implications for mineral separation through flotation. Int. J. Miner. Process. 2012, 114, 16–26. [Google Scholar] [CrossRef]
- Wang, J.; Liu, Q.; Zeng, H. Understanding copper activation and xanthate adsorption on sphalerite by ToF-SIMS, XPS and in-situ SECM. J. Phys. Chem. C 2013, 117, 20089–20097. [Google Scholar] [CrossRef]
- Ejtemaei, M.; Nguyen, A.V. A comparative study of the attachment of air bubbles onto sphalerite and pyrite surfaces activated by copper sulphate. Miner. Eng. 2017, 109, 14–20. [Google Scholar] [CrossRef]
- Wittstock, G.; Kartio, I.; Hirsch, D.; Kunze, S.; Szargan, R. Oxidation of galena in acetate buffer investigated by atomic force microscopy and photoelectron spectroscopy. Langmuir 1996, 12, 5709–5721. [Google Scholar] [CrossRef]
- Kartio, I.; Wittstock, G.; Laajalehto, K.; Hirsch, D.; Simola, J.; Laiho, T.; Szargan, R.; Suoninen, E. Detection of elemental sulphur on galena oxidized in acidic solution. Int. J. Miner. Process. 1997, 51, 293–301. [Google Scholar] [CrossRef]
- De Giudici, G.; Ricci, P.; Lattanzi, P.; Anedda, A. Dissolution of the (001) surface of galena: An in situ assessment of surface speciation by fluid-cell micro-Raman spectroscopy. Am. Mineral. 2007, 92, 518–524. [Google Scholar] [CrossRef]
- Hampton, M.A.; Plackowski, C.; Nguyen, A.V. Physical and chemical analysis of elemental sulfur formation during galena surface oxidation. Langmuir 2011, 27, 4190–4201. [Google Scholar] [CrossRef] [PubMed]
- Palyanova, G.A.; Mikhlin, Y.L.; Karmanov, N.S.; Kokh, K.A.; Seryotkin, Y.V. Visible and “invisible” forms of gold and silver in the crystallization products of melts in the Fe–S–Ag–Au system: Experimental data. Doklady Earth Sci. 2017, 474, 636–640. [Google Scholar] [CrossRef]
- Goh, S.W.; Buckley, A.N.; Lamb, R.N.; Woods, R. The ability of static secondary ion mass spectrometry to discriminate submonolayer from multilayer adsorption of thiol collectors. Miner. Eng. 2006, 19, 571–581. [Google Scholar] [CrossRef]
- Ruano, G.; Pomiro, F.; Ferrón, J. Surface chemical reactions induced on pyrite by ion bombardment. Surf. Sci. 2018, 667, 138–147. [Google Scholar] [CrossRef]
- Galvez-Martinez, S.; Escamilla-Roa, E.; Zorzano, M.-P.; Mateo-Marti, E. Ar+ ion bombardment dictates glycine adsorption on pyrite (100) surface: X-ray photoemission spectroscopy and DFT approach. Appl. Surf. Sci. 2020, 530, 147182. [Google Scholar] [CrossRef]
- Woicik, J.C. (Ed.) Hard X-ray Photoelectron Spectroscopy (HAXPES); Springer: Cham, Switzerland, 2016. [Google Scholar]
- Brown, G.E., Jr.; Sturchio, N.C. An overview of synchrotron radiation applications to low temperature geochemistry and environmental science. Rev. Mineral. Geochem. 2002, 49, 1–115. [Google Scholar] [CrossRef]
- Nesbitt, H.W.; Muir, I.J. X-ray photoelectron spectroscopic study of a pristine pyrite surface reacted with water vapour and air. Geochim. Cosmochim. Acta 1994, 58, 4667–4679. [Google Scholar] [CrossRef]
- Nesbitt, H.W.; Muir, I.J.; Pratt, A.R. Oxidation of arsenopyrite by air and air-saturated, distilled water, and implications for mechanism of oxidation. Geochim. Cosmochim. Acta 1995, 59, 1773–1786. [Google Scholar] [CrossRef]
- Nesbitt, H.W.; Bancroft, G.M.; Pratt, A.R.; Scaini, M.J. Sulfur and iron surface states on fractured pyrite surfaces. Am. Mineral. 1998, 83, 1067–1076. [Google Scholar] [CrossRef]
- Schaufuss, A.G.; Nesbitt, H.W.; Scaini, M.J.; Hoechst, H.; Bancroft, M.G.; Szargan, R. Reactivity of surface sites on fractured arsenopyrite (FeAsS) toward oxygen. Am. Miner. 2000, 85, 1754–1766. [Google Scholar] [CrossRef]
- Nesbitt, H.W.; Schaufuss, A.; Sciani, M.; Hochst, H.; Bancroft, G.M.; Szargan, R. Monitoring fundamental reactions at NiAsS surfaces by synchrotron radiation X-ray photoelectron spectroscopy: As and S air oxidation by consecutive reaction schemes. Geochim. Cosmochim. Acta 2003, 67, 845–858. [Google Scholar] [CrossRef]
- Harmer, S.L.; Pratt, A.R.; Nesbitt, W.H.; Fleet, M.E. Sulfur species at chalcopyrite (CuFeS2) fracture surfaces. Am. Mineral. 2004, 89, 1026–1032. [Google Scholar] [CrossRef]
- Von Oertzen, G.U.; Harmer, S.L.; Skinner, W.M. XPS and ab initio calculation of surface states of sulfide minerals: Pyrite, chalcopyrite and molybdenite. Mol. Simul. 2006, 32, 1207–1212. [Google Scholar] [CrossRef]
- Harmer, S.L.; Nesbitt, H.W.; Skinner, W.M.; Buckley, A.N.; Pratt, A. ARXPS and SXPS evidence for surface stabilization of sphalerite Zn1-xFexS (110) surfaces. ECS Trans. 2010, 28, 81–90. [Google Scholar] [CrossRef]
- Kartio, I.; Laajalehto, K.; Suoninen, E. Application of electron spectroscopy to characterization of mineral surfaces in flotation studies. Colloids Surf. A Physicochem. Eng. Asp. 1994, 93, 149–158. [Google Scholar] [CrossRef]
- Laajalehto, K.; Kartio, I.; Suoninen, E. XPS and SR-XPS techniques applied to sulphide mineral surfaces. Int. J. Miner. Process. 1997, 51, 163–170. [Google Scholar] [CrossRef]
- Kartio, I.; Laajalehto, K.; Kaurila, T.; Suoninen, E. A study of galena (PbS) surfaces under controlled potential in pH 4.6 solution by synchrotron radiation excited photoelectron spectroscopy. Appl. Surf. Sci. 1996, 93, 167–177. [Google Scholar] [CrossRef]
- Schaufuß, A.G.; Nesbitt, H.W.; Kartio, I.; Laajalehto, K.; Bancroft, G.M.; Szargan, R. Incipient oxidation of fractured pyrite surfaces in air. J. Electron Spectrosc. Rel. Phenom. 1998, 96, 69–82. [Google Scholar] [CrossRef]
- Schaufuß, A.G.; Nesbitt, H.W.; Kartio, I.; Laajalehto, K.; Bancroft, G.M.; Szargan, R. Reactivity of surface chemical states on fractured pyrite. Surf. Sci. 1998, 411, 321–328. [Google Scholar] [CrossRef]
- Kartio, I.; Laajalehto, K.; Suoninen, E. Characterization of the ethyl xanthate adsorption layer on galena (PbS) by synchrotron radiation excited photoelectron spectroscopy. Colloids Surf. A Physicochem. Eng. Asp. 1999, 154, 97–101. [Google Scholar] [CrossRef]
- Nesbitt, H.W.; Scaini, M.; Hochst, H.; Bancroft, G.M.; Schaufuss, A.G.; Szargan, R. Synchrotron XPS evidence for Fe2+-S and Fe3+-S surface species on pyrite fracture-surfaces, and their 3D electronic states. Am. Mineral. 2000, 85, 850–857. [Google Scholar] [CrossRef]
- Nesbitt, H.W.; Schaufuss, A.G.; Scaini, M.; Bancroft, G.M.; Szargan, R. XPS measurement of fivefold and sixfold coordinated sulfur in pyrrhotites and evidence for millerite and pyrrhotite surface species. Am. Mineral. 2001, 86, 318–326. [Google Scholar] [CrossRef]
- Goh, S.W.; Buckley, A.N.; Skinner, W.M.; Fan, L.J. An X-ray photoelectron and absorption spectroscopic investigation of the electronic structure of cubanite, CuFe2S3. Phys. Chem. Miner. 2010, 37, 389–405. [Google Scholar] [CrossRef]
- Acres, R.G.; Harmer, S.L.; Beattie, D.A. Synchrotron XPS studies of solution exposed chalcopyrite, bornite, and heterogeneous chalcopyrite with bornite. Int. J. Miner. Process. 2010, 94, 43–51. [Google Scholar] [CrossRef]
- Acres, R.G.; Harmer, S.L.; Beattie, D.A. Synchrotron XPS, NEXAFS, and ToF-SIMS studies of solution exposed chalcopyrite and heterogeneous chalcopyrite with pyrite. Miner. Eng. 2010, 23, 928–936. [Google Scholar] [CrossRef]
- Yang, Y.; Harmer, S.; Chen, M. Synchrotron X-ray photoelectron spectroscopic study of the chalcopyrite leached by moderate thermophiles and mesophiles. Miner. Eng. 2014, 69, 185–195. [Google Scholar] [CrossRef]
- Yang, Y.; Harmer, S.; Chen, M. Synchrotron-based XPS and NEXAFS study of surface chemical species during electrochemical oxidation of chalcopyrite. Hydrometallurgy 2015, 156, 89–98. [Google Scholar] [CrossRef]
- Pettifer, Z.E.; Quinton, J.S.; Skinner, W.M.; Harmer, S.L. New interpretation and approach to curve fitting synchrotron X-ray photoelectron spectra of (Fe, Ni)9S8 fracture surfaces. Appl. Surf. Sci. 2020, 504, 144458. [Google Scholar] [CrossRef]
- Von Oertzen, G.U.; Skinner, W.M.; Nesbitt, H.W.; Pratt, A.R.; Buckley, A.N. Cu adsorption on pyrite (100): Ab initio and spectroscopic studies. Surf. Sci. 2007, 601, 5794–5799. [Google Scholar] [CrossRef]
- Weiland, C.; Rumaiz, A.K.; Pianetta, P.; Woicik, J.C. Recent applications of hard X-ray photoelectron spectroscopy. J. Vac. Sci. Technol. A 2016, 34, 030801. [Google Scholar] [CrossRef]
- Regoutz, A.; Mascheck, M.; Wiell, T.; Eriksson, S.K.; Liljenberg, C.; Tetzner, K.; Williamson, B.A.D.; Scanlon, D.O.; Palmgren, P. A novel laboratory-based hard X-ray photoelectron spectroscopy system. Rev. Sci. Instrum. 2018, 89, 073105. [Google Scholar] [CrossRef] [PubMed]
- Siol, S.; Mann, J.; Newman, J.; Miyayama, T.; Watanabe, K.; Schmutz, P.; Cancellieri, C.; Jeurgens, L.P. Concepts for chemical state analysis at constant probing depth by lab-based XPS/HAXPES combining soft and hard X-ray sources. Surf. Interface Anal. 2020. [Google Scholar] [CrossRef]
- Nicol, M.; Miki, H.; Velásquez-Yévenes, L. The dissolution of chalcopyrite in chloride solutions: Part 3. Mechanisms. Hydrometallurgy 2010, 103, 86–95. [Google Scholar] [CrossRef]
- Pugaev, D.; Nicol, M.; Senanayake, G. The mechanisms of the passivation of sulfide minerals in oxidative leaching processes. In Proceedings of the 6th Southern African Base Metals Conference, Phalaborwa, South Africa, 18–21 July 2011; pp. 39–48. [Google Scholar]
- Li, Y.; Wei, Z.; Qian, G.; Li, J.; Gerson, A.R. Kinetics and mechanisms of chalcopyrite dissolution at controlled redox potential of 750 mV in sulfuric acid solution. Minerals 2016, 6, 83. [Google Scholar] [CrossRef]
- Crundwell, F.K. The semiconductor mechanism of dissolution and the pseudopassivation of chalcopyrite. Can. Metall. Q. 2015, 54, 279–288. [Google Scholar] [CrossRef]
- O’Connor, G.M.; Eksteen, J.J. A critical review of the passivation and semiconductor mechanisms of chalcopyrite leaching. Miner. Eng. 2020, 154, 106401. [Google Scholar] [CrossRef]
- Kiskinova, M. Spectromicroscopy studies with high spatial resolution. Surf. Rev. Lett. 2000, 7, 447–453. [Google Scholar] [CrossRef]
- Abyaneh, M.K.; Gregoratti, L.; Amati, M.; Dalmiglio, M.; Kiskinova, M. Scanning photoelectron microscopy: A powerful technique for probing micro and nano-structures. J. Surf. Sci. Nanotechnol. 2011, 9, 158–162. [Google Scholar] [CrossRef]
- Mino, L.; Borfecchia, E.; Segura-Ruiz, J.; Giannini, C.; Martinez-Criado, G. Materials characterization by synchrotron X-ray microprobes and nanoprobes. Rev. Modern Phys. 2018, 90, 025007. [Google Scholar] [CrossRef]
- Schofield, P.F.; Smith, A.D.; Scholl, A.; Doran, A.; Covey-Crump, S.J.; Young, A.T.; Ohldag, H. Chemical and oxidation-state imaging of mineralogical intergrowths: The application of X-ray photo-emission electron microscopy (XPEEM). Coord. Chem. Rev. 2014, 277–278, 31–43. [Google Scholar] [CrossRef]
- Acres, R.G.; Harmer, S.L.; Beattie, D.A. Synchrotron PEEM and ToF-SIMS study of oxidized heterogeneous pentlandite, pyrrhotite and chalcopyrite. J. Synchrotron Rad. 2010, 17, 606–615. [Google Scholar] [CrossRef] [PubMed]
- Kalegowda, Y.; Chan, Y.-L.; Wei, D.-H.; Harmer, S.L. X-PEEM, XPS and ToF-SIMS characterisation of xanthate induced chalcopyrite flotation: Effect of pulp potential. Surf. Sci. 2015, 635, 70–77. [Google Scholar] [CrossRef]
- Acres, R.G.; Harmer, S.L.; Shui, H.W.; Chen, C.-H.; Beattie, D.A. Synchrotron scanning photoemission microscopy of homogeneous and heterogeneous metal sulfide. J. Synchrotron Rad. 2011, 18, 649–657. [Google Scholar] [CrossRef]
- Chandra, A.P.; Gerson, A.R. Pyrite (FeS2) oxidation: A sub-micron synchrotron investigation of the initial steps. Geochim. Cosmochim. Acta 2011, 75, 6239–6254. [Google Scholar] [CrossRef]
- Li, Y.; Chandra, A.P.; Gerson, A.R. Scanning photoelectron microscopy studies of freshly fractured chalcopyrite exposed to O2 and H2O. Geochim. Cosmochim. Acta 2014, 133, 372–386. [Google Scholar] [CrossRef]
- Li, Y.; Qian, G.; Brown, P.L.; Gerson, A.R. Chalcopyrite dissolution: Scanning photoelectron microscopy examination of the evolution of sulfur species with and without added iron or pyrite. Geochim. Cosmochim. Acta 2017, 212, 33–47. [Google Scholar] [CrossRef]
- Shchukarev, A. XPS at solid–aqueous solution interface. Adv. Colloid Interface Sci. 2016, 122, 149–157. [Google Scholar] [CrossRef]
- Salmeron, M.; Schlögl, R. Ambient pressure photoelectron spectroscopy: A new tool for surface science and nanotechnology. Surf. Sci. Rep. 2008, 63, 169–199. [Google Scholar] [CrossRef]
- Arble, C.; Jia, M.; Newberg, J.T. Lab-based ambient pressure X-ray photoelectron spectroscopy from past to present. Surf. Sci. Rep. 2018, 73, 37–57. [Google Scholar] [CrossRef]
- Head, A.R.; Bluhm, H. Ambient pressure X-ray photoelectron spectroscopy. In Encyclopedia of Interfacial Chemistry; Wandelt, K., Ed.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 13–27. [Google Scholar] [CrossRef]
- Patel, D.I.; Roychowdhury, T.; Jain, V.; Shah, D.; Avval, T.G.; Chatterjee, S.; Bahr, S.; Dietrich, P.; Meyer, M.; Thißen, A.; et al. Introduction to near-ambient pressure X-ray photoelectron spectroscopy characterization of various materials. Surf. Sci. Spectra 2019, 26, 016801. [Google Scholar] [CrossRef]
- Braun, A. In situ photoelectron spectroscopy. In Encyclopedia of Interfacial Chemistry; Wandelt, K., Ed.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 264–279. [Google Scholar] [CrossRef]
- Brown, M.A.; Jordan, I.; Redondo, A.B.; Kleibert, A.; Wörner, H.J.; van Bokhoven, J.A. In situ photoelectron spectroscopy at the liquid/nanoparticle interface. Surf. Sci. 2013, 610, 1–6. [Google Scholar] [CrossRef]
- Starr, D.E.; Favaro, M.; Abdi, F.F.; Bluhm, H.; Crumlin, E.J.; van de Krol, R. Combined soft and hard X-ray ambient pressure photoelectron spectroscopy studies of semiconductor/electrolyte interfaces. J. Electron Spectrosc. Rel. Phenom. 2017, 221, 106–115. [Google Scholar] [CrossRef]
- Endo, R.; Watanabe, D.; Shimomura, M.; Masuda, T. In situ X-ray photoelectron spectroscopy using a conventional Al-Kα source and an environmental cell for liquid samples and solid-liquid interfaces. Appl. Phys. Lett. 2019, 114, 173702. [Google Scholar] [CrossRef]
- Burger, K.; Fluck, E. X-ray-photoelectron spectroscopy (ESCA) investigations in coordination chemistry, I. Solvation of SbCl5 studied in quick-frozen solutions. Inorg. Nucl. Chem. Lett. 1974, 10, 171–177. [Google Scholar] [CrossRef]
- Shchukarev, A.; Sjöberg, S. XPS with fast-frozen samples: A renewed approach to study the real mineral/solution interface. Surf. Sci. 2005, 584, 106–112. [Google Scholar] [CrossRef]
- Shchukarev, A. Electrical double layer at the mineral-aqueous solution interface as probed by XPS with fast-frozen samples. J. Electron Spectrosc. Relat. Phenom. 2010, 176, 13–17. [Google Scholar] [CrossRef]
- Shchukarev, A.; Ramstedt, M. Cryo-XPS: Probing intact interfaces in nature and life. Surf. Interface Anal. 2017, 49, 349–356. [Google Scholar] [CrossRef]
- Kozin, P.A.; Shchukarev, A.; Boily, J.-F. Electrolyte ion binding at iron oxyhydroxide mineral surfaces. Langmuir 2013, 29, 12129–12137. [Google Scholar] [CrossRef]
- Wan, M.; Shchukarev, A.; Lohmayer, R.; Planer-Friedrich, B.; Peiffer, S. Occurrence of surface polysulfides during the interaction between ferric (hydr)oxides and aqueous sulfide. Environ. Sci. Technol. 2014, 48, 5076–5084. [Google Scholar] [CrossRef]
- Khoshkhoo, M.; Dopson, M.; Shchukarev, A.; Sandström, Å. Chalcopyrite leaching and bioleaching: An X-ray photoelectron spectroscopic (XPS) investigation on the nature of hindered dissolution. Hydrometallurgy 2014, 149, 220–227. [Google Scholar] [CrossRef]
- Hampton, M.A.; Nguyen, A.V. Accumulation of dissolved gases at hydrophobic surfaces in water and sodium chloride solutions: Implications for coal flotation. Miner. Eng. 2009, 22, 786–792. [Google Scholar] [CrossRef]
- Xing, Y.; Gui, X.; Cao, Y. The hydrophobic force for bubble–particle attachment in flotation—A brief review. Phys. Chem. Chem. Phys. 2017, 19, 24421–24435. [Google Scholar] [CrossRef] [PubMed]
- Owens, C.L.; Schach, E.; Rudolph, M.; Nash, G.R. Surface nanobubbles on the carbonate mineral dolomite. RSC Adv. 2018, 8, 35448–35452. [Google Scholar] [CrossRef]
- Owens, C.L.; Schach, E.; Heinig, T.; Rudolph, M.; Nash, G.R. Surface nanobubbles on the rare earth fluorcarbonate mineral synchysite. J. Colloid Interface Sci. 2019, 552, 66–71. [Google Scholar] [CrossRef]
- Yu, Y.; Ma, L.; Cao, M.; Liu, Q. Slime coatings in froth flotation: A review. Miner. Eng. 2017, 114, 26–36. [Google Scholar] [CrossRef]
- Peng, Y.; Grano, S. Effect of iron contamination from grinding media on the flotation of sulphide minerals of different particle size. Int. J. Miner. Process. 2010, 97, 1–6. [Google Scholar] [CrossRef]
- Mikhlin, Y.; Vorobyev, S.; Romanchenko, A.; Karasev, S.; Karacharov, A.; Zharkov, S. Ultrafine particles derived from mineral processing: A case study of the Pb-Zn sulfide ore with emphasis on lead-bearing colloids. Chemosphere 2016, 147, 60–66. [Google Scholar] [CrossRef]
- Mikhlin, Y.; Romanchenko, A.; Vorobyev, S.; Karasev, S.; Volochaev, M.; Kamenskiy, E.; Burdakova, E. Ultrafine particles in ground sulfide ores: A comparison of four Cu-Ni ores from Siberia, Russia. Ore Geol. Rev. 2017, 81, 1–9. [Google Scholar] [CrossRef]
- Bremmell, K.E.; Fornasiero, D.; Ralston, J. Pentlandite-lizardite interactions and implications for their separation by flotation. Colloids Surf. A Physicochem. Eng. Asp. 2005, 252, 207–212. [Google Scholar] [CrossRef]
- Liu, D.; Zhang, G.; Chen, Y.; Chen, W.; Gao, Y. A novel method to limit the adverse effect of fine serpentine on the flotation of pyrite. Minerals 2018, 8, 582. [Google Scholar] [CrossRef]
- Kelly, M.A. Historical perspectives on charging issues in XPS. J. Electron Spectrosc. Rel. Phenom. 2010, 176, 7–9. [Google Scholar] [CrossRef]
- Yasuno, S. Charge compensation in hard X-ray photoelectron spectroscopy by electron beam of several kilo-electron-volts. J. Surf. Anal. 2019, 26, 202–203. [Google Scholar]
| Technique | Possibilities and Findings | Drawbacks | Mineral Samples |
|---|---|---|---|
| Conventional XPS | Analysis of surface reaction products and adsorbates, non-stoichiometry | Loss of volatile species; decay of solid/water interface | numerous minerals, ores |
| Freeze-drying | Detection of volatile species (S, dixanthogens, etc.) | Decay of solid/water interface | PbS [44,56] FeS2 [99,100] |
| Fast-freezing | Characterization of volatile species and solid/water interfaces | Uncertainty about the effect of freezing | CuFeS2 [68,102,186], FeS2, PbS [102], ZnS [103] |
| Soft SR-XPS (hν = 100–1000 eV) | Tunable excitation energy and surface sensitivity; high spectral sensitivity and resolution | Need of access to SR facilities, potential sample damage and loss of volatile species, decay of solid/water interface | PbS [56,138,139,140,143], FeS2 [58,130,132,141,142,144,152], CuFeS2 [135,136,147,148,149,150], ZnS [137], FeAsS [131,133], NiAsS [134], Fe1-xS [145], Cu5FeS4 [147], (Fe, Ni)9S8 [151] |
| HAXPES (hν = 2000–10000 eV) | Non-destructive depth profiling | Mainly buried layers and interfaces, need of SR facility access (mostly) | Fe1-xS, FeS2 [89], CuFeS2 [90] |
| SPEM | Lateral resolution down to 100 nm | Strict requirements to samples; access to SR facilities | FeS2 [168], CuFeS2 [169,170] |
| PEEM | Lateral resolution down to 10 nm | Limited chemical information; access to SR facilities (mostly) | CuFeS2 [165,166,167], (Ni,Fe)9S8 [165], Fe1-xS [165], FeS2 [167] |
| AP-XPS | N situ analysis of solid/gas and solid/liquid interfaces | Lack of appropriate techniques; need of SR facility access (mostly) | − |
© 2020 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mikhlin, Y. X-ray Photoelectron Spectroscopy in Mineral Processing Studies. Appl. Sci. 2020, 10, 5138. https://doi.org/10.3390/app10155138
Mikhlin Y. X-ray Photoelectron Spectroscopy in Mineral Processing Studies. Applied Sciences. 2020; 10(15):5138. https://doi.org/10.3390/app10155138
Chicago/Turabian StyleMikhlin, Yuri. 2020. "X-ray Photoelectron Spectroscopy in Mineral Processing Studies" Applied Sciences 10, no. 15: 5138. https://doi.org/10.3390/app10155138
APA StyleMikhlin, Y. (2020). X-ray Photoelectron Spectroscopy in Mineral Processing Studies. Applied Sciences, 10(15), 5138. https://doi.org/10.3390/app10155138





