Low Temperature Decomposition of Polystyrene
Abstract
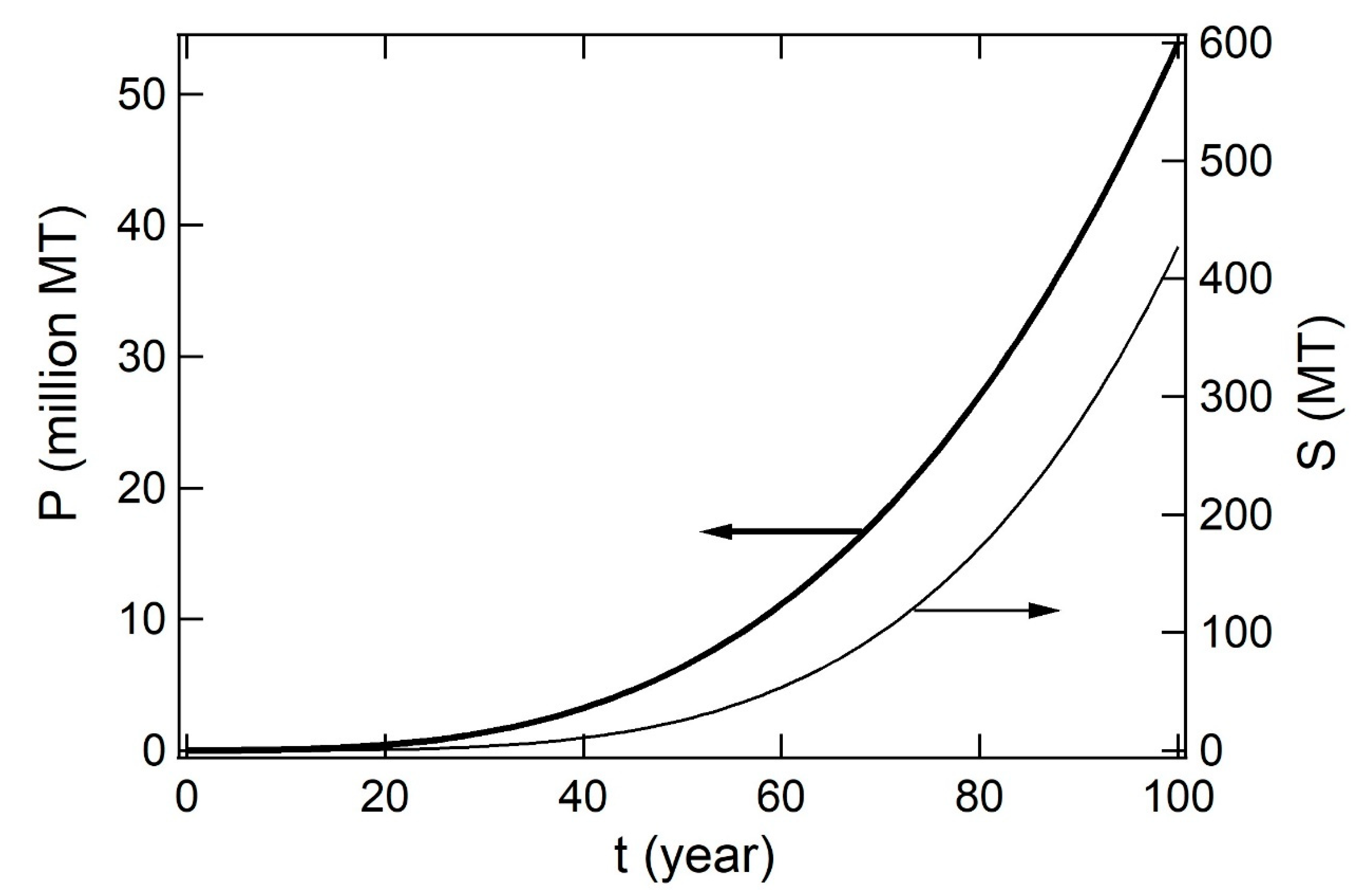
1. Introduction
2. Experimental
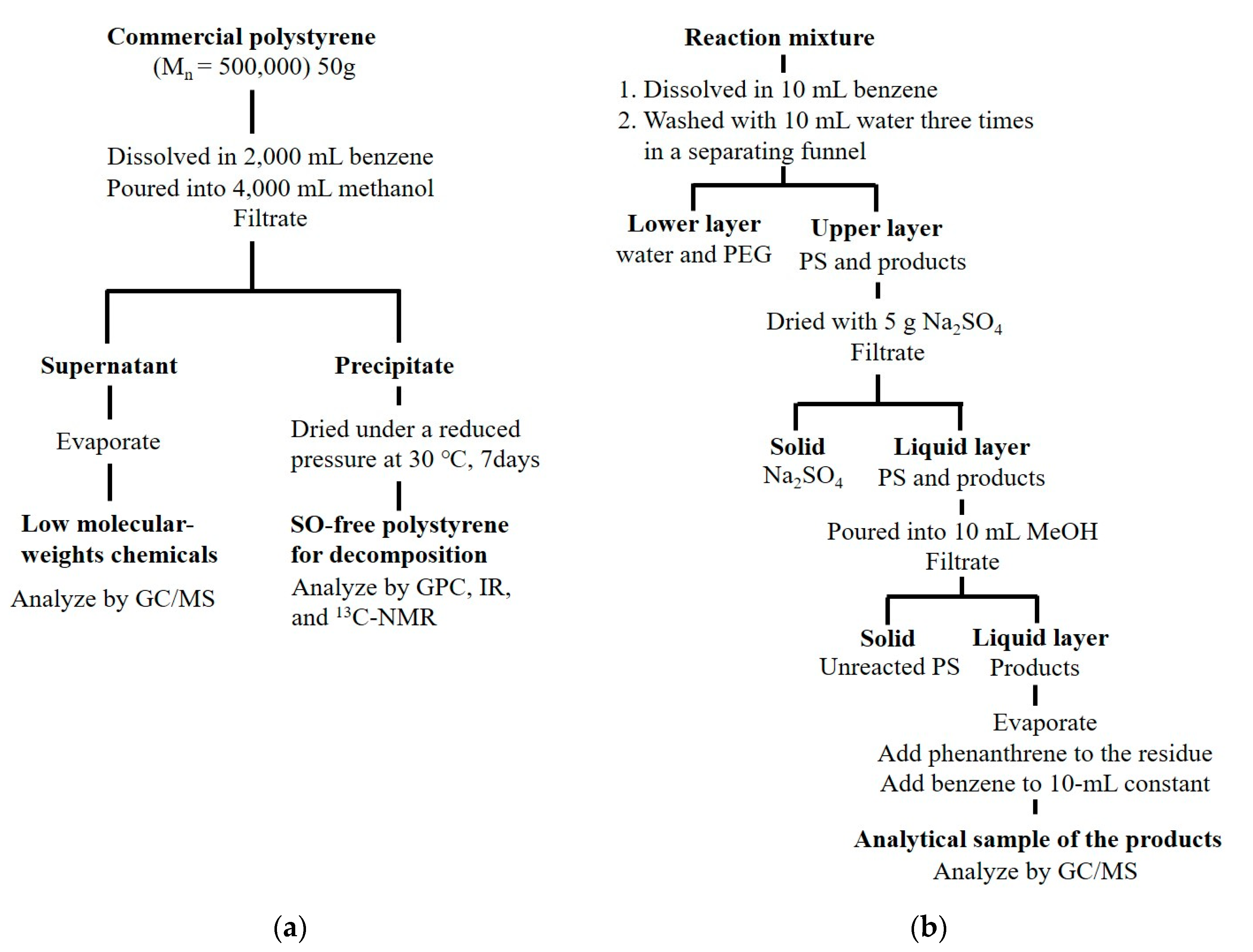
2.1. Preparation of Reaction Samples
2.2. Reagent
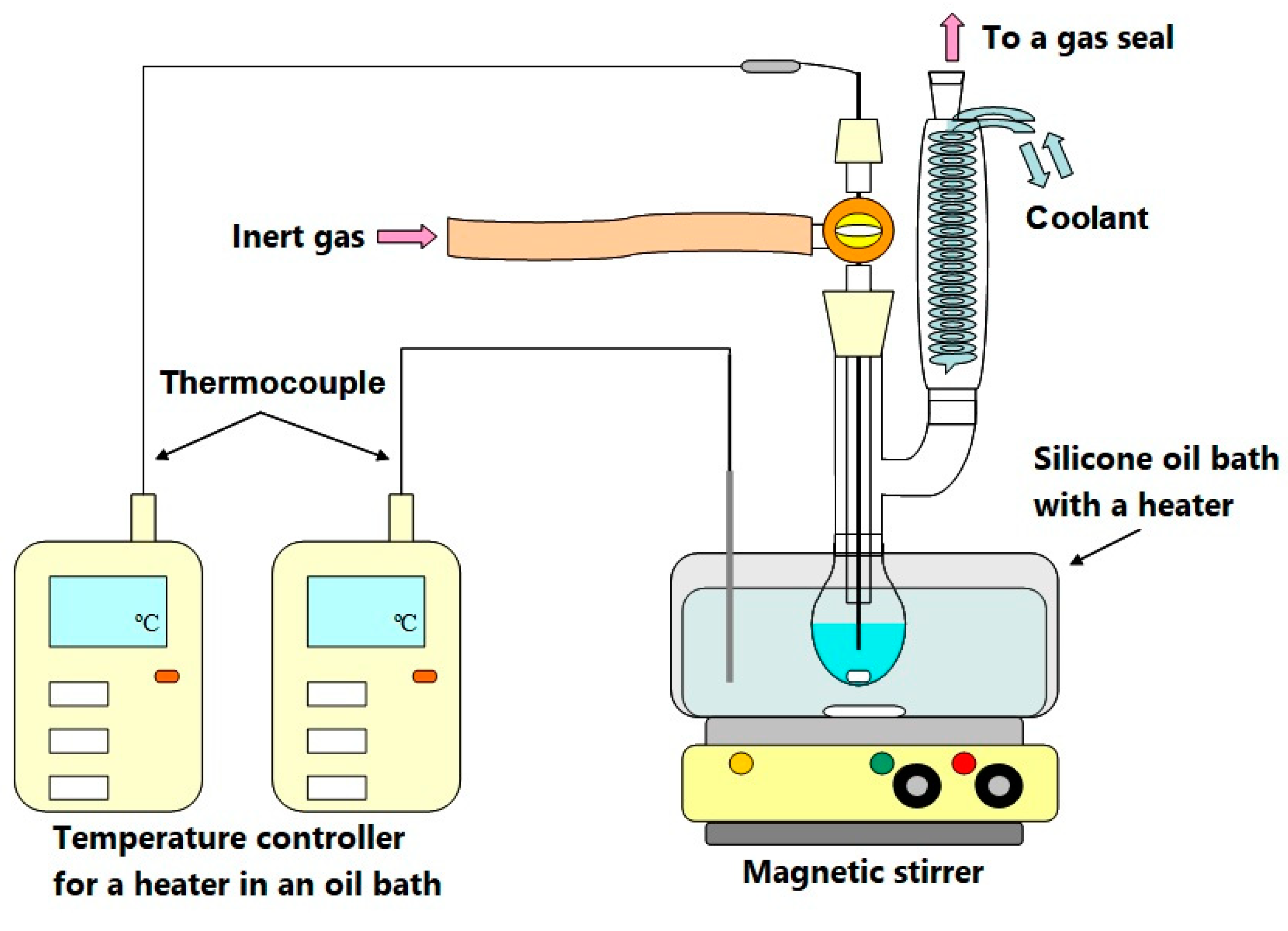
2.3. Decomposition Method
2.4. Apparatus and Operating Conditions
3. Results and Discussion
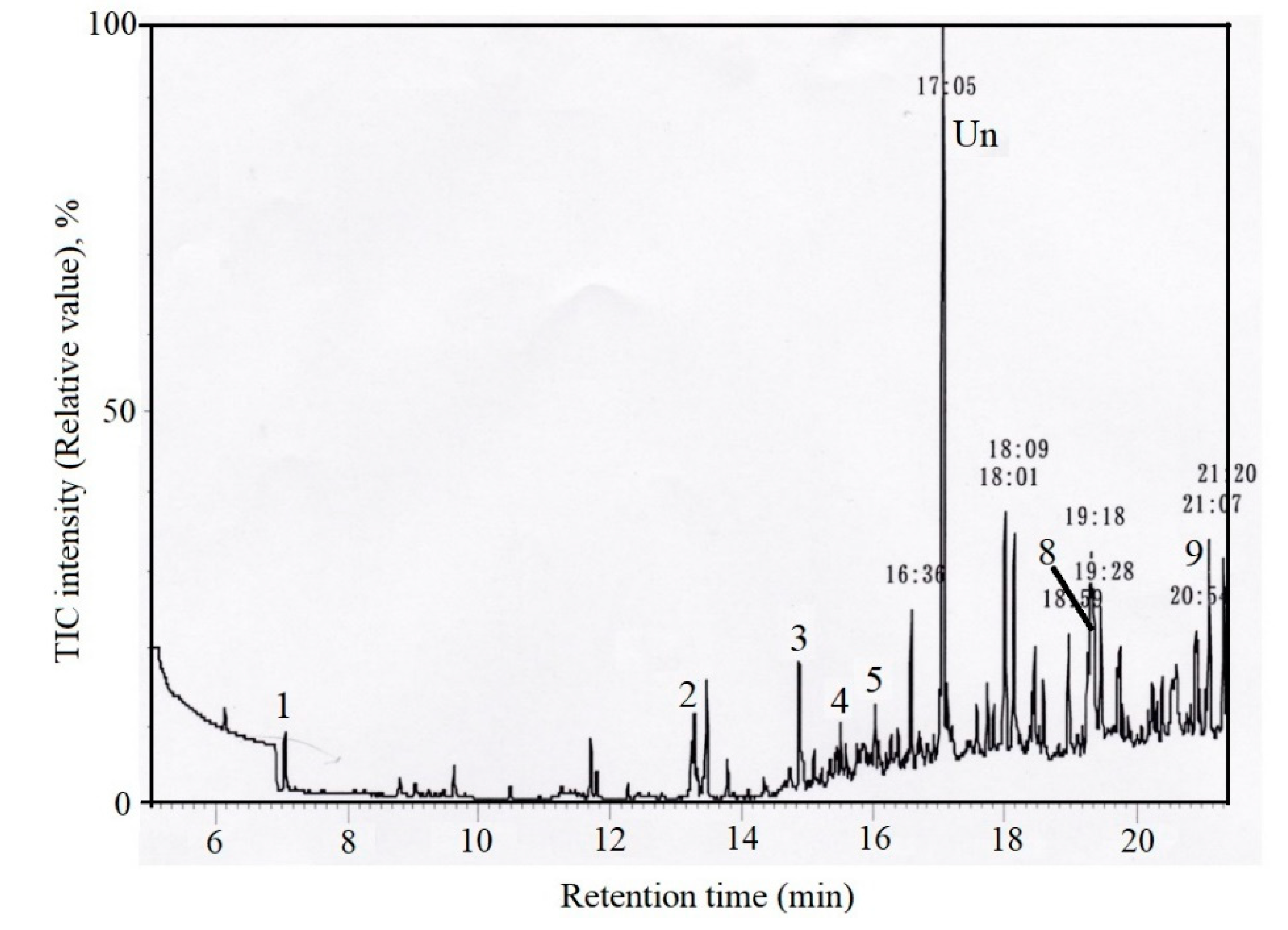
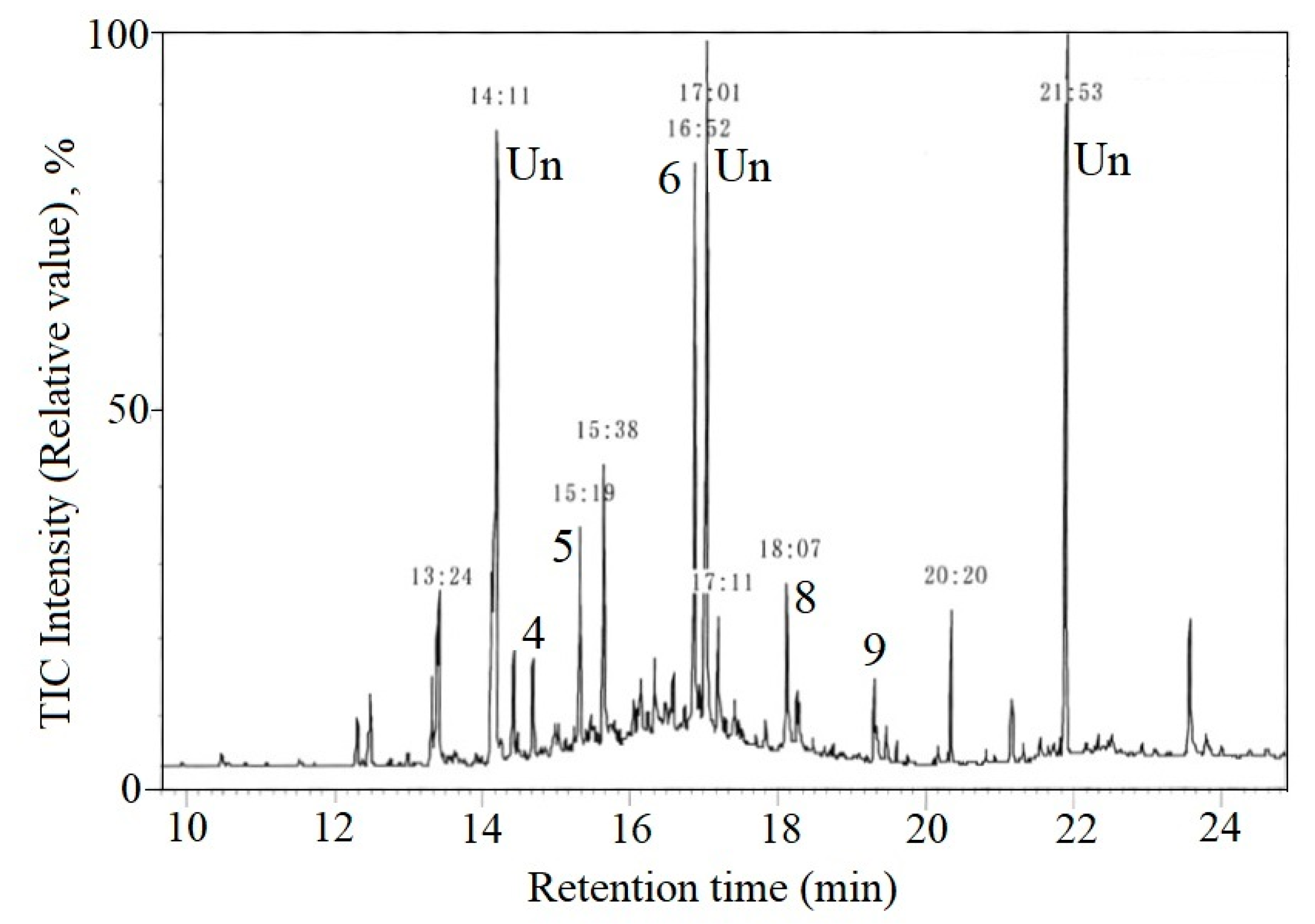
3.1. Accuracy
3.2. Effects of Temperature and Time
3.3. Degree of PS Degradation in Ocean, Determined from Rate Constant of ST Formation
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Plastics Europe. The Compelling Facts about Plastics 2006 (Plastics Europe, 2008). Available online: https://www.plasticseurope.org/application/files/2815/1689/9283/2006compelling_fact_PubJan2008.pdf (accessed on 20 June 2020).
- Plastics Europe. Plastics-the Facts 2016 (Plastics Europe, 2016). Available online: https://www.plasticseurope.org/application/files/4315/1310/4805/plastic-the-fact-2016.pdf (accessed on 20 June 2020).
- Carpenter, E.D.; Smith, K.L., Jr. Plastics on the Sargasso Sea surface. Science 1972, 175, 1240–1241. [Google Scholar] [CrossRef] [PubMed]
- Moore, C.J.; Moore, S.L.; Leecaster, M.K.; Weisberg, S.B. A comparison of plastic and plankton in the north pacific central gyre. Mar. Pollut. Bull. 2001, 42, 1297–1300. [Google Scholar] [CrossRef]
- Thompson, R.C.; Olsen, Y.; Mitchel, R.P.; Davis, A.; Rowland, S.J.; John, A.W.G.; McGonigle, D.; Russell, A.E. Lost at sea: Where is all the plastic? Science 2004, 304, 838. [Google Scholar] [CrossRef] [PubMed]
- Takada, H. Call for pellets! International pellet watch global monitoring of POPs using beached plastic resin pellets. Mar. Pollut. Bull. 2006, 52, 1547–1548. [Google Scholar] [CrossRef]
- Law, K.L. Plastics in the marine environment. Annu. Rev. Mar. Sci. 2017, 9, 205–229. [Google Scholar] [CrossRef]
- Isobe, A.; Iwasaki, S.; Uchida, K.; Tokai, T. Abundance of non-conservative microplastics in the upper ocean from 1957 to 2066. Nat. Commun. 2019, 10, 417. [Google Scholar] [CrossRef] [PubMed]
- Law, K.L.; Thompson, R.C. Microplastics in the sea. Science 2014, 345, 144–145. [Google Scholar] [CrossRef]
- Jennings, S.; Melin, F.; Blanchard, J.L.; Forster, R.M.; Dulvy, N.K.; Wilson, R.W. Global-scale predictions of community and ecosystem properties from simple ecological theory. Proc. R. Soc. B 2008, 275, 1375–1383. [Google Scholar] [CrossRef]
- Saido, K.; Amamiya, K.; Sato, H.; Okabe, A.; Ogawa, N.; Kamaya, Y.; Kogure, K.; Nishimura, M.; Okukawa, K.; Kusui, T. Analysis of styrene oligomer contaminations generated from marine debris polystyrene on the coast of Okinawa. Bunseki Kagaku 2012, 61, 629–636. [Google Scholar] [CrossRef]
- Kwon, B.G.; Kiozumi, K.; Chung, S.-Y.; Kodera, Y.; Kim, J.-O.; Saido, K. Global styrene oligomers monitoring as new chemical contamination from polystyrene plastic marine pollution. J. Hazard. Mater. 2015, 300, 359–367. [Google Scholar] [CrossRef]
- Kwon, B.G.; Chung, S.-Y.; Park, S.-S.; Saido, K. Qualitative assessment to determine internal and external factors influencing the origin of styrene oligomers pollution by polystyrene plastic in coastal marine environments. Environ. Pollut. 2018, 234, 167–173. [Google Scholar] [CrossRef] [PubMed]
- Amamiya, K.; Saido, K.; Chung, S.-Y.; Hiaki, T.; Lee, D.S.; Kwon, B.-G. Evidence of transport of styrene oligomers originated from polystyrene plastic to oceans by runoff. Sci. Total Environ. 2019, 667, 57–63. [Google Scholar] [CrossRef] [PubMed]
- Amamiya, K.; Koizumi, K.; Yamada, K.; Hiaki, T.; Kusui, T.; Saido, K. Analysis of Drifting Polystyrene Degradation Surround Japan. Austin J. Environ. Txicol. 2020, 6, id1030. [Google Scholar]
- Staudinger, H.; Steinhofer, A. Über hochpolymere Verbindungen. 107. Beiträge zur Kenntnis der Polystyrole. Justus Liebigs Ann. der Chem. 1935, 517, 35–53. [Google Scholar] [CrossRef]
- Madorsky, S.L. Rate of thermal degradation of polystyrene and polyethylene in a vacuum. J. Polym. Sci. 1952, 9, 133–156. [Google Scholar] [CrossRef]
- John, J.R.M.; Eddy, J.; Callaerts, A.; Buekens, A. Kinetic study of thermal decomposition of polystyrene by means of pyrolysis gas chromatography. Macro. Chem. Rpid Commun. 1982, 3, 349–356. [Google Scholar] [CrossRef]
- Faravelli, F.; Pinciroli, M.; Pisano, F.; Bozzano, G.; Dente, M.; Ranizi, E.G. Themal degradation of polystyrene. J. Anal. Appl. Pyrolysis 2000, 60, 103–121. [Google Scholar] [CrossRef]
- Dewangga, P.B.; Rochmadi; Purnomo, C.W. Pyrolysis of polystyrene plastic waste using bentonite catalyst. Earth Environ. Sci. 2019, 399, 0120110. [Google Scholar] [CrossRef]
- Saido, K.; Taguchi, H.; Kodera, Y.; Ishihara, Y.; Ryu, I.-J.; Chung, S.-Y. Novel method for polystyrene reactions at low temperature. Macromol. Res. 2003, 11, 87–91. [Google Scholar] [CrossRef]
- Saido, K.; Kodera, Y.; Taguchi, H.; Tomono, K.; Ishihara, Y.; Kuroki, Y. New trimer depolymerization pathway in thermal degradation of polystyrene. 230th ACS Polym. Prep 2005, 46, 685. [Google Scholar]
- Saido, K.; Motohashi, S.; Kuroki, T.; Ikemura, T.; Kirisawa, M. Preparation and properties of heat-resistant phthalic ester plasticizers derived from the thermal decomposition products of waste polystyrene. Ind. Eng. Chem. Prod. Res. Dev. 1985, 24, 102–106. [Google Scholar] [CrossRef]
- Shibasaki, Y.; Yang, M.; Sun, L.; Shi, L. Role of back-biting in the thermal degradation of vinyl polymers. Polym. Recycl. 2000, 5, 189–204. [Google Scholar]
- Lafeuille, J.-L.; Buniak, M.-L.; Vioujas, M.-C.; Lefevre, S. Natural formation of styrene by cinnamon mold flora. J. Food Sci. 2009, 74, 276–283. [Google Scholar] [CrossRef] [PubMed]
- Jambeck, J.R.; Geyer, R.; Wilcox, C.; Siegler, T.R.; Perryman, M.; Andrady, A.; Narayan, R.; Law, K.L. Plastic waste inputs from land into the ocean. Science 2015, 347, 768–771. [Google Scholar] [CrossRef] [PubMed]

| Apparatus | JEOL JMS-AM II with GC/MS (HP6890) |
| Selected Ion (SI, m/z) | 78, 104, 105, 152, 178, 193, 196, 207, 208, 312 |
| Injection | 1 μL |
| Column | DB-1, L 30 m, ID 0.32 mm, Thick 0.25 μm |
| Injection method | Spitless |
| Injection temperature | 250 °C |
| Column temperature | Initial temp. 40 °C (holding 5 min), max temp. 290 °C (holding 5 min), program rate 15 °C/min |
| Interface temperature | 250 °C |
| Ion source temperature | 200 °C |
| Ion acceleration current | 70 eV |
| Current | 300 μA |
| PM voltage | 600 V |
| Carrier gas | He, 1.4 mL/min |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kimukai, H.; Kodera, Y.; Koizumi, K.; Okada, M.; Yamada, K.; Hiaki, T.; Saido, K. Low Temperature Decomposition of Polystyrene. Appl. Sci. 2020, 10, 5100. https://doi.org/10.3390/app10155100
Kimukai H, Kodera Y, Koizumi K, Okada M, Yamada K, Hiaki T, Saido K. Low Temperature Decomposition of Polystyrene. Applied Sciences. 2020; 10(15):5100. https://doi.org/10.3390/app10155100
Chicago/Turabian StyleKimukai, Hideki, Yoichi Kodera, Koushirou Koizumi, Masaki Okada, Kazunori Yamada, Toshihiko Hiaki, and Katsuhiko Saido. 2020. "Low Temperature Decomposition of Polystyrene" Applied Sciences 10, no. 15: 5100. https://doi.org/10.3390/app10155100
APA StyleKimukai, H., Kodera, Y., Koizumi, K., Okada, M., Yamada, K., Hiaki, T., & Saido, K. (2020). Low Temperature Decomposition of Polystyrene. Applied Sciences, 10(15), 5100. https://doi.org/10.3390/app10155100






