Investigation on Gas Hydrates Formation and Dissociation in Multiphase Gas Dominant Transmission Pipelines
Abstract
1. Introduction
2. Methodology
2.1. Materials
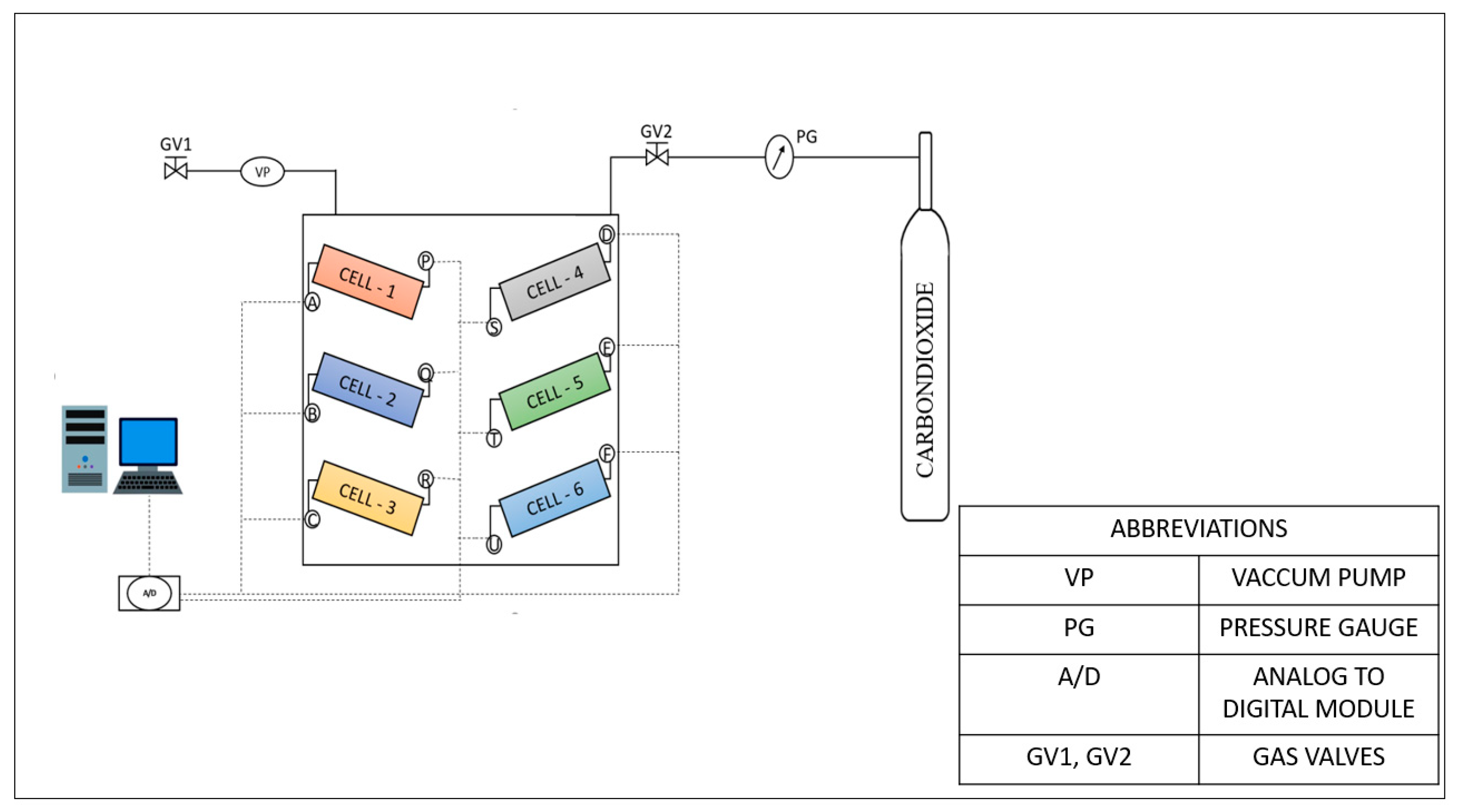
2.2. Experimental Setup
2.3. Experimental Procedure
2.3.1. Materials Characterization
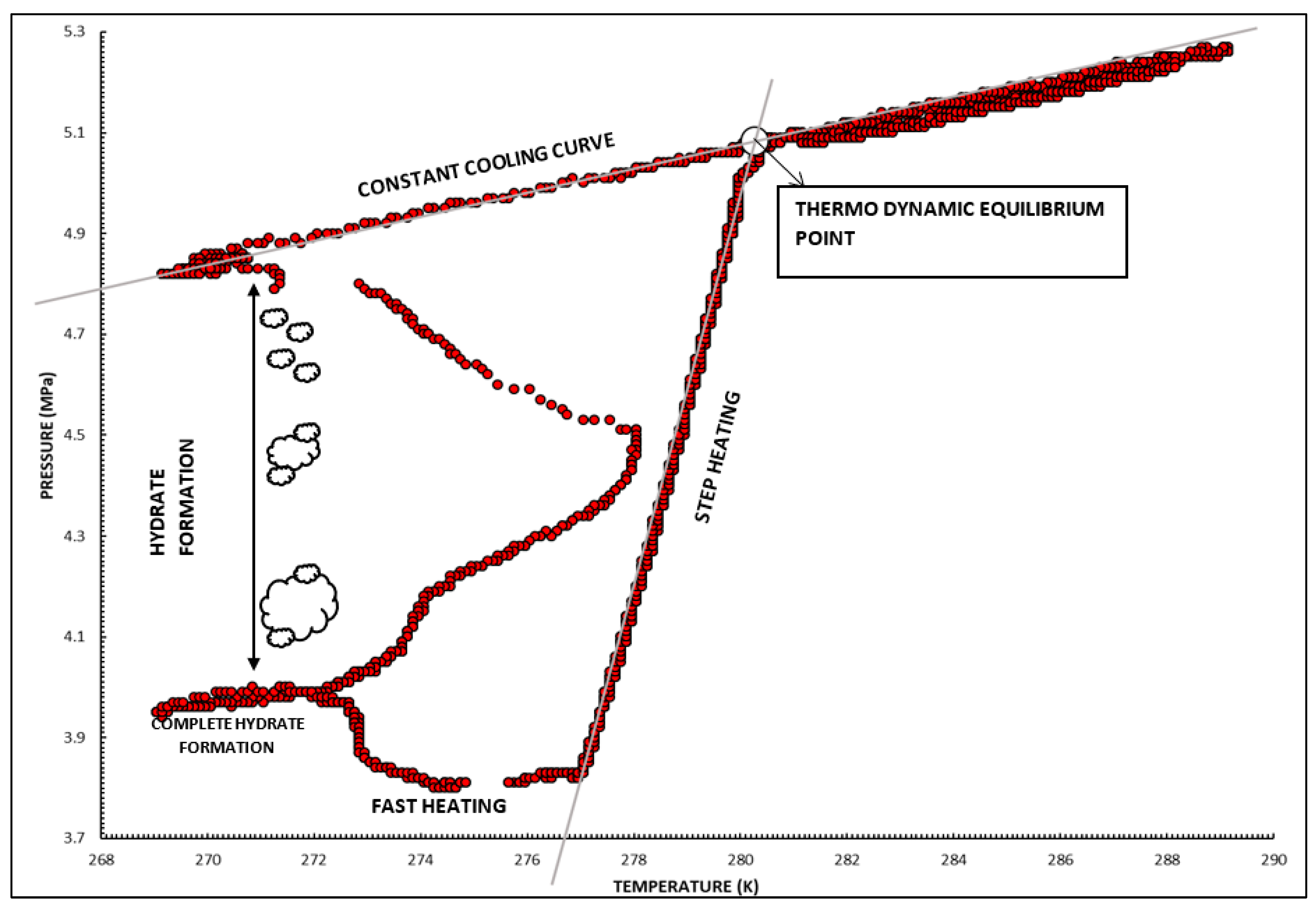
2.3.2. To Determine Thermodynamic Phase Behavior
2.3.3. To Determine Gas Hydrates Kinetics
3. Results and Discussion
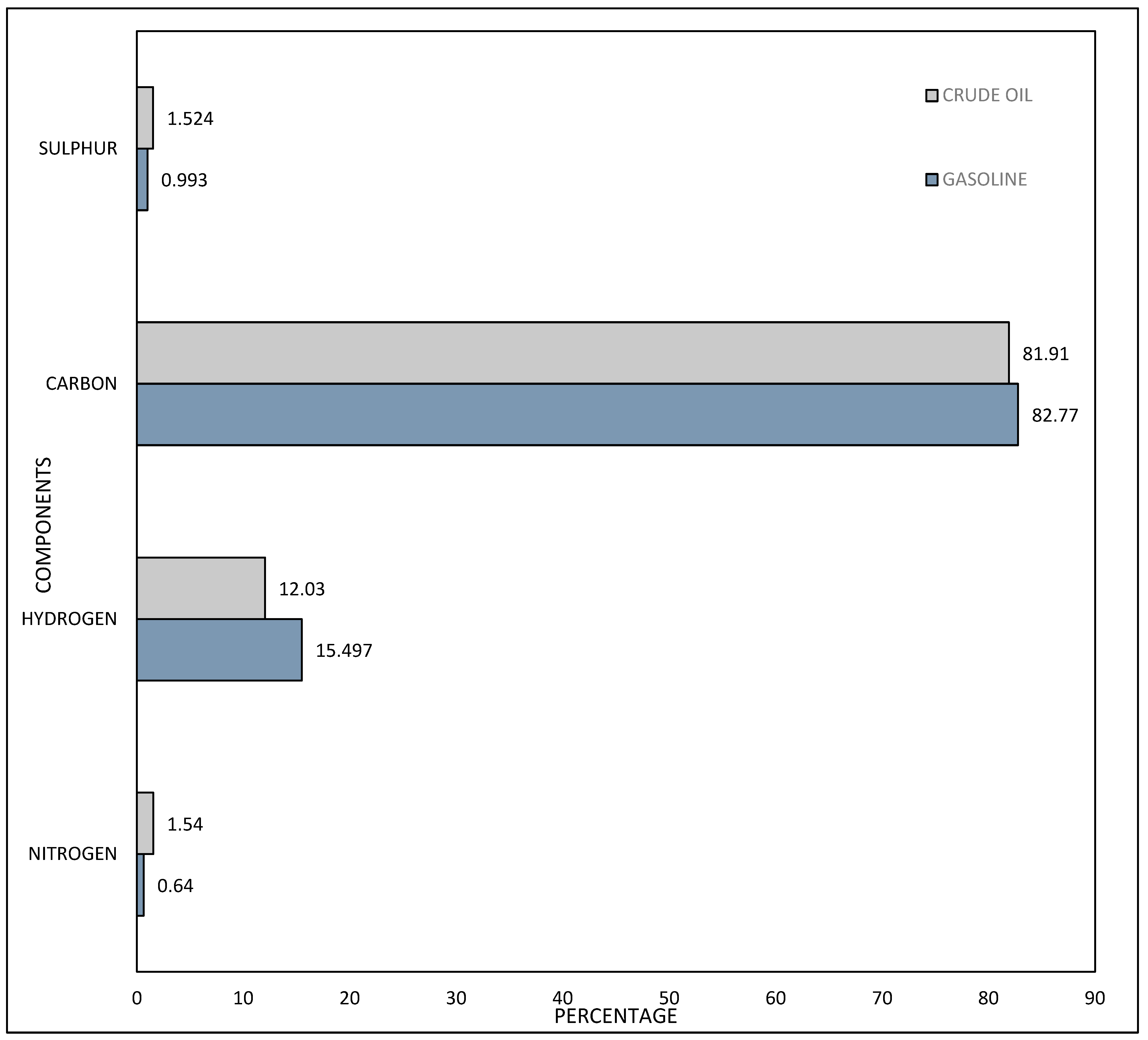
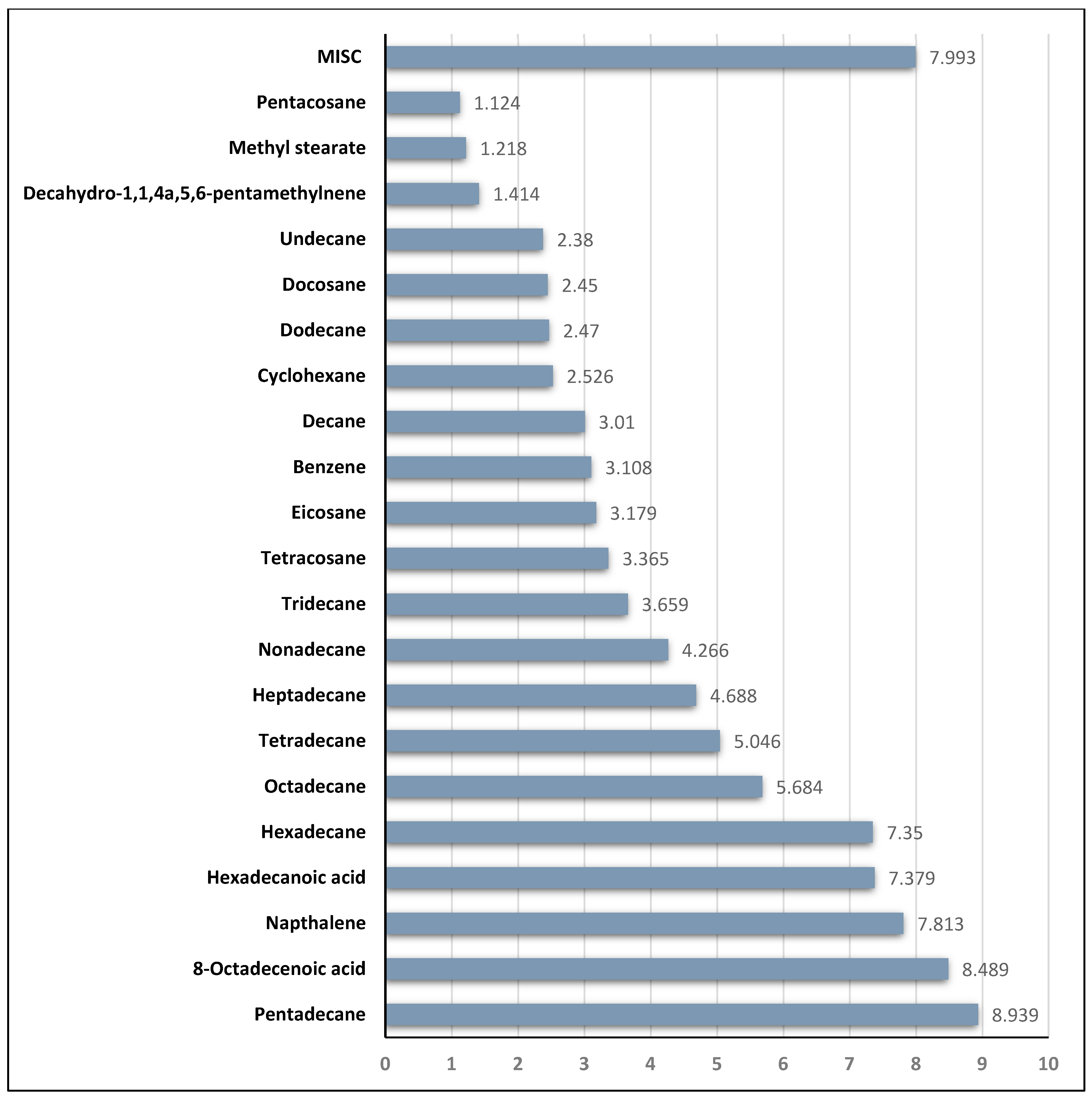
3.1. Carbon Chain Analysis in Crude Oil and Gasoline
3.2. Thermodynamic Equilibrium Conditions
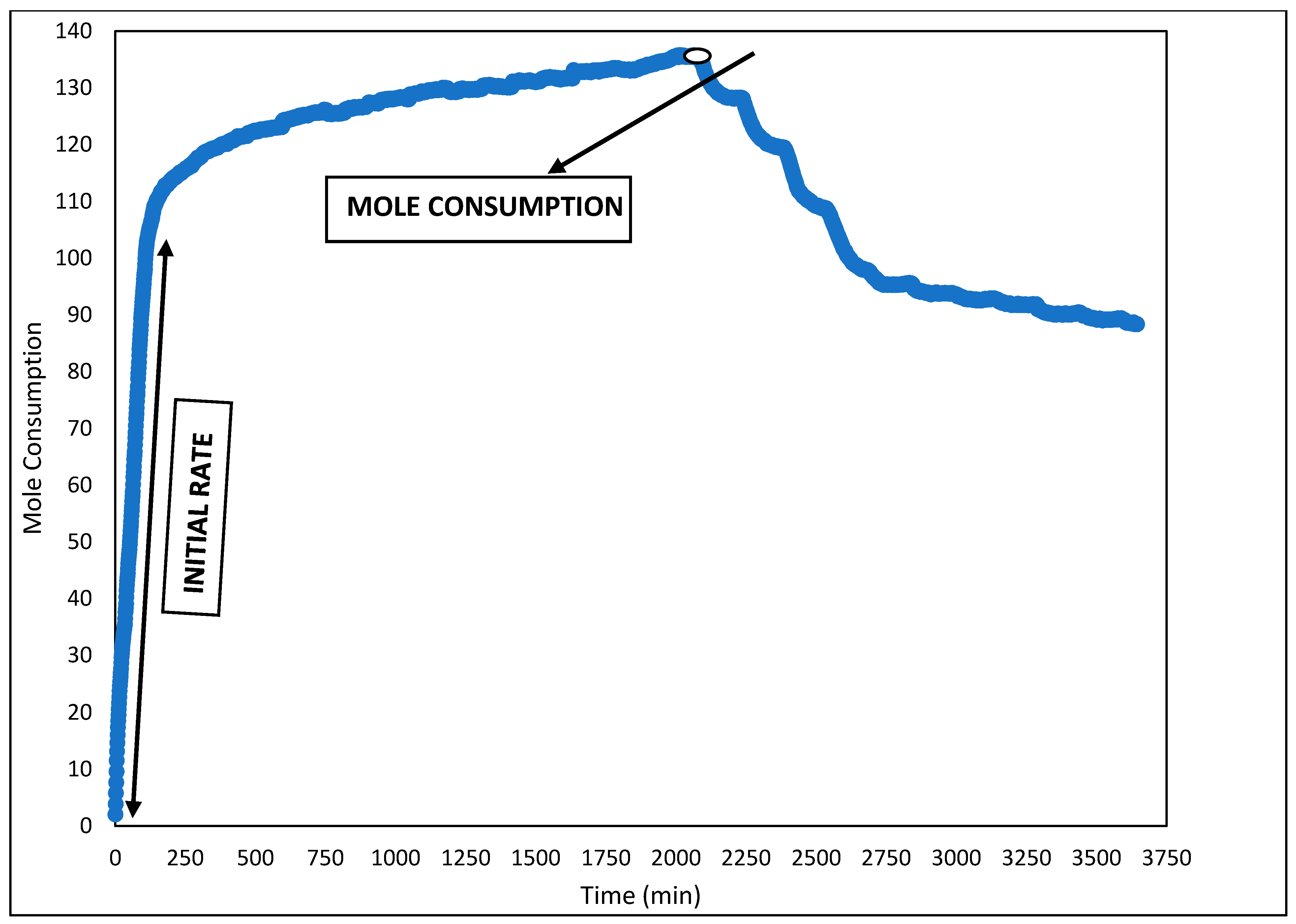
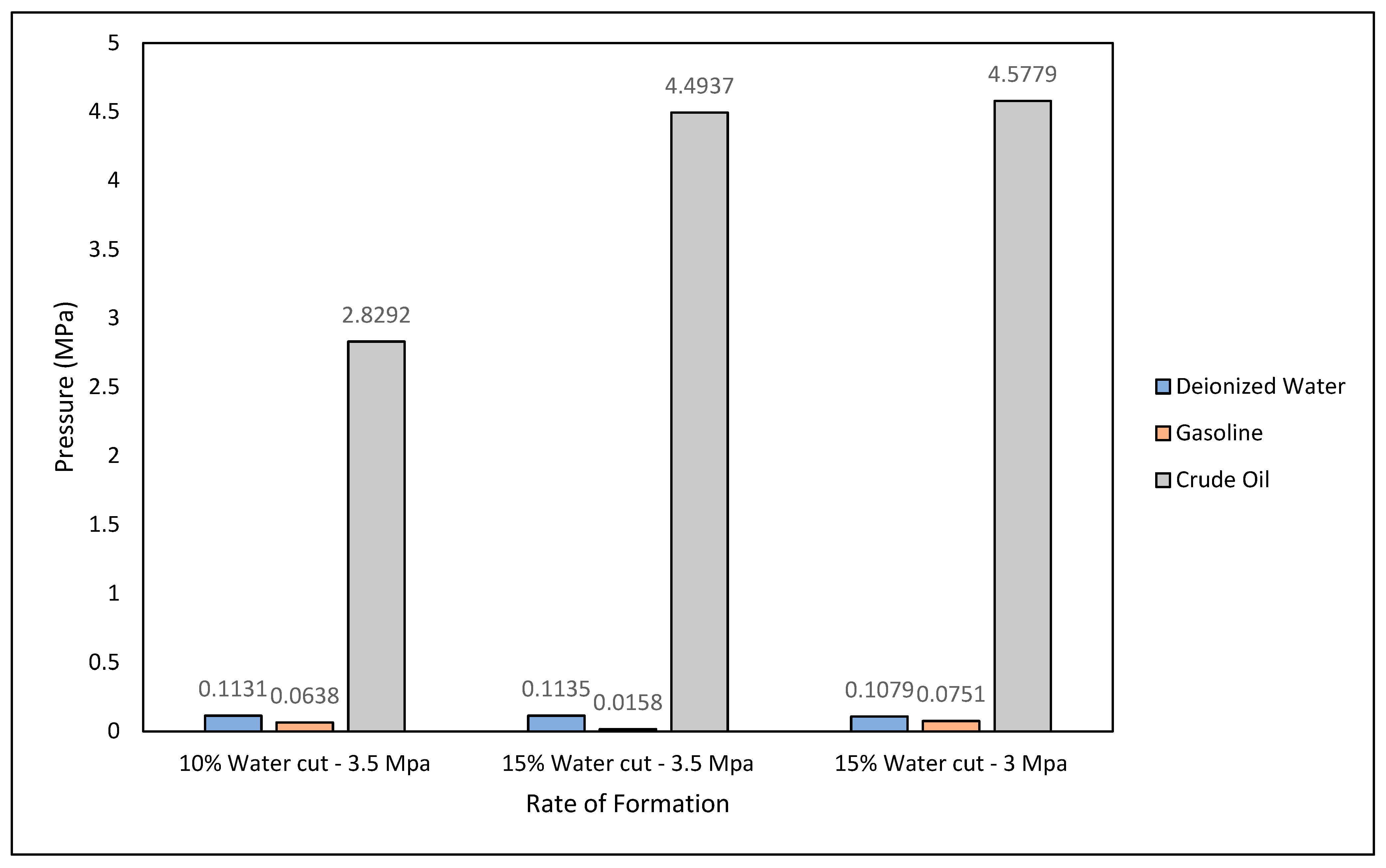
3.3. For Evaluation of Gas Hydrates Kinetics
3.3.1. Hydrate Formation Rate
3.3.2. Calculation of Kinetic Parameters
Induction Time
Initial Rate of Gas Consumption
CO2 Gas Consumption and Gas Uptake
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Griffith, P. Multiphase Flow in Pipes. J. Pet. Technol. 1984, 36, 361–367. [Google Scholar] [CrossRef]
- Qasim, A.; Khan, M.S.; Lal, B.; Ismail, M.C.; Rostani, K. Quaternary ammonium salts as thermodynamic hydrate inhibitors in the presence and absence of monoethylene glycol for methane hydrates. Fuel 2020, 259, 116219. [Google Scholar] [CrossRef]
- Khan, M.S.; Lal, B.; Keong, L.K.; Ahmed, I. Tetramethyl ammonium chloride as dual functional inhibitor for methane and carbon dioxide hydrates. Fuel 2019, 236, 251–263. [Google Scholar] [CrossRef]
- Charlton, T.B.; Di Lorenzo, M.; Zerpa, L.E.; Koh, C.A.; Johns, M.L.; May, E.F.; Aman, Z.M. Simulating Hydrate Growth and Transport Behavior in Gas-Dominant Flow. Energy Fuels 2018, 32, 1012–1023. [Google Scholar] [CrossRef]
- Garg, S.; Shariff, A.M.; Shaikh, M.S.; Lal, B.; Suleman, H.; Faiqa, N. Experimental data, thermodynamic and neural network modeling of CO2 solubility in aqueous sodium salt of l-phenylalanine. J. CO2 Util. 2017, 19, 146–156. [Google Scholar] [CrossRef]
- Mu, L.; Li, S.; Ma, Q.L.; Zhang, K.; Sun, C.Y.; Chen, G.J.; Liu, B.; Yang, L.Y. Experimental and modeling investigation of kinetics of methane gas hydrate formation in water-in-oil emulsion. Fluid Phase Equilibria 2014, 362, 28–34. [Google Scholar] [CrossRef]
- Partoon, B.; Sahith, S.J.K.; Lal, B.; Bin Maulud, A.S. Gas hydrate models. In Chemical Additives for Gas Hydrates; Springer: Cham, Switzerland, 2020; pp. 67–85. [Google Scholar] [CrossRef]
- Erickson, D.; Brown, T. Hydrate occurrence in multiphase flowlines. Ann. N. Y. Acad. Sci. 1994, 715, 40–58. [Google Scholar] [CrossRef]
- Nygaard, H.F. Hydrate Properties in Multiphase Transportation Systems. Available online: https://www.onepetro.org/general/SPE-20290-MS (accessed on 21 May 2020).
- Zheng, D.; Che, D.; Liu, Y. Experimental investigation on gas-liquid two-phase slug flow enhanced carbon dioxide corrosion in vertical upward pipeline. Corros. Sci. 2008, 50, 3005–3020. [Google Scholar] [CrossRef]
- Jakobsen, T.; Sjöblom, J.; Ruoff, P. Kinetics of gas hydrate formation in w/o-emulsions the model system trichlorofluoromethane/water/non-ionic surfactant studied by means of dielectric spectroscopy. Colloids Surf. A Physicochem. Eng. Asp. 1996, 112, 73–84. [Google Scholar] [CrossRef]
- Jassim, E.; Abdi, M.A.; Muzychka, Y. A new approach to investigate hydrate deposition in gas-dominated flowlines. J. Nat. Gas Sci. Eng. 2010, 2, 163–177. [Google Scholar] [CrossRef]
- Talatori, S.; Barth, T. Rate of hydrate formation in crude oil/gas/water emulsions with different water cuts. J. Pet. Sci. Eng. 2012, 80, 32–40. [Google Scholar] [CrossRef]
- Xiang, C.; Peng, B.; Liu, H.; Sun, C.; Chen, G.; Sun, B. Hydrate Formation/Dissociation in (Natural Gas + Water + Diesel Oil) Emulsion Systems. Energies 2013, 1009–1022. [Google Scholar] [CrossRef]
- Kakati, H.; Kar, S.; Mandal, A.; Laik, S. Methane hydrate formation and dissociation in oil-in-water emulsion. Energy Fuels 2014, 28, 4440–4446. [Google Scholar] [CrossRef]
- Chen, J.; Liu, J.; Chen, G.J.; Sun, C.Y.; Jia, M.L.; Liu, B.; Si, S.; Ren, N. Insights into methane hydrate formation, agglomeration, and dissociation in water + diesel oil dispersed system. Energy Convers. Manag. 2014, 86, 886–891. [Google Scholar] [CrossRef]
- Daraboina, N.; Pachitsas, S.; Von Solms, N. Natural gas hydrate formation and inhibition in gas/crude oil/aqueous systems. Fuel 2015, 148, 186–190. [Google Scholar] [CrossRef]
- Wang, Y.; Fan, S.; Lang, X. Reviews of gas hydrate inhibitors in gas-dominant pipelines and application of kinetic hydrate inhibitors in China. Chin. J. Chem. Eng. 2019, 27, 2118–2132. [Google Scholar] [CrossRef]
- Chaudhari, P.; Zerpa, L.E.; Sum, A.K. A correlation to quantify hydrate plugging risk in oil and gas production pipelines based on hydrate transportability parameters. J. Nat. Gas Sci. Eng. 2018, 58, 152–161. [Google Scholar] [CrossRef]
- Bavoh, C.B.; Md Yuha, Y.B.; Tay, W.H.; Ofei, T.N.; Lal, B.; Mukhtar, H. Experimental and modelling of the impact of quaternary ammonium salts/ionic liquid on the rheological and hydrate inhibition properties of xanthan gum water-based muds for drilling gas hydrate-bearing rocks. J. Pet. Sci. Eng. 2019, 183, 106468. [Google Scholar] [CrossRef]
- Daimaru, T.; Yamasaki, A.; Yanagisawa, Y. Effect of surfactant carbon chain length on hydrate formation kinetics. J. Pet. Sci. Eng. 2007, 56, 89–96. [Google Scholar] [CrossRef]
- Wei, Y. Current technologies and prospects of shale gas development in China. J. Pet. Environ. Biotechnol. 2017, 8, 7463. [Google Scholar] [CrossRef]
- Nashed, O.; Partoon, B.; Lal, K.M.; Sabil, A.M. Investigation of functionalized carbon nanotubes’ performance on carbon dioxide hydrate formation. Energy 2019, 174, 602–610. [Google Scholar] [CrossRef]
- Khan, M.S.; Cornelius, B.B.; Lal, B.; Bustam, M.A. Kinetic Assessment of Tetramethyl Ammonium Hydroxide (Ionic Liquid) for Carbon Dioxide, Methane and Binary Mix Gas Hydrates. Recent Adv. Ion. Liq. 2018. [Google Scholar] [CrossRef]
- Bavoh, C.B.; Nashed, O.; Khan, M.S.; Partoon, B.; Lal, B.; Sharif, A.M. The impact of amino acids on methane hydrate phase boundary and formation kinetics. J. Chem. Thermodyn. 2018, 117, 48–53. [Google Scholar] [CrossRef]
- Mohammadi, A.H.; Richon, D. Gas hydrate phase equilibrium in the presence of ethylene glycol or methanol aqueous solution. Ind. Eng. Chem. Res. 2010, 49, 8865–8869. [Google Scholar] [CrossRef]
- Adams, C.W.M. Chemistry and Physics of Lipids. Nature 1967, 213, 974. [Google Scholar] [CrossRef]
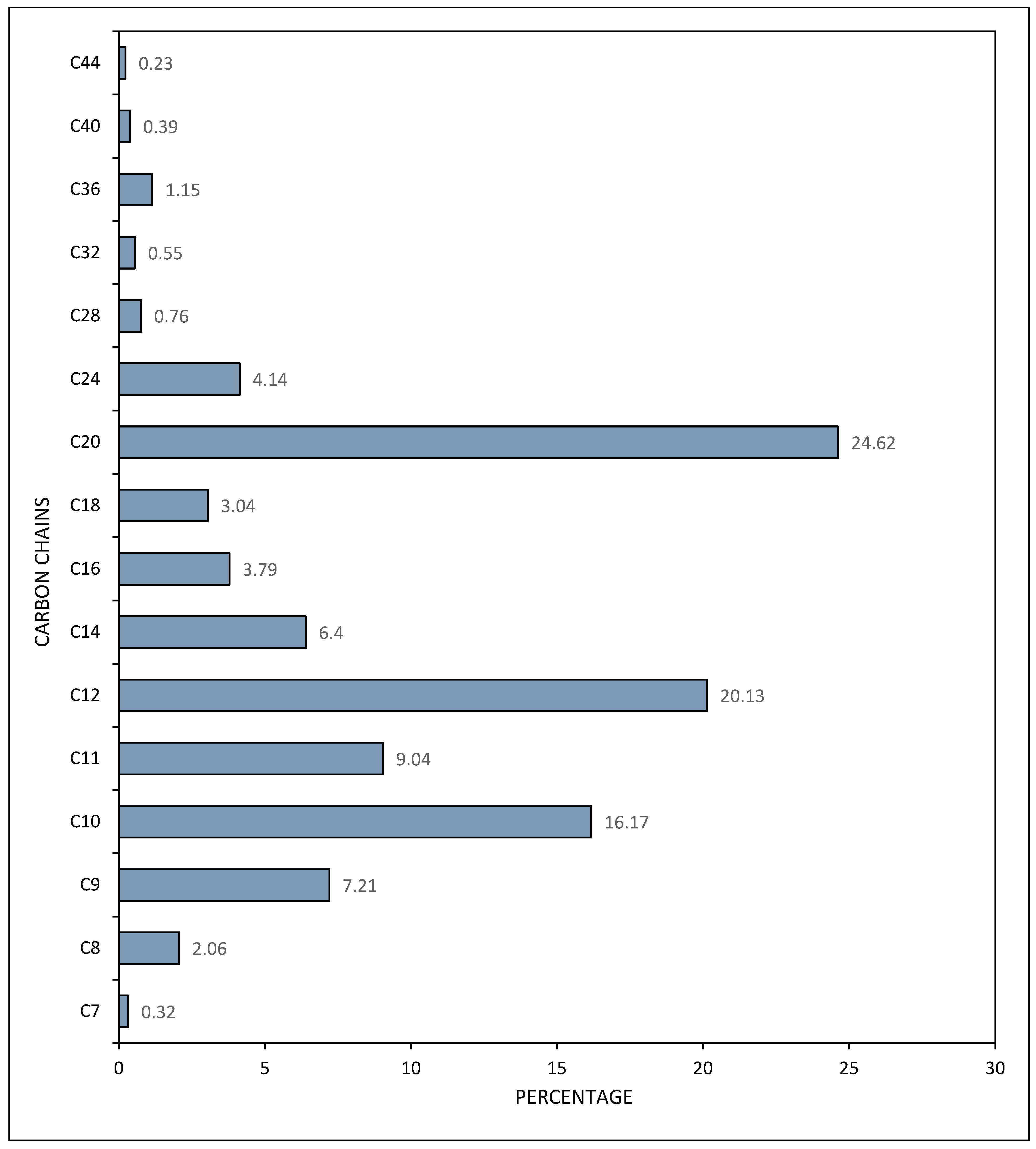
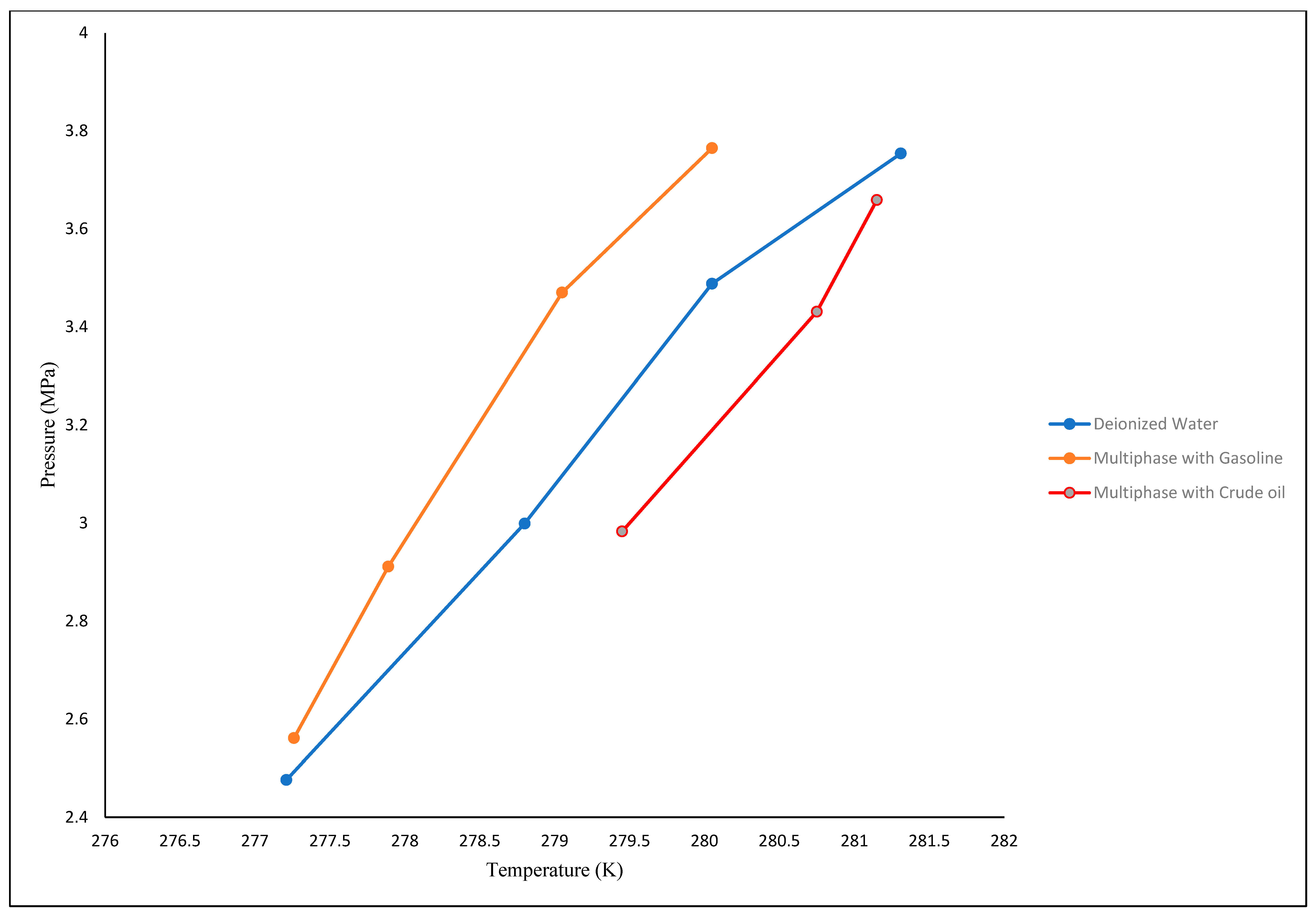

| System | Initial Pressure | Initial Temperature | Equilibrium Pressure | Equilibrium Temperature |
|---|---|---|---|---|
| Deionized water + Carbon dioxide | 2.5 | 285.15 | 2.476 | 277.21 |
| 3 | 285.15 | 2.999 | 278.80 | |
| 3.5 | 285.15 | 3.488 | 280.05 | |
| 3.8 | 285.15 | 3.754 | 281.31 | |
| Gasoline + Deionized Water + Carbon dioxide | 2.5 | 285.15 | 2.561 | 277.26 |
| 3 | 285.15 | 2.911 | 277.89 | |
| 3.5 | 285.15 | 3.47 | 279.05 | |
| 3.8 | 285.15 | 3.765 | 280.05 | |
| Crude Oil + Deionized Water + Carbon dioxide | 3 | 285.15 | 2.983 | 279.45 |
| 3.5 | 285.15 | 3.431 | 280.75 | |
| 3.8 | 285.15 | 3.659 | 281.15 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sahith, S.J.K.; Pedapati, S.R.; Lal, B. Investigation on Gas Hydrates Formation and Dissociation in Multiphase Gas Dominant Transmission Pipelines. Appl. Sci. 2020, 10, 5052. https://doi.org/10.3390/app10155052
Sahith SJK, Pedapati SR, Lal B. Investigation on Gas Hydrates Formation and Dissociation in Multiphase Gas Dominant Transmission Pipelines. Applied Sciences. 2020; 10(15):5052. https://doi.org/10.3390/app10155052
Chicago/Turabian StyleSahith, Sayani Jai Krishna, Srinivasa Rao Pedapati, and Bhajan Lal. 2020. "Investigation on Gas Hydrates Formation and Dissociation in Multiphase Gas Dominant Transmission Pipelines" Applied Sciences 10, no. 15: 5052. https://doi.org/10.3390/app10155052
APA StyleSahith, S. J. K., Pedapati, S. R., & Lal, B. (2020). Investigation on Gas Hydrates Formation and Dissociation in Multiphase Gas Dominant Transmission Pipelines. Applied Sciences, 10(15), 5052. https://doi.org/10.3390/app10155052






