A CNN CADx System for Multimodal Classification of Colorectal Polyps Combining WL, BLI, and LCI Modalities
Abstract
1. Introduction
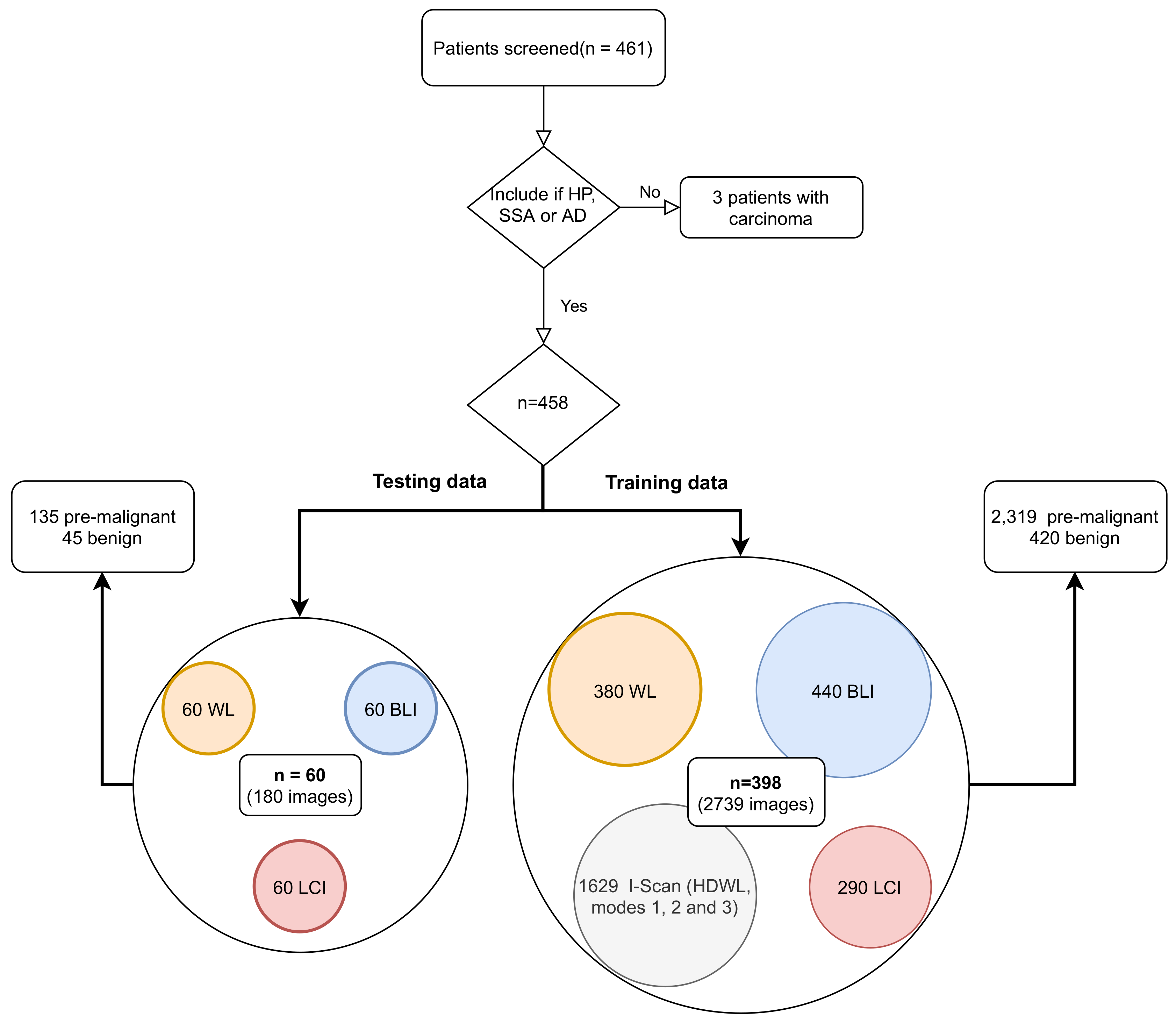
- Our dataset is improved with 458 new patients resulting in a total of 2919 images obtained from three different hospitals.
- We perform a benchmark with mostly used state-of-the-art colorectal polyp deep learning architectures, in order to train an end-to-end CNN, evaluated with a test set of 60 patients obtained with WL, BLI, and LCI.
- We build a CADx system to classify CRPs between benign and pre-malignant and we compare our results with the knowledge and expertise of 19 endoscopists (13 novices and 6 experts).
- We present a probability score to the endoscopists, which is computed from the average prediction of WL, BLI, and LCI.
- Our developed CADx systems provides explainable visual data from the CNN to contribute to smooth decision-making.
2. Materials and Methods
2.1. Patient Inclusion and Data Acquisition
2.2. Data Preprocessing
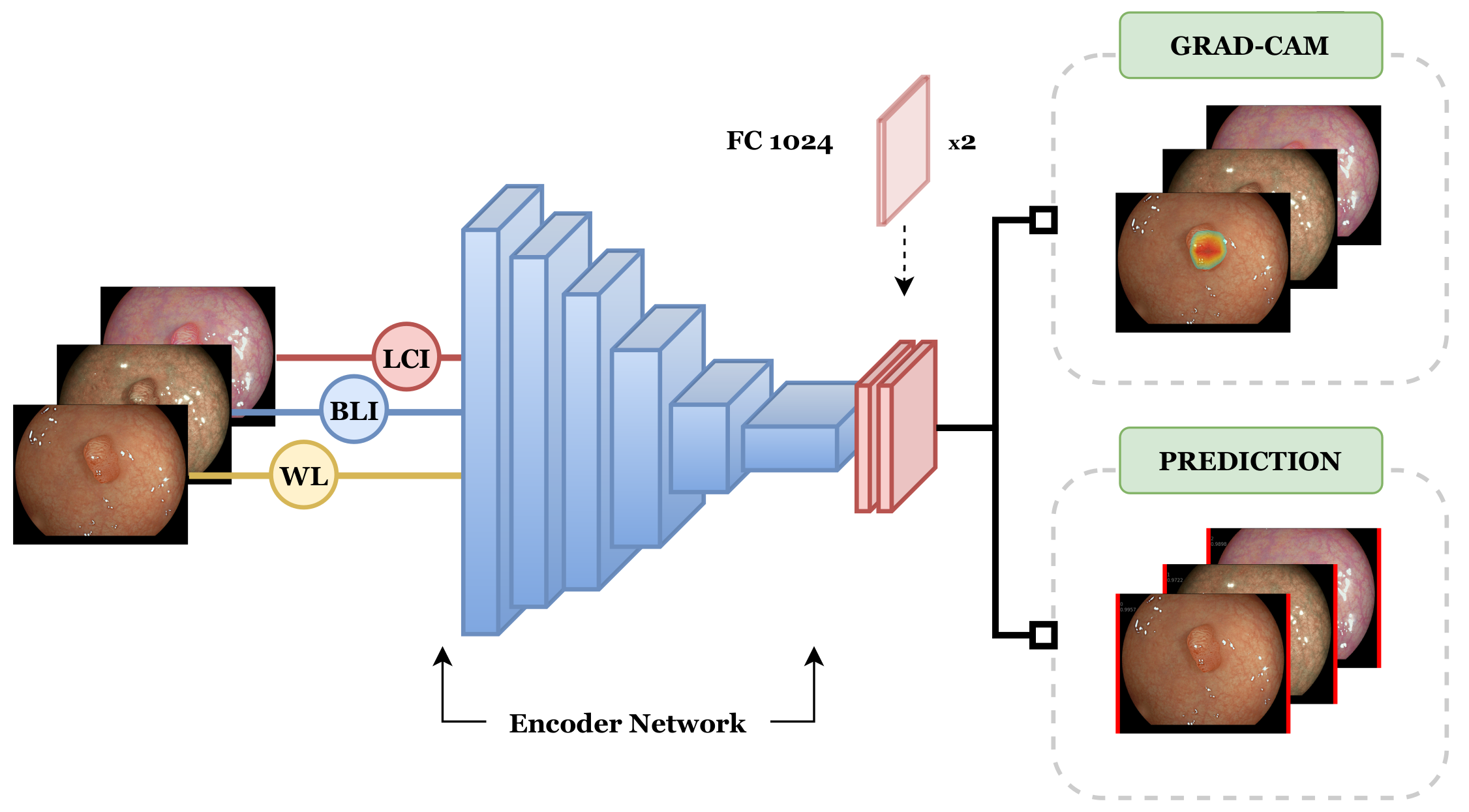
2.3. Network Architectures
2.4. Training
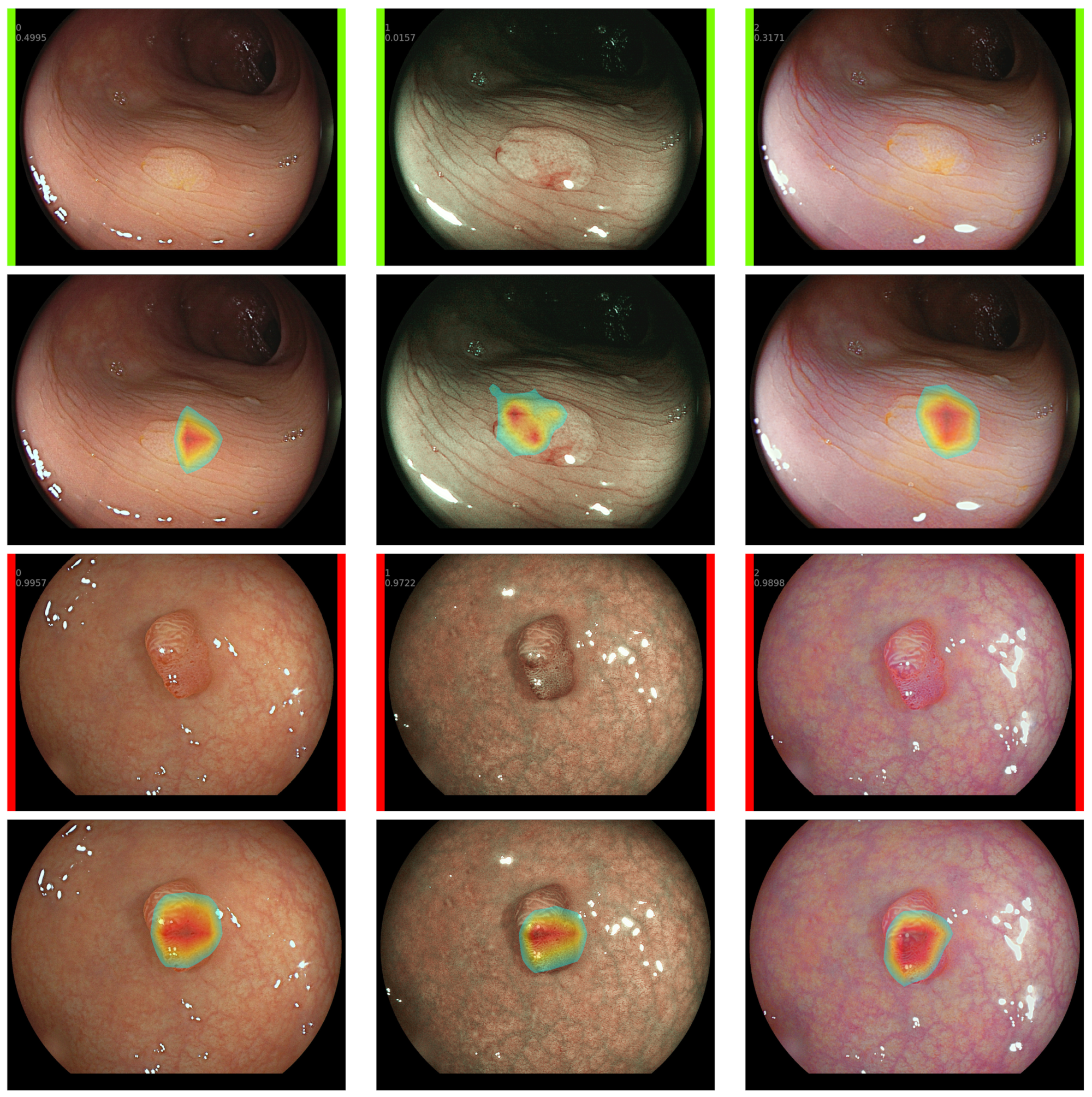
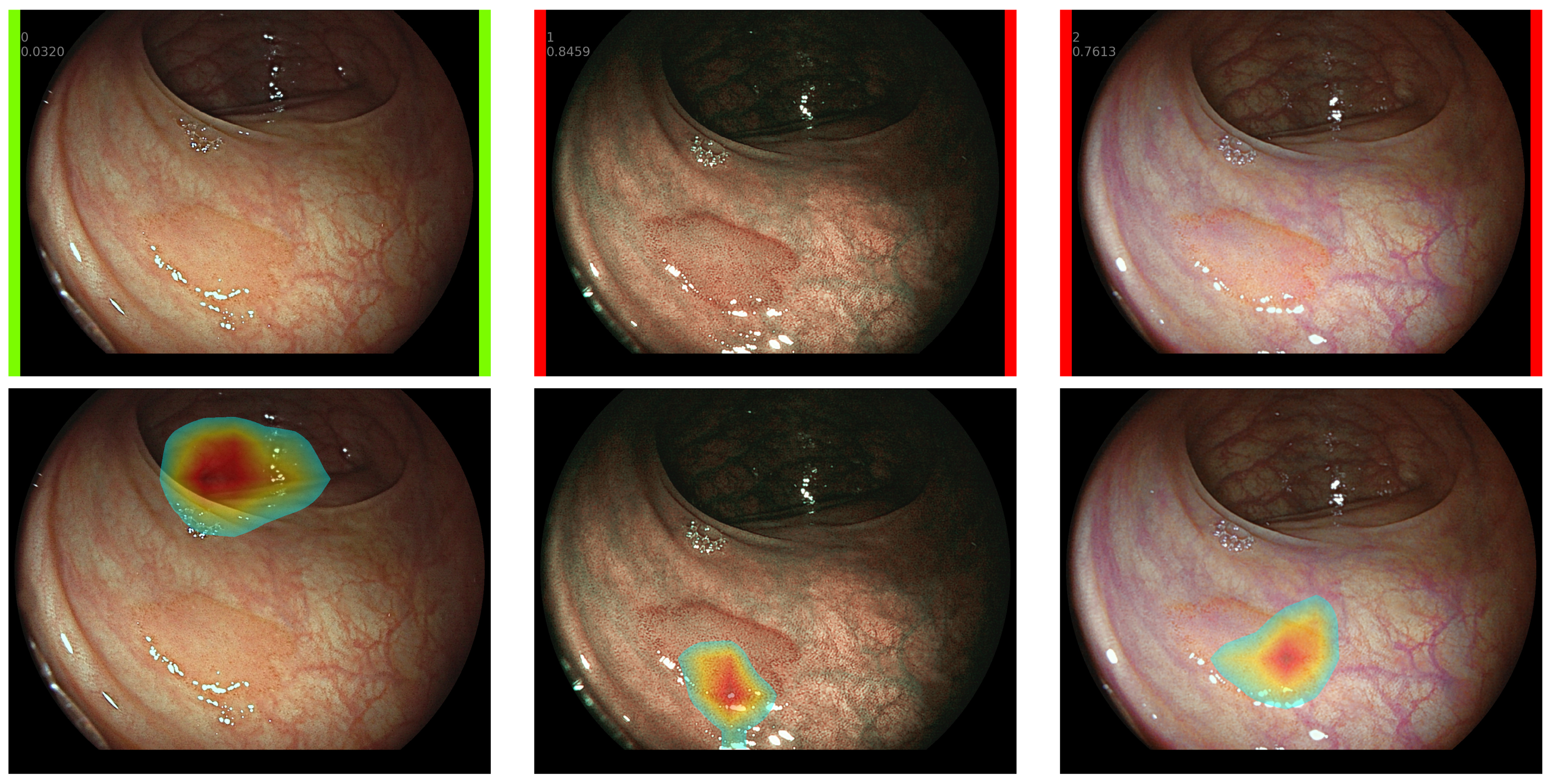
2.5. Explainable CADx System
2.6. Clinical Benchmark
3. Evaluation and Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA A Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef]
- Ferlay, J.; Colombet, M.; Soerjomataram, I.; Mathers, C.; Parkin, D.M.; Piñeros, M.; Znaor, A.; Bray, F. Estimating the global cancer incidence and mortality in 2018: GLOBOCAN sources and methods. Int. J. Cancer 2019, 144, 1941–1953. [Google Scholar] [CrossRef] [PubMed]
- Jass, J.R. Classification of colorectal cancer based on correlation of clinical, morphological and molecular features. Histopathology 2017, 50, 113–130. [Google Scholar] [CrossRef] [PubMed]
- Vleugels, J.L.A.; Dijkgraaf, M.G.W.; Hazewinkel, Y.; Wanders, L.K.; Fockens, P.; Dekker, E. Effects of Training and Feedback on Accuracy of Predicting Rectosigmoid Neoplastic Lesions and Selection of Surveillance Intervals by Endoscopists Performing Optical Diagnosis of Diminutive Polyps. Gastroenterology 2018, 154, 1682–1693.e1681. [Google Scholar] [CrossRef] [PubMed]
- Ignjatovic, A.; East, J.E.; Suzuki, N.; Vance, M.; Guenther, T.; Saunders, B.P. Optical diagnosis of small colorectal polyps at routine colonoscopy (Detect InSpect ChAracterise Resect and Discard; DISCARD trial): A prospective cohort study. Lancet Oncol. 2009, 10, 1171–1178. [Google Scholar] [CrossRef]
- Hassan, C.; Pickhardt, P.J.; Rex, D.K. A Resect and Discard Strategy Would Improve Cost-Effectiveness of Colorectal Cancer Screening. Clin. Gastroenterol. Hepatol. 2010, 8, 865–869.e3. [Google Scholar] [CrossRef]
- Tsuji, S.; Takeda, Y.; Tsuji, K.; Yoshida, N.; Takemura, K.; Yamada, S.; Doyama, H. Clinical outcomes of the “resect and discard” strategy using magnifying narrow-band imaging for small (<10 mm) colorectal polyps. Endosc. Int. Open 2018, 6, E1382–E1389. [Google Scholar]
- Neumann, H.; Neumann Sen, H.; Vieth, M.; Bisschops, R.; Thieringer, F.; Rahman, K.F.; Gamstätter, T.; Tontini, G.E.; Galle, P.R. Leaving colorectal polyps in place can be achieved with high accuracy using blue light imaging (BLI). United Eur. Gastroenterol. J. 2018, 6, 1099–1105. [Google Scholar] [CrossRef]
- Kandel, P.; Wallace, M.B. Should We Resect and Discard Low Risk Diminutive Colon Polyps. Clin. Endosc. 2019, 52, 239. [Google Scholar] [CrossRef]
- Har-Noy, O.; Katz, L.; Avni, T.; Battat, R.; Bessissow, T.; Yung, D.E.; Engel, T.; Koulaouzidis, A.; Eliakim, R.; Ben-Horin, S.; et al. Chromoendoscopy, narrow-band imaging or white light endoscopy for neoplasia detection in inflammatory bowel diseases. Dig. Dis. Sci. 2017, 62, 2982–2990. [Google Scholar] [CrossRef]
- East, J.E.; Guenther, T.; Kennedy, R.H.; Saunders, B.P. Narrow band imaging avoids potential chromoendoscopy risks. Gut 2007, 56, 1168–1169. [Google Scholar] [PubMed]
- Vişovan, I.I.; Tanțău, M.; Pascu, O.; Ciobanu, L.; Tanțău, A. The role of narrow band imaging in colorectal polyp detection. Bosn. J. Basic Med. Sci. 2017, 17, 152. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Bisschops, R.; Hassan, C.; Bhandari, P.; Coron, E.; Neumann, H.; Pech, O.; Correale, L.; Repici, A. BASIC (BLI Adenoma Serrated International Classification) classification for colorectal polyp characterization with blue light imaging. Endoscopy 2018, 50, 211–220. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, N.; Dohi, O.; Inoue, K.; Yasuda, R.; Murakami, T.; Hirose, R.; Inoue, K.; Naito, Y.; Inada, Y.; Ogiso, K.; et al. Blue Laser Imaging, Blue Light Imaging, and Linked Color Imaging for the Detection and Characterization of Colorectal Tumors. Gut Liver 2019, 13, 140–148. [Google Scholar] [CrossRef]
- Yu, L.; Chen, H.; Dou, Q.; Qin, J.; Heng, P.A. Integrating Online and Offline Three-Dimensional Deep Learning for Automated Polyp Detection in Colonoscopy Videos. IEEE J. Biomed. Health Inform. 2017, 21, 65–75. [Google Scholar] [CrossRef]
- Wang, D.; Zhang, N.; Sun, X.; Zhang, P.; Zhang, C.; Cao, Y.; Liu, B. AFP-Net: Realtime Anchor-Free Polyp Detection in Colonoscopy. In Proceedings of the ICTAI 2019, Portland, OR, USA, 4–6 November 2019; pp. 636–643. [Google Scholar]
- Zhang, X.; Chen, F.; Yu, T.; An, J.; Huang, Z.; Liu, J.; Hu, W.; Wang, L.; Duan, H.; Si, J. Real-time gastric polyp detection using convolutional neural networks. Real-time gastric polyp detection using convolutional neural networks. PLoS ONE 2019, 14, e0214133. [Google Scholar]
- Sornapudi, S.; Meng, F.; Yi, S. Region-Based Automated Localization of Colonoscopy and Wireless Capsule Endoscopy Polyps. Appl. Sci. 2019, 9, 2404. [Google Scholar] [CrossRef]
- Qadir, H.A.; Balasingham, I.; Solhusvik, J.; Bergsland, J.; Aabakken, L.; Shin, Y. Improving Automatic Polyp Detection Using CNN by Exploiting Temporal Dependency in Colonoscopy Video. IEEE J. Biomed. Health Inform. 2020, 24, 180–193. [Google Scholar] [CrossRef]
- Urban, G.; Tripathi, P.; Alkayali, T.; Mittal, M.; Jalali, F.; Karnes, W.; Baldi, P. Deep Learning Localizes and Identifies Polyps in Real Time With 96% Accuracy in Screening Colonoscopy. Gastroenterology 2018, 155, 1069–1078. [Google Scholar] [CrossRef]
- Wang, P.; Berzin, T.M.; Glissen-Brown, J.R.; Bharadwaj, S.; Becq, A.; Xiao, X.; Liu, P.; Li, L.; Song, Y.; Zhang, D.; et al. Real-time automatic detection system increases colonoscopic polyp and adenoma detection rates: A prospective randomised controlled study. Gut 2019, 68, 1813–1819. [Google Scholar] [CrossRef]
- Gross, S.; Stehle, T.; Behrens, A.; Auer, R.; Aach, T.; Winograd, R.; Trautwein, C.; Tischendorf, J. A comparison of blood vessel features and local binary patterns for colorectal polyp classification. SPIE Med. Imaging 2009, 7260, 72602Q. [Google Scholar]
- Tischendorf, J.J.W.; Gross, S.; Winograd, R.; Hecker, H.; Auer, R.; Behrens, A.; Trautwein, C.; Aach, T.; Stehle, T. Computer-aided classification of colorectal polyps based on vascular patterns: A pilot study. Endoscopy 2010, 42, 203–207. [Google Scholar] [CrossRef]
- Gross, S.; Trautwein, C.; Behrens, A.; Winograd, R.; Palm, S.; Lutz, H.; Schirin-Sokhan, R.; Hecker, H.; Aach, T.; Tischendorf, J. Computer-based classification of small colorectal polyps by using narrow-band imaging with optical magnification. Gastrointest. Endosc. 2011, 74, 1354–1359. [Google Scholar] [CrossRef] [PubMed]
- Tamaki, T.; Yoshimuta, J.; Kawakami, M.; Raytchev, B.; Kaneda, K.; Yoshida, S.; Takemura, Y.; Onji, K.; Miyaki, R.; Tanaka, S. Computer-aided colorectal tumor classification in NBI endoscopy: Using local features. Med. Image Anal. 2013, 17, 78–100. [Google Scholar] [CrossRef] [PubMed]
- Scheeve, T.; Schreuder, R.M.; van der Sommen, F.; IJspeert, J.E.; Dekker, E.; Schoon, E.J. Computer-aided classification of colorectal polyps using blue-light and linked-color imaging. In Medical Imaging 2019: Computer-Aided Diagnosis; International Society for Optics and Photonics: Bellingham, WA, USA, 2019; Volume 10950, p. 1095012. [Google Scholar]
- Tamaki, T.; Yoshimuta, J.; Kawakami, M.; Raytchev, B.; Kaneda, K.; Yoshida, S.; Takemura, Y.; Onji, K.; Miyaki, R.; Tanaka, S. Computer-aided colorectal tumor classification in NBI endoscopy: Using CNN features. Med. Image Anal. 2016, 17, 78–100. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Zheng, Y.; Mak, W.C.T.; Yu, R.; Wong, S.H.; Lau, J.; Poon, C. Automatic Detection and Classification of Colorectal Polyps by Transferring Low-level CNN Features from Non-Medical Domain. IEEE J. Biomed. Health Inform. 2016, 21, 41–47. [Google Scholar] [CrossRef]
- Ribeiro, E.; Häfner, M.; Wimmer, G.; Tamaki, T.; Tischendorf, J.J.; Yoshida, S.; Tanaka, S.; Uhl, A. Exploring texture transfer learning for colonic polyp classification via convolutional neural networks. In Proceedings of the ISBI 2017, Melbourne, Australia, 18–21 April 2017; pp. 1044–1048. [Google Scholar]
- Murata, M.; Usami, H.; Iwahori, Y.; Aili, W. Polyp Classification Using Multiple CNN-SVM Classifiers from Endoscope Images. In Proceedings of the PATTERNS 2017, Athens, Greece, 19–23 February 2017; pp. 109–112. [Google Scholar]
- Kominami, Y.; Yoshida, S.; Tanaka, S.; Sanomura, Y.; Hirakawa, T.; Raytchev, B.; Tamaki, T.; Koide, T.; Kaneda, K.; Chayama, K. Computer-aided diagnosis of colorectal polyp histology by using a real-time image recognition system and narrow-band imaging magnifying colonoscopy. Gastrointest. Endosc. 2016, 83, 643–649. [Google Scholar] [CrossRef]
- Mori, Y.; Kudo, S.E.; Misawa, M.; Saito, Y.; Ikematsu, H.; Hotta, K.; Ohtsuka, K.; Urushibara, F.; Kataoka, S.; Ogawa, Y.; et al. Real-Time Use of Artificial Intelligence in Identification of Diminutive Polyps During Colonoscopy: A Prospective Study. Ann. Intern. Med. 2018, 169, 357–366. [Google Scholar] [CrossRef]
- Chen, P.J.; Lin, M.C.; Lai, M.J.; Lin, J.C.; Lu, H.H.; Tseng, V.S. Accurate Classification of Diminutive Colorectal Polyps Using Computer-Aided Analysis. Gastroenterology 2018, 154, 568–575. [Google Scholar] [CrossRef]
- Byrne, M.F.; Chapados, N.; Soudan, F.; Oertel, C.; Linares-Pérez, M.; Kelly, R.; Iqbal, N.; Chandelier, F.; Rex, D.K. Real-time differentiation of adenomatous and hyperplastic diminutive colorectal polyps during analysis of unaltered videos of standard colonoscopy using a deep learning model. Gut 2019, 68, 94–100. [Google Scholar] [CrossRef]
- Fonolla, R.; Van Der Sommen, F.; Schreuder, R.M.; Schoon, E.J.; De With, P.H.N. Multi-modal classification of polyp malignancy using CNN features with balanced class augmentation. In Proceedings of the ISBI 2019, Venice, Italy, 8–11 April 2019; pp. 74–78. [Google Scholar]
- Tan, M.; Le, Q. Rethinking Model Scaling for Convolutional Neural Networks. Proceedings of the 36th International Conference on Machine Learning. PMLR 2019, 97, 6105–6114. [Google Scholar]
- Selvaraju, R.R.; Cogswell, M.; Das, A.; Vedantam, R.; Parikh, D.; Batra, D. Grad-CAM: Visual Explanations from Deep Networks via Gradient-Based Localization. Int. Comput. Vis. 2019, 128, 336–359. [Google Scholar] [CrossRef]
- Subramaniam, S.; Hayee, B.; Aepli, P.; Schoon, E.; Stefanovic, M.; Kandiah, K.; Thayalasekaran, S.; Alkandari, A.; Bassett, P.; Coron, E.; et al. Optical diagnosis of colorectal polyps with Blue Light Imaging using a new international classification. United Eur. Gastroenterol. J. 2019, 7, 316–325. [Google Scholar] [CrossRef] [PubMed]
| Accuracy (%) | Specificity (%) | Sensitivity (%) | AUC | ||
|---|---|---|---|---|---|
| Intuition (±SD) | |||||
| Novices (n = 13) | N.A | ||||
| Experts (n = 6) | N.A | ||||
| Total (n = 19) | N.A | ||||
| BASIC (±SD) | |||||
| Novices (n = 13) | N.A | ||||
| Experts (n = 6) | N.A | ||||
| Total (n = 19) | N.A | ||||
| CADx | |||||
| VGG16 | 85.0 | 84.4 | 86.7 | 0.90 | |
| ResNet50V2 | 83.3 | 93.3 | 80.0 | 0.92 | |
| ResNet101V2 | 88.3 | 93.3 | 73.3 | 0.94 | |
| Xception | 85.0 | 84.4 | 86.7 | 0.93 | |
| InceptionResNetV2 | 85.0 | 86.7 | 80.0 | 0.94 | |
| EfficientNetB4 | 95.0 | 93.3 | 95.6 | 0.97 |
| Hyperplastic | Adenoma | Sessile Serrated | |||
|---|---|---|---|---|---|
| Surface | |||||
| Presence of mucus | No | No | Yes | ||
| Regular/irregular | Regular | Regular or irregular | Regular or irregular | ||
| Pseudodepression | No | Yes | No | ||
| Depression | No | No | No | ||
| Pit Pattern | |||||
| Featureless | Yes | No | No | ||
| Type | Round | Not round | Round pits with/without dark spots | ||
| Distribution | Homogenous | Homogenous or heteregenous without focal loss | Homogenous or heteregenous | ||
| Vessels | |||||
| Present? | Yes/No | Yes | Yes/No | ||
| Type if present | Lacy | Pericryptal | Pericryptal |
| Accuracy (%) | Specificity (%) | Sensitivity (%) | AUC | |
|---|---|---|---|---|
| WL | 86.7 (78.1–95.3) | 86.7 (78.1–95.3) | 86.7 (78.1–95.3) | 0.94 (0.86–0.99) |
| BLI | 93.3 (87.0–99.6) | 93.3 (87.0–99.6) | 93.3 (87.0–99.6) | 0.96 (0.88–1.00) |
| LCI | 90.0 (82.4–97.6) | 80.0 (69.9–90.1) | 93.3 ( 83.2–100) | 0.89 (0.79–0.97) |
| Combined | 95.0 (89.5–100) | 93.3 (87.0–99.6) | 95.6 (89.2–100) | 0.97 (0.92–1.00) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fonollà, R.; E. W. van der Zander, Q.; Schreuder, R.M.; Masclee, A.A.M.; Schoon, E.J.; van der Sommen, F.; de With, P.H.N. A CNN CADx System for Multimodal Classification of Colorectal Polyps Combining WL, BLI, and LCI Modalities. Appl. Sci. 2020, 10, 5040. https://doi.org/10.3390/app10155040
Fonollà R, E. W. van der Zander Q, Schreuder RM, Masclee AAM, Schoon EJ, van der Sommen F, de With PHN. A CNN CADx System for Multimodal Classification of Colorectal Polyps Combining WL, BLI, and LCI Modalities. Applied Sciences. 2020; 10(15):5040. https://doi.org/10.3390/app10155040
Chicago/Turabian StyleFonollà, Roger, Quirine E. W. van der Zander, Ramon M. Schreuder, Ad A. M. Masclee, Erik J. Schoon, Fons van der Sommen, and Peter H. N. de With. 2020. "A CNN CADx System for Multimodal Classification of Colorectal Polyps Combining WL, BLI, and LCI Modalities" Applied Sciences 10, no. 15: 5040. https://doi.org/10.3390/app10155040
APA StyleFonollà, R., E. W. van der Zander, Q., Schreuder, R. M., Masclee, A. A. M., Schoon, E. J., van der Sommen, F., & de With, P. H. N. (2020). A CNN CADx System for Multimodal Classification of Colorectal Polyps Combining WL, BLI, and LCI Modalities. Applied Sciences, 10(15), 5040. https://doi.org/10.3390/app10155040






