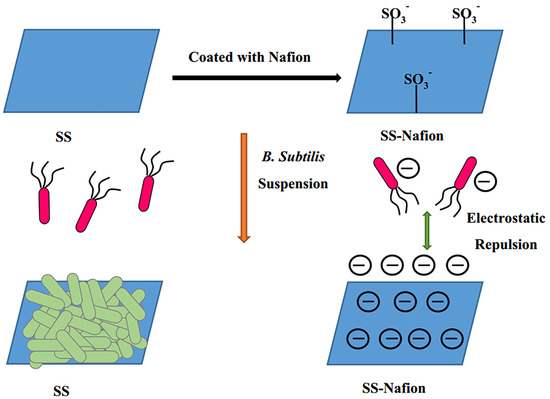
The Effectiveness of Nafion-Coated Stainless Steel Surfaces for Inhibiting Bacillus Subtilis Biofilm Formation
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
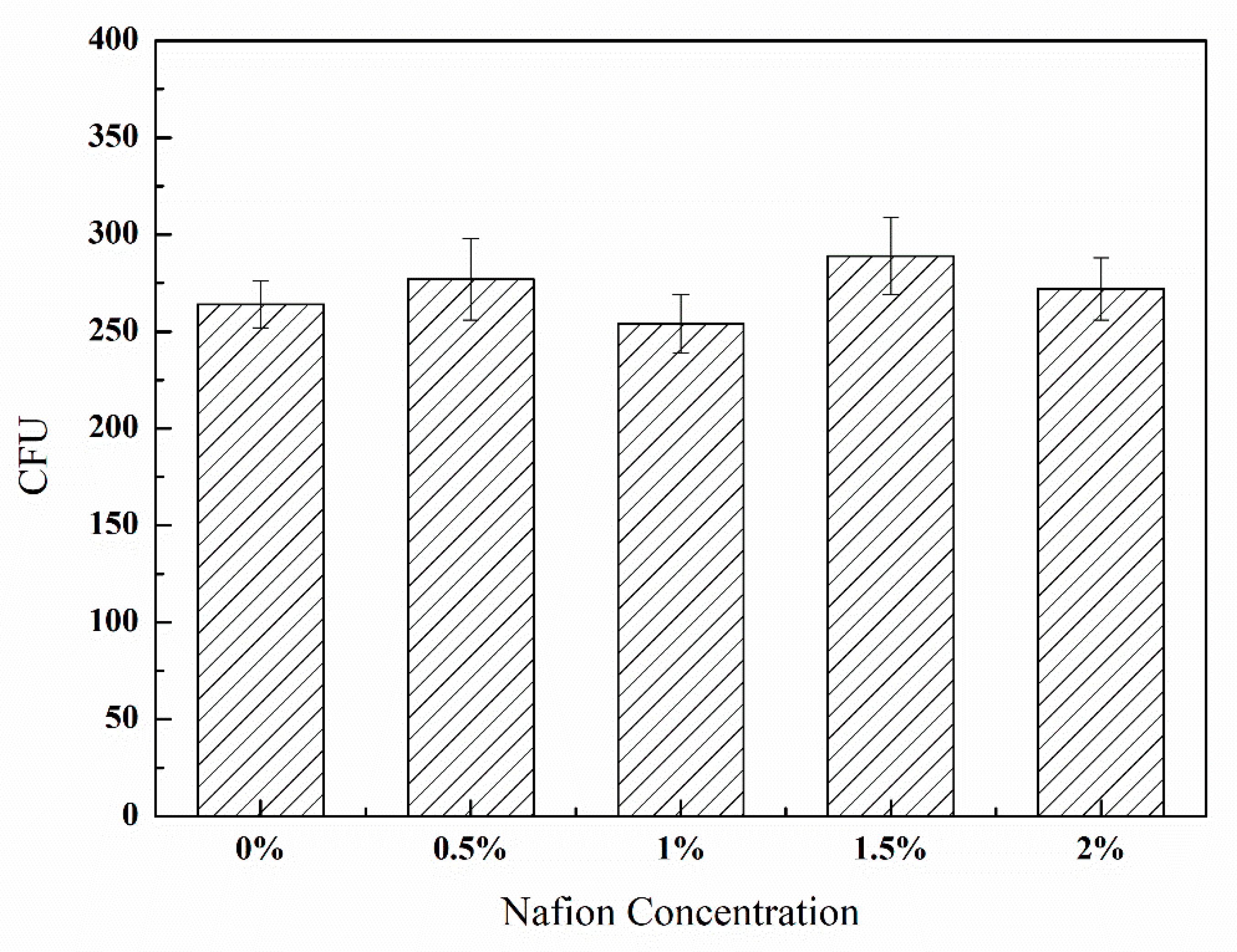
2.2. Toxcity Test of Nafion Solution to B. subtilis
2.3. Preparation of Nafion-Coated Stainless Steel Discs
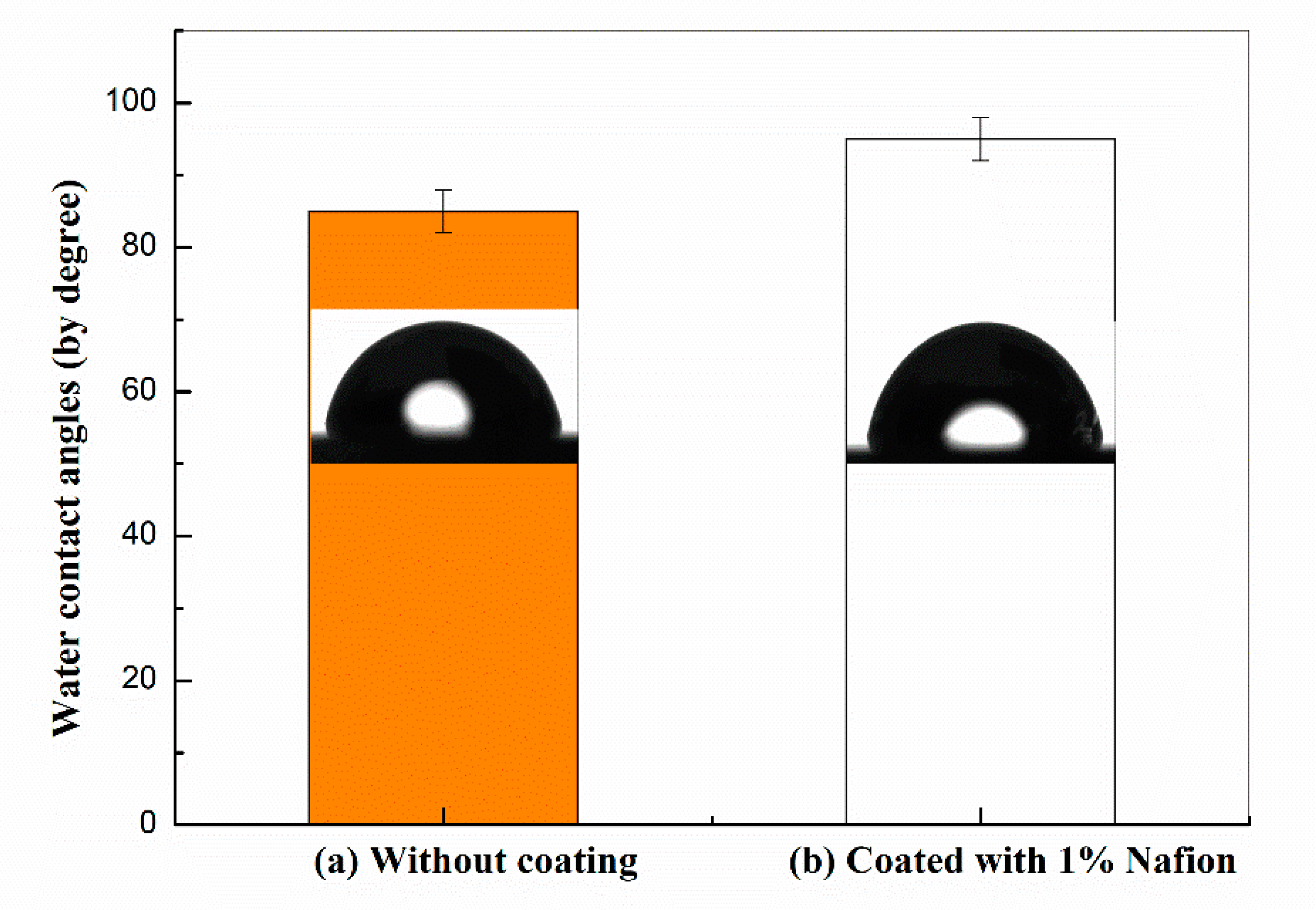
2.4. Surface Characterization
2.5. Biofilm Formation Assay and Quantification
3. Results
3.1. Toxicity of Nafion Solution to B. subtilis
3.2. Surface Fabrication and Their Characterization of Nafion-Coated Stainless Steel
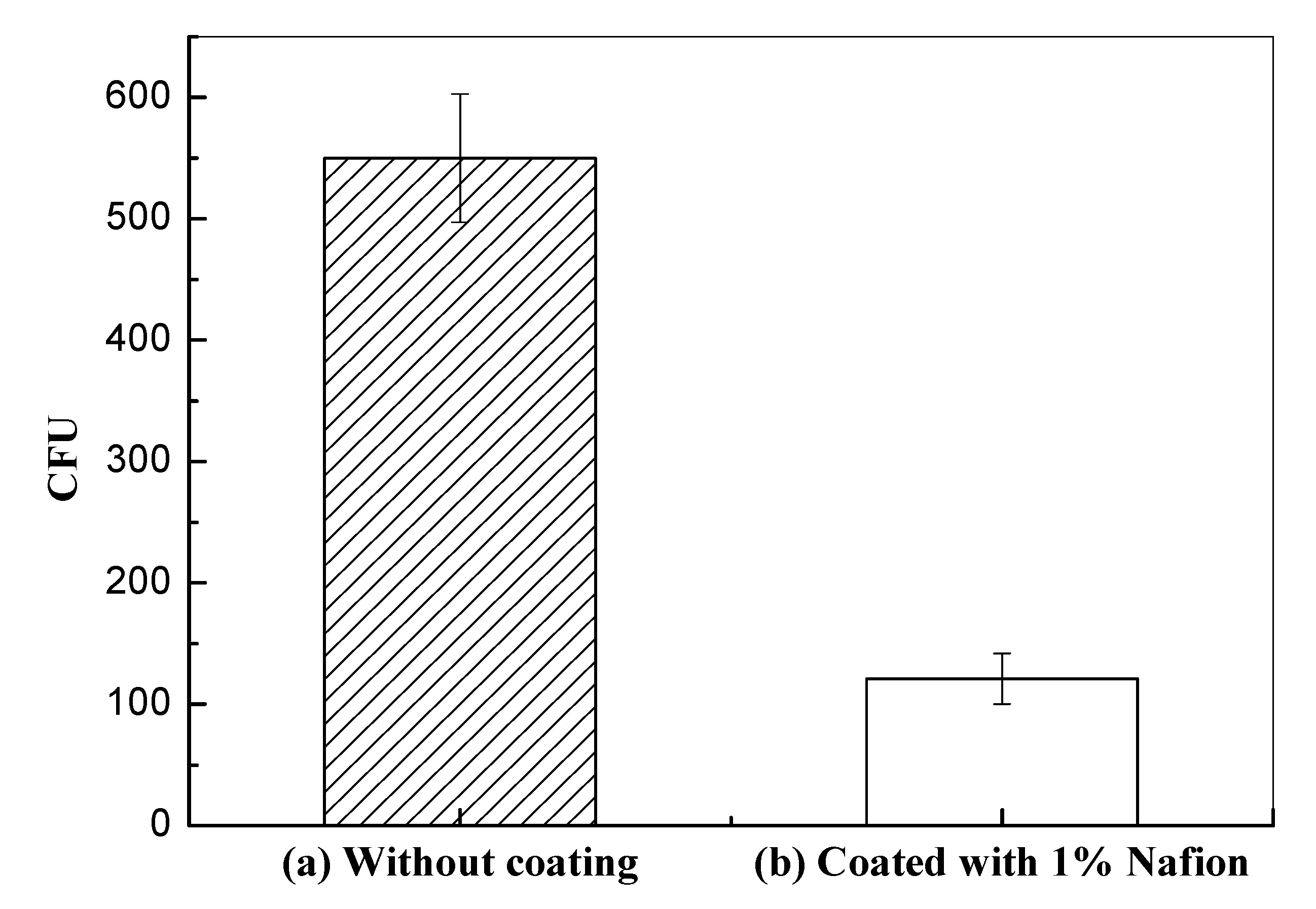
3.3. Antibacterial Adhesion of Nafion-Coated Stainless Steel Surfaces
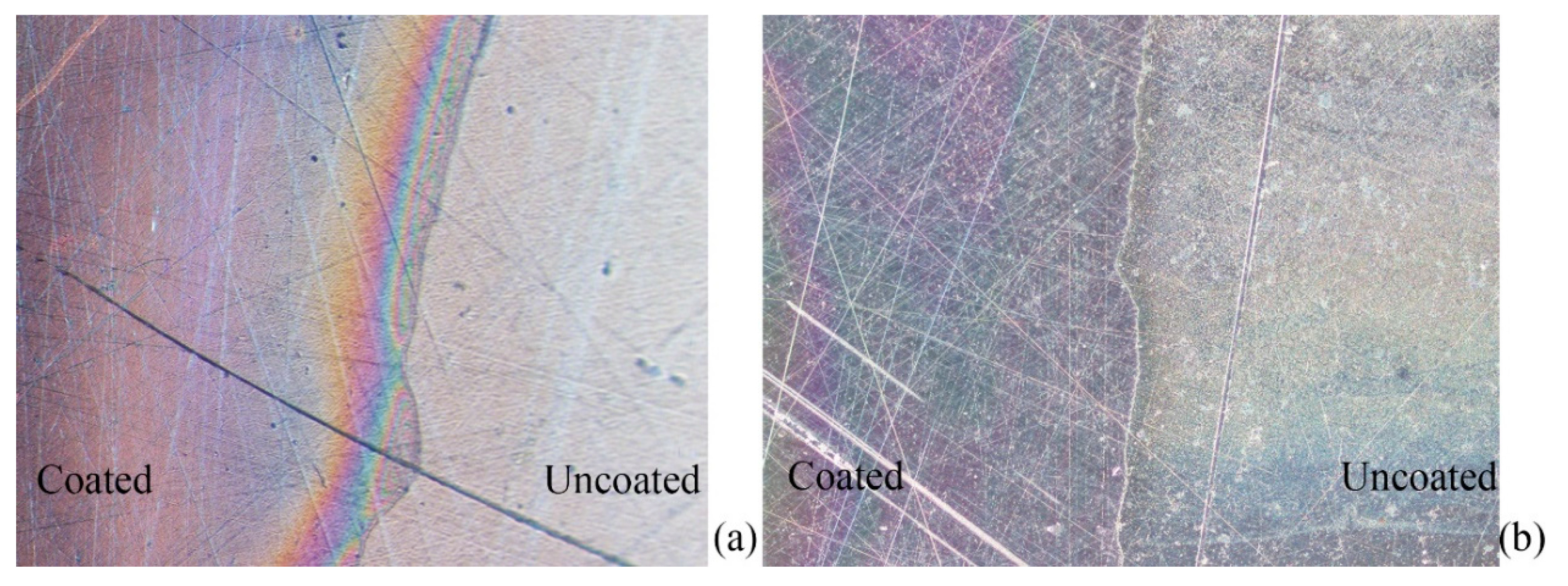
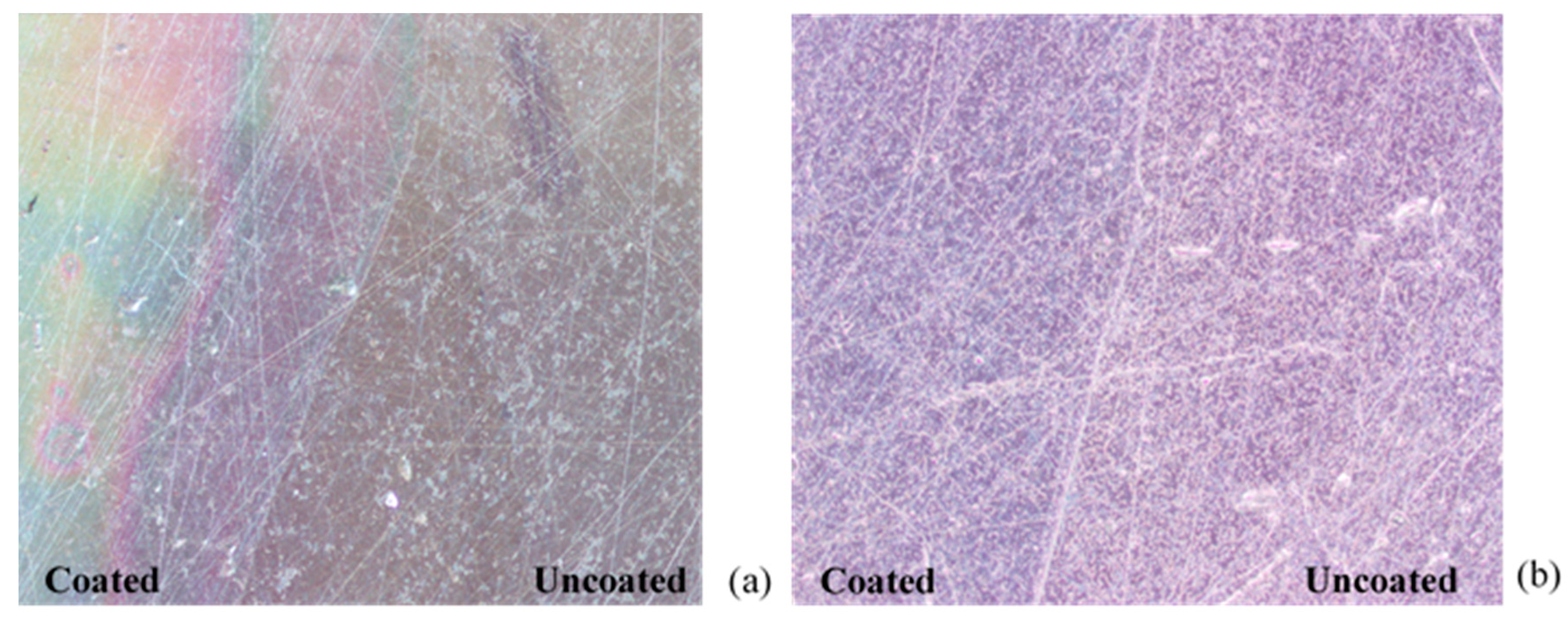
3.4. Durability of Antibacterial Properties
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Flemming, H.C.; Wingender, J.; Szewzyk, U.; Steinberg, P.; Rice, S.A.; Kjelleberg, S. Biofilms: An emergent form of bacterial life. Nat. Rev. Microbiol. 2016, 14, 563–575. [Google Scholar] [CrossRef]
- Arciola, C.R.; Campoccia, D.; Montanaro, L. Implant infections: Adhesion, biofilm formation and immune evasion. Nat. Rev. Microbiol. 2018, 18, 397–409. [Google Scholar] [CrossRef] [PubMed]
- Koo, H.; Allan, R.N.; Howlin, R.P.; Stoodley, P.; Stoodley, L.H. Targeting microbial biofilms: Current and prospective therapeutic strategies. Nat. Rev. Microbiol. 2017, 15, 740–755. [Google Scholar] [CrossRef] [PubMed]
- Kip, N.; Veen, J.A. The dual role of microbes in corrosion. ISME J. 2015, 9, 542–551. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Wang, H.; Hu, C.; Yang, M.; Hu, H.; Niu, J. Characteristics of biofilms and iron corrosion scales with ground and surface waters in drinking water distribution systems. Corros. Sci. 2015, 90, 331–339. [Google Scholar] [CrossRef]
- Torretta, S.; Drago, L.; Marchisio, P.; Ibba, T.; Pignataro, L. Role of biofilms in children with chronic adenoiditis and middle ear disease. J. Clin. Med. 2019, 8, 671. [Google Scholar] [CrossRef] [PubMed]
- Costerton, J.W.; Stewart, P.S.; Greenberg, E.P. Bacterial biofilms: A common course of persistent infections. Science 1999, 284, 1318–1322. [Google Scholar] [CrossRef]
- Dejea, C.M.; Wick, E.C.; Hechenbleikner, E.M.; White, J.R.; Welch, J.L.M.; Rossetti, B.J.; Peterson, S.N.; Snesrud, E.C.; Borisy, G.G.; Lazarev, M.; et al. Microbiota organization is a distinct feature of proximal colorectal cancers. Proc. Natl. Acad. Sci. USA 2014, 111, 18321–18326. [Google Scholar] [CrossRef]
- Henriques, P.C.; Borges, I.; Pinto, A.M.; Magalhaes, F.D.; Goncalves, I.C. Fabrication and antimicrobial performance of surfaces integrating graphene-based materials. Carbon 2018, 132, 709–732. [Google Scholar] [CrossRef]
- Vestby, L.K.; Grønseth, T.; Simm, R.; Nesse, L.L. Bacterial biofilm and its role in the pathogenesis of disease. Antibiotics 2020, 9, 59. [Google Scholar] [CrossRef]
- Alexandra, M.B.; Marta, F.G. Poly (ionic liquid)s as antimicrobial materials. Eur. Pol. J. 2018, 105, 135–149. [Google Scholar]
- Liao, C.; Li, Y.; Tjong, S.C. Anti-bacterial Activities of Aliphatic Polyester Nanocomposites with Silver Nanoparticles and/or Graphene Oxide Sheets. Nanomaterials 2019, 9, 1102. [Google Scholar] [CrossRef] [PubMed]
- Pallavicini, P.; Dacarro, G.; Diaz-Fernandez, Y.A.; Taglietti, A. Coordination chemistry of surface-grafted ligands for anti-bacterial materials. Coordin. Chem. Rev. 2014, 275, 37–53. [Google Scholar] [CrossRef]
- Li, L.; Molin, S.; Yang, L.; Ndoni, S. Sodium Dodecyl Sulfate (SDS)-Loaded Nanoporous Polymer as Anti-bacterial Surface Coating Material. Int. J. Mol. Sci. 2013, 14, 3050–3064. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, W.; Zhai, Z.; Gao, C. Adaptive anti-bacterial biomaterial surfaces and their applications. Mater. Today Bio 2019, 2, 100017. [Google Scholar] [CrossRef] [PubMed]
- Kaur, R.; Liu, S. Anti-bacterial surface design–Contact kill. Prog. Surf. Sci. 2016, 91, 136–153. [Google Scholar] [CrossRef]
- Hochella, M.F.; Eggleston, C.M.; Elings, V.B.; Parks, G.A.; Brown, G.E.; Wu, C.M.; Kjoller, K. Mineralogy in two dimensions: Scanning tunneling microscopy of semiconducting minerals with implications on geochemical reactivity. Am. Mineral. 1989, 74, 1233–1246. [Google Scholar]
- Arnold, J.W.; Bailey, G.W. Surface finishes on stainless steel reduce bacterial attachment and early biofilm formation: Scanning electron and atomic force microscopy study. Poult. Sci. 2000, 79, 1839–1845. [Google Scholar] [CrossRef]
- Saeki, D.; Nagashima, Y.; Sawad, I.; Matsuyama, H. Effect of hydrophobicity of polymer materials used for water purification membranes on biofilm formation dynamics. Colloid Surf. A 2016, 506, 622–628. [Google Scholar] [CrossRef]
- Boudarel, H.; Mathias, J.; Blaysat, B.; Grédiac, M. Towards standardized mechanical characterization of microbial biofilms: Analysis and critical review. NPJ Biofilms Microbiomes 2018, 17, 1–15. [Google Scholar] [CrossRef]
- Rodríguez-Melcón, C.; Alonso-Calleja, C.; Capita, R. Architecture and viability of the biofilms formed by nine Listeria strains on various hydrophobic and hydrophilic materials. Appl. Sci. 2019, 9, 5256. [Google Scholar]
- Flint, S.H.; Brooks, J.D.; Bremer, P.J. Properties of the stainless steel substrate, influencing the adhesion of thermo-resistant streptococci. J. Food. Eng. 2000, 43, 235–242. [Google Scholar] [CrossRef]
- Ammar, Y.; Swailes, D.; Bridgens, B.; Chen, J. Influence of surface roughness on the initial formation of biofilm. Surf. Coat. Technol. 2015, 284, 410–416. [Google Scholar] [CrossRef]
- Preedy, E.; Perni, S.; Nipiĉ, D.; Bohinc, K.; Prokopovich, P. Surface roughness mediated adhesion forces between borosilicate glass and gram-positive bacteria. Langmuir 2014, 30, 9466–9476. [Google Scholar] [CrossRef]
- Zhang, Q.; Yu, Z.; Jin, S.; Liu, C.; Li, Y.; Guo, D.; Hu, M.; Ruan, R.; Liu, Y. Role of surface roughness in the algal short-term cell adhesion and long-term biofilm cultivation under dynamic flow condition. Algal Res. 2020, 46, 101787. [Google Scholar] [CrossRef]
- Pereni, C.I.; Zhao, Q.; Liu, Y.; Abel, E. Surface free energy effect on bacterial retention. Colloid Surf. B 2006, 48, 143–147. [Google Scholar] [CrossRef]
- Khan, M.M.T.; Chapman, T.; Cochran, K.; Schuler, A.J. Attachment surface energy effects on nitrification and estrogen removal rates by biofilms for improved wastewater treatment. Water Res. 2013, 7, 2190–2198. [Google Scholar] [CrossRef]
- Cerca, N.; Pier, G.B.; Vilanova, M.; Oliveira, R.; Azeredo, J. Quantitative analysis of adhesion and biofilm formation hydrophilic and hydrophobic surfaces of clinical isolates of Staphylococcus epidermidis. Res. Microbiol. 2005, 156, 506–514. [Google Scholar] [CrossRef]
- Song, D.; Zhang, W.; Cheng, W.; Jia, B.; Wang, P.; Sun, Z.; Ma, J.; Zhai, X.; Qi, J.; Liu, C. Micro fine particles deposition on gravity-driven ultrafiltration membrane to modify the surface properties and biofilm compositions: Water quality improvement and biofouling mitigation. Chem. Eng. J. 2020, 393, 123270. [Google Scholar] [CrossRef]
- Jindal, S.; Anand, S.; Huang, K.; Goddard, J.; Metzger, L.; Amamcharla, J. Evaluation of modified stainless steel surfaces targeted to reduce biofilm formation by common milk sporeformers. J. Dairy Sci. 2016, 12, 9502–9513. [Google Scholar] [CrossRef]
- Agarwall, S.V.; Ellepola, K.; Costa, M.C.F.; Fechine, G.J.M.; Morin, J.L.P.; Neto, A.H.C.; Seneviratne, C.J.; Rosa, V. Hydrophobicity of graphene as a driving force for inhibiting biofilm formation of pathogenic bacteria and fungi. Dent. Mater. 2019, 35, 403–413. [Google Scholar] [CrossRef]
- Boulange-Peterman, L. Processes of bioadhesion on stainless steel surfaces and cleanability: A review with special reference to the food industry. Biofouling 1996, 10, 275–300. [Google Scholar] [CrossRef]
- Huang, Y.; Zheng, Y.; Li, J.; Liao, Q.; Fu, Q.; Xia, A.; Fu, J.; Sun, Y. Enhancing microalgae biofilm formation and growth by fabricating microgrooves onto the substrate surface. Bioresour. Technol. 2018, 261, 36–43. [Google Scholar] [CrossRef]
- Guo, C.; Duan, D.; Sun, Y.; Han, Y.; Zhao, S. Enhancing Scenedesmus obliquus biofilm growth and CO2 fixation in a gas-permeable membrane photobioreactor integrated with additional rough surface. Alagal Res. 2019, 43, 101620. [Google Scholar] [CrossRef]
- Eginton, P.J.; Gibson, H.; Holah, J.; Handley, P.S.; Gilbert, P. The influence of substratum properties on the attachment of bacterial cells. Colloid Surf. B 1995, 5, 153–159. [Google Scholar] [CrossRef]
- Han, A.; Tsoi, J.K.H.; Matinlinna, J.P.; Chen, Z. Influence of grit-blasting and hydrofluoric acid etching treatment on surface characteristics and biofilm formation on zirconia. Coatings 2017, 7, 130. [Google Scholar] [CrossRef]
- Ha, K.Y.; Chung, Y.G.; Ryoo, S.J. Adherence and biofilm formation of Staphylococcus Epidermidis and Mycobacterium Tuberculosis on various spinal implants. Spine 2005, 30, 38–43. [Google Scholar] [CrossRef]
- Zhao, Q.; Liu, Y.; Wang, C.; Wang, S.; Müller-Steinhagen, H. Effect of surface free energy on the adhesion of biofouling and crystalline fouling. Chem. Eng. Sci. 2005, 60, 4858–4865. [Google Scholar] [CrossRef]
- Liu, Y.; Zhao, Q. Influence of surface energy of modified surfaces on bacterial adhesion. Biophys. Chem. 2005, 117, 39–45. [Google Scholar] [CrossRef]
- Jonssona, A.S.; Jonsson, B. The influence of nonionic and ionic surfactants on hydrophobic and hydrophilic ultrafiltration membranes. J. Membr. Sci. 1991, 56, 49–76. [Google Scholar] [CrossRef]
- Brink, L.E.S.; Elbers, S.J.G.; Robbertson, T.; Both, P. The anti-fouling action of polymers pre-adsorbed on ultrafiltration and microfiltration membranes. J. Membr. Sci. 1993, 76, 281–291. [Google Scholar] [CrossRef]
- Bakker, D.P.; Huijs, F.M.; de Vries, J.; Klijnstra, J.W.; Busscher, H.J.; van der Mei, H.C. Bacterial deposition to fluoridated and non-fluoridated polyurethane coatings with different elastic modulus and surface tension in a parallel plate and a stagnation point flow chamber. Colloids Surf. B 2003, 32, 179–190. [Google Scholar] [CrossRef]
- Boulange-Peterman, L.; Baroux, B.; Bellon-Fontaine, M.N. The influence of metallic surface wettability on bacterial adhesion. J. Adhes. Sci. Technol. 1993, 7, 221–230. [Google Scholar] [CrossRef]
- Terada, A.; Okuyama, K.; Nishikawa, M.; Tsuneda, S.; Hosomi, M. The effect of surface charge property on Escherichia coli initial adhesion and subsequent biofilm formation. Biotechnol. Bioeng. 2012, 109, 1745–1754. [Google Scholar] [CrossRef] [PubMed]
- Schwegmann, H.; Feitz, A.J.; Frimmel, F.H. Influence of the zeta potential on the sorption and toxicity of iron oxide nanoparticles on S. cerevisiae and E. coli. J. Colloid Interface Sci. 2010, 347, 43–48. [Google Scholar] [CrossRef]
- Zheng, Y.; Huang, Y.; Xia, A.; Qian, F.; Wei, C. A rapid inoculation method for microalgae biofilm cultivation based on microalgae-microalgae co-flocculation and zeta-potential adjustment. Bioresource Technol. 2019, 278, 272–278. [Google Scholar] [CrossRef]
- Kannan, A.; Karumanchi, S.L.; Ramalingam, S.; Gautam, P. Quantitative study on the effect of calcium and magnesium palmitate on the formation of Pseudomonas aeruginosa biofilm. J. Microbiol. Immunol. 2016, 49, 988–991. [Google Scholar] [CrossRef]
- Song, B.; Leff, L.G. Influence of magnesium ions on biofilm formation by Pseudomonas fluorescens. Microbiol. Res. 2006, 161, 355–361. [Google Scholar] [CrossRef]
- Kurniawan, A.; Tsuchiya, Y.; Eda, S.; Morisaki, H. Characterization of the internal ion environment of biofilms based on charge density and shape of ion. Colloids Surf. B 2015, 136, 22–26. [Google Scholar] [CrossRef]
- Kreve, S.; Reis, A.C. Influence of the electrostatic condition of the titanium surface on bacterial adhesion: A systematic review. J. Prosthet. Dent. 2020, (in press). [CrossRef]
- Graham, M.V.; Cady, N.C. Nano and microscale topographies for the prevention of bacterial surface fouling. Coatings 2014, 4, 37–59. [Google Scholar] [CrossRef]
- Skoog, S.A.; Kumar, G.; Narayan, R.J.; Goering, P.L. Biological responses to immobilized microscale and nanoscale surface topographies. Pharmacol. Ther. 2018, 182, 33–55. [Google Scholar] [CrossRef] [PubMed]
- Hochbaum, A.I.; Aizenberg, J. Bacteria pattern spontaneously on periodic nanostructure arrays. Nano Lett. 2010, 10, 3717–3721. [Google Scholar] [CrossRef]
- Crawford, R.J.; Webb, H.K.; Truong, V.K.; Hasan, J.; Ivanova, E.P. Surface topographical factors influencing bacterial attachment. Adv. Colloid Interface Sci. 2012, 179, 142–149. [Google Scholar] [CrossRef]
- Nguyen, D.H.K.; Pham, V.T.H.; Truong, V.K.; Sbarski, I.; Wang, J.; Balcytis, A.; Juodkazis, S.; Mainwaring, D.E.; Crawford, R.J.; Ivanova, E.P. Role of topological scale in the differential fouling of Pseudomonas aeruginosa and Staphylococcus aureus bacterial cells on wrinkled gold-coated polystyrene surfaces. Nanoscale 2018, 10, 5089–5096. [Google Scholar] [CrossRef]
- Tripathy, A.; Sen, P.; Su, B.; Briscoe, W.H. Natural and bioinspired nanostructured bactericidal surfaces. Adv. Colloid Interface Sci. 2017, 248, 85–104. [Google Scholar] [CrossRef]
- Elbourne, A.; Coyle, V.E.; Truong, V.K.; Sabri, Y.; Kandjani, A.E.; Bhargava, S.K.; Ivanova, E.P.; Crawford, R.J. Multi-directional electrodeposited gold nanospikes for anti-bacterial surface applications. Nanoscale Adv. 2018. [Google Scholar] [CrossRef]
- Díaz, C.; Schilardi, P.L.; Salvarezza, R.C.; Fernández Lorenzo de Mele, M. Nano/microscale order affects the early stages of biofilm formation on metal surfaces. Langmuir 2007, 23, 11206–11210. [Google Scholar] [CrossRef]
- Bruzaud, J.; Tarrade, J.; Celia, E.; Darmanin, T.; De Givenchy, E.T.; Guittard, F.; Herry, J.M.; Guilbaud, M.; Bellon-Fontaine, M.N. The design of superhydrophobic stainless steel surfaces by controlling nanostructures: A key parameter to reduce the implantation of pathogenic bacteria. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 73, 40–47. [Google Scholar] [CrossRef]
- Hasan, J.; Jain, S.; Padmarajan, R.; Purighalla, S.; Sambandamurthy, V.K.; Chatterjee, K. Multi-scale surface topography to minimize adherence and viability of nosocomial drug-resistant bacteria. Mater. Des. 2018, 140, 332–344. [Google Scholar] [CrossRef]
- Ludecke, C.; Roth, M.; Yu, W.; Horn, U.; Bossert, J.; Jandt, K.D. Nanorough titanium surfaces reduce adhesion of Escherichia coli and Staphylococcus aureus via nano adhesion points. Colloids Surf. B 2016, 145, 617–625. [Google Scholar] [CrossRef]
- Luan, Y.; Liu, S.; Pihl, M.; van der Mei, H.C.; Liu, J.; Hizal, F.; Choi, C.; Chen, H.; Ren, Y.; Busscher, H.J. Bacterial interactions with nanostructured surfaces. Curr. Opin. Colloid Interface Sci. 2018, 38, 170–189. [Google Scholar] [CrossRef]
- Modaresifar, K.; Azizian, S.; Ganjian, M.; Fratila-Apachitei, L.E.; Zadpoor, A.A. Bactericidal effects of nanopatterns: A systematic review. Acta Biomater. 2019, 83, 29–36. [Google Scholar] [CrossRef]
- Lin, N.; Berton, P.; Moraes, C.; Rogers, R.D.; Tufenkji, N. Nanodarts, nanoblades, and nanospikes: Mechano-bactericidal nanostructures and where to find them. Adv. Colloid Interface Sci. 2018, 252, 55–68. [Google Scholar] [CrossRef]
- Bohinc, K.; Drazic, G.; Abram, A.; Jevšnik, M.; Jeršek, B.; Nipič, D.; Kurinčič, M.; Raspor, P. Metal surface characteristics dictate bacterial adhesion capacity. Int. J. Adhes. Adhes. 2016, 68, 39–46. [Google Scholar] [CrossRef]
- Bekmurzayeva, A.; Duncanson, W.J.; Azevedo, H.S.; Kanayeva, D. Surface modification of stainless steel for biomedical applications: Revisiting a century-old material. Mater. Sci. Eng. 2018, 93, 1073–1089. [Google Scholar] [CrossRef]
- Ogawa, A.; Takakura, K.; Hirai, N.; Kanematsu, H.; Kuroda, D.; Kougo, T.; Sano, K.; Terada, S. Biofilm formation plays a crucial rule in the initial step of carbon steel corrosion in air and water environments. Materials 2020, 13, 923. [Google Scholar] [CrossRef]
- Zhang, J.; Li, W.; Chen, J.; Qi, W.; Wang, F.; Zhou, Y. Impact of biofilm formation and detachment on the transmission of bacterial antibiotic resistance in drinking water distribution systems. Chemosphere 2018, 203, 368–380. [Google Scholar] [CrossRef]
- Hoyer, B.; Florence, T.M.; Batley, G.E. Application of polymer-coated glassy carbon electrodes in anodic stripping voltammetry. Anal. Chem. 1987, 59, 1608–1614. [Google Scholar] [CrossRef]
- Zhong, L.J.; Pang, L.Q.; Che, L.M.; Wu, X.; Chen, X. Nafion coated stainless steel for anti-biofilm application. Colloids Surf. B 2013, 111, 252–256. [Google Scholar] [CrossRef]
- Madigan, M.T.; Martinko, J.M.; Bender, K.S.; Buckley, D.H.; Stahl, D.A. Chapter 2, Microbial Cell Structure and Function. In Brock Biology of Microorganisms, 14th ed.; Pearson Education: New York, NY, USA, 2014; pp. 25–67. [Google Scholar]
- Turner, R.F.B.; Harrison, D.J.; Rojotte, R.V. Preliminary in vivo biocompatibility studies on perfluorosulphonic acid polymer membranes for biosensor applications. Biomaterials 1991, 12, 361–368. [Google Scholar] [CrossRef]
- Dai, Z.; Mohwald, H. Highly stable and biocompatible Nafion-Based capsules with controlled permeability for low-molecular-weight species. Chem. Eur. J. 2002, 8, 4751–4755. [Google Scholar] [CrossRef]
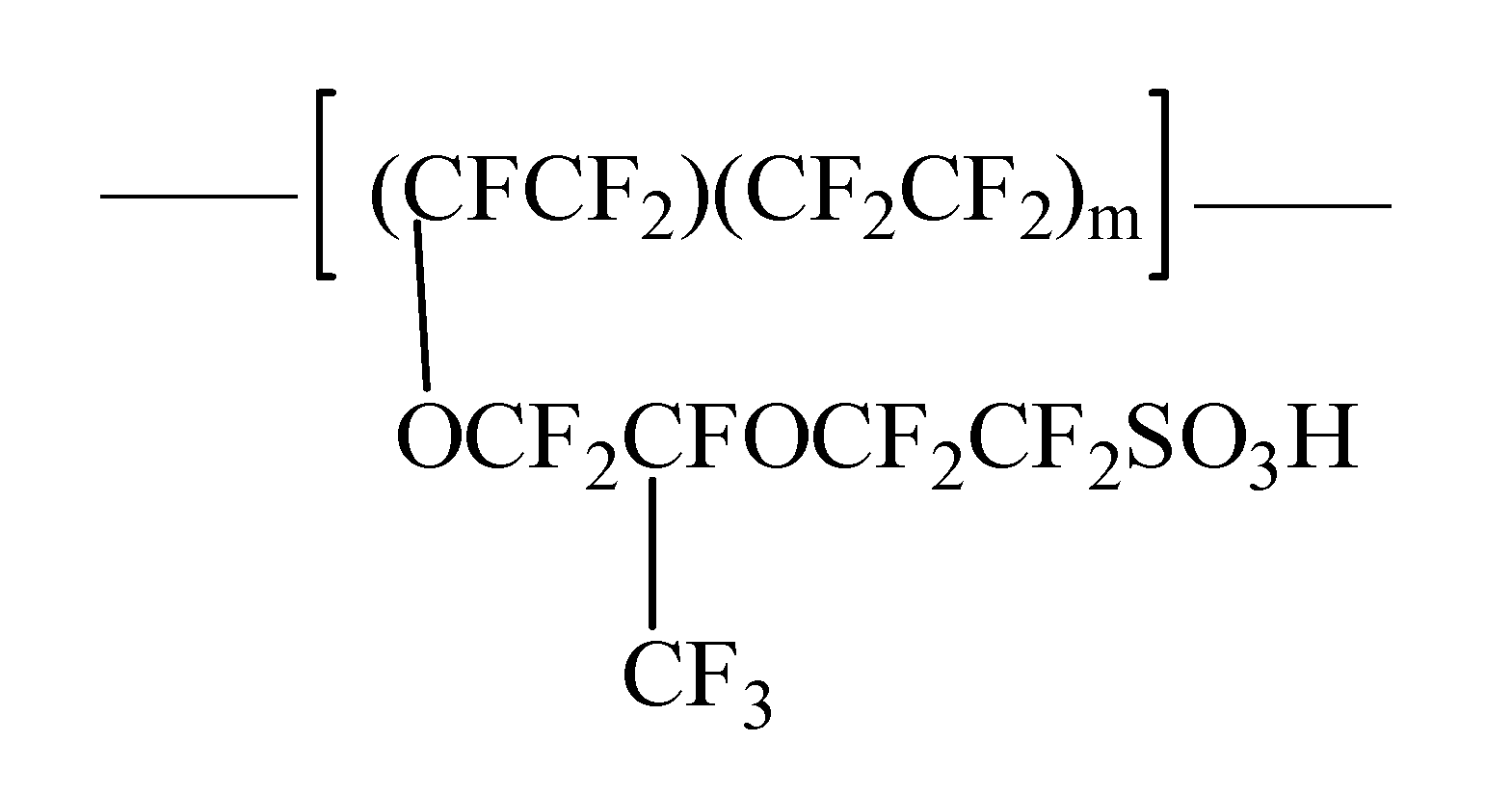

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhong, L.; Song, Y.; Zhou, S. The Effectiveness of Nafion-Coated Stainless Steel Surfaces for Inhibiting Bacillus Subtilis Biofilm Formation. Appl. Sci. 2020, 10, 5001. https://doi.org/10.3390/app10145001
Zhong L, Song Y, Zhou S. The Effectiveness of Nafion-Coated Stainless Steel Surfaces for Inhibiting Bacillus Subtilis Biofilm Formation. Applied Sciences. 2020; 10(14):5001. https://doi.org/10.3390/app10145001
Chicago/Turabian StyleZhong, Lijuan, Yibo Song, and Shufeng Zhou. 2020. "The Effectiveness of Nafion-Coated Stainless Steel Surfaces for Inhibiting Bacillus Subtilis Biofilm Formation" Applied Sciences 10, no. 14: 5001. https://doi.org/10.3390/app10145001
APA StyleZhong, L., Song, Y., & Zhou, S. (2020). The Effectiveness of Nafion-Coated Stainless Steel Surfaces for Inhibiting Bacillus Subtilis Biofilm Formation. Applied Sciences, 10(14), 5001. https://doi.org/10.3390/app10145001