Advances in Translational Nanotechnology: Challenges and Opportunities
Abstract
1. Introduction and Historical Perspective
2. Progress in Various Nanotechnology Disciplines
2.1. Nanotechnology Applications in Engineering
2.1.1. Optics, Photonics and Plasmonics
2.1.2. Electronics
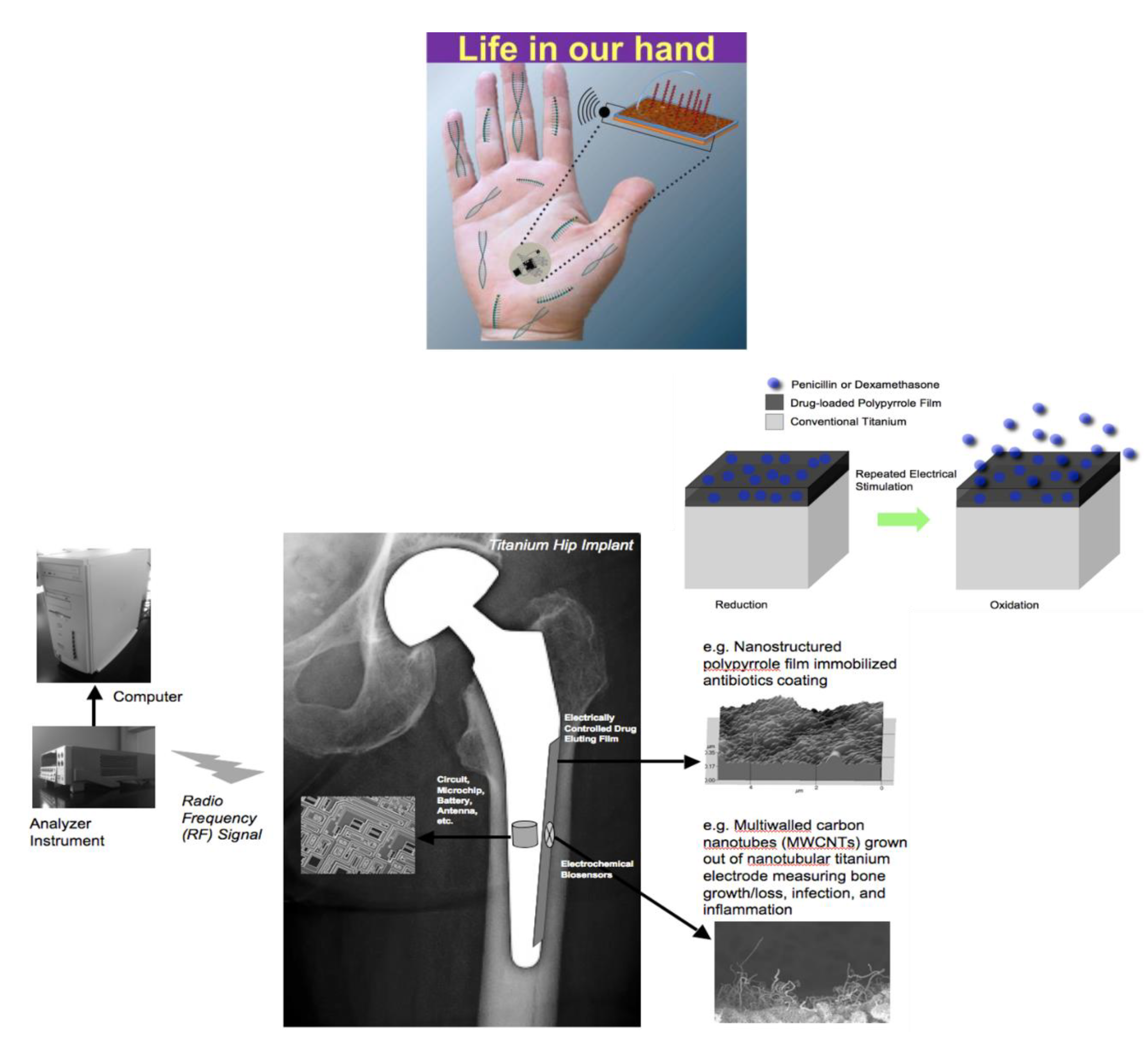
2.2. Biomedical Devices
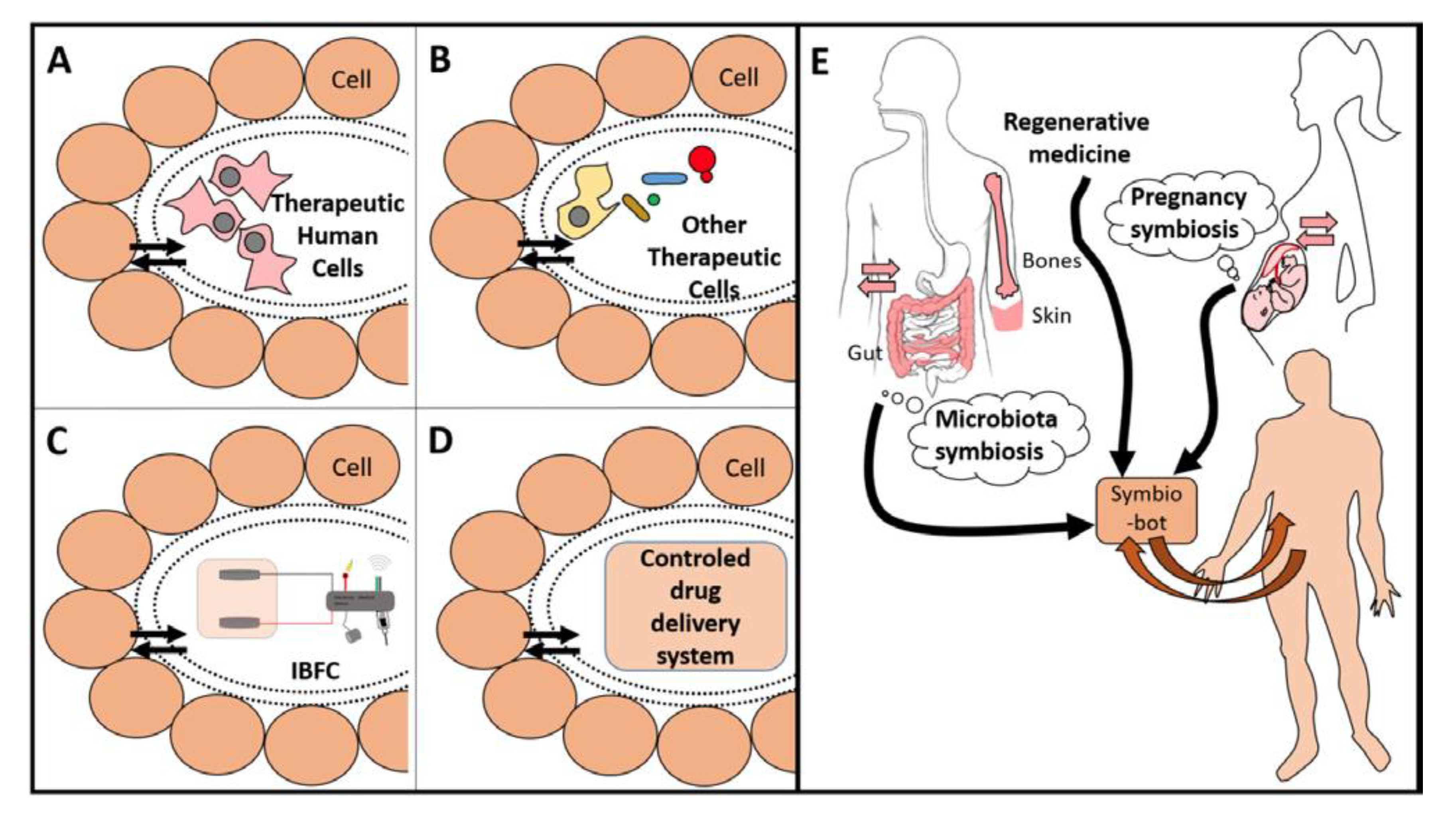
2.3. Nano-Biotechnology
2.4. Nanotherapeutics
2.4.1. Experimental Nanotherapies
2.4.2. NanoVaccines and Therapies
2.4.3. Drug Discovery Platforms
2.5. Nanodiagnostics and Nanotheranostics
2.6. Tissue Engineering
2.7. Bioprinting
2.8. Environmental Nanotechnology
3. Challenges and Future Directions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- NNI Supplement to the President’s 2020 Budget. Available online: https://www.nano.gov/2020BudgetSupplement (accessed on 11 June 2020).
- Alcaraz, J.-P.; Menassol, G.; Penven, G.; Thélu, J.; El Ichi, S.; Zebda, A.; Cinquin, P.; Martin, D.K. Challenges for the Implantation of Symbiotic Nanostructured Medical Devices. Appl. Sci. 2020, 10, 2923. [Google Scholar] [CrossRef]
- Wang, Y.; Guo, L. Nanomaterial-Enabled Neural Stimulation. Front. Neurosci. 2016, 10, 69. [Google Scholar] [CrossRef]
- Deisseroth, K. Optogenetics: 10 years of microbial opsins in neuroscience. Nat. Neurosci. 2015, 18, 1213–1225. [Google Scholar] [CrossRef]
- Colombo, E.; Feyen, P.; Antognazza, M.R.; Lanzani, G.; Benfenati, F. Nanoparticles: A Challenging Vehicle for Neural Stimulation. Front. Neurosci. 2016, 10, 105. [Google Scholar] [CrossRef] [PubMed]
- Carvalho-de-Souza, J.L.; Treger, J.S.; Dang, B.; Kent, S.B.; Pepperberg, D.R.; Bezanilla, F. Photosensitivity of neurons enabled by cell-targeted gold nanoparticles. Neuron 2015, 86, 207–217. [Google Scholar] [CrossRef] [PubMed]
- Marino, A.; Arai, S.; Hou, Y.; Sinibaldi, E.; Pellegrino, M.; Chang, Y.T.; Mazzolai, B.; Mattoli, V.; Suzuki, M.; Ciofani, G. Piezoelectric Nanoparticle-Assisted Wireless Neuronal Stimulation. ACS Nano 2015, 9, 7678–7689. [Google Scholar] [CrossRef]
- Chen, R.; Romero, G.; Christiansen, M.G.; Mohr, A.; Anikeeva, P. Wireless magnetothermal deep brain stimulation. Science 2015, 347, 1477–1480. [Google Scholar] [CrossRef]
- Eom, K.; Kim, J.; Choi, J.M.; Kang, T.; Chang, J.W.; Byun, K.M.; Jun, S.B.; Kim, S.J. Enhanced infrared neural stimulation using localized surface plasmon resonance of gold nanorods. Small 2014, 10, 3853–3857. [Google Scholar] [CrossRef]
- Yong, J.; Needham, K.; Brown, W.G.; Nayagam, B.A.; McArthur, S.L.; Yu, A.; Stoddart, P.R. Gold-nanorod-assisted near-infrared stimulation of primary auditory neurons. Adv. Healthc. Mater. 2014, 3, 1862–1868. [Google Scholar] [CrossRef]
- Chen, S.; Weitemier, A.Z.; Zeng, X.; He, L.; Wang, X.; Tao, Y.; Huang, A.J.Y.; Hashimotodani, Y.; Kano, M.; Iwasaki, H.; et al. Near-infrared deep brain stimulation via upconversion nanoparticle-mediated optogenetics. Science 2018, 359, 679–684. [Google Scholar] [CrossRef]
- Yoo, S.; Hong, S.; Choi, Y.; Park, J.H.; Nam, Y. Photothermal inhibition of neural activity with near-infrared-sensitive nanotransducers. ACS Nano 2014, 8, 8040–8049. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Luo, R.; Lin, X.; Jadhav, A.D.; Zhang, Z.; Yan, L.; Chan, C.Y.; Chen, X.; He, J.; Chen, C.H.; et al. Remote modulation of neural activities via near-infrared triggered release of biomolecules. Biomaterials 2015, 65, 76–85. [Google Scholar] [CrossRef] [PubMed]
- Pappas, T.C.; Wickramanyake, W.M.; Jan, E.; Motamedi, M.; Brodwick, M.; Kotov, N.A. Nanoscale engineering of a cellular interface with semiconductor nanoparticle films for photoelectric stimulation of neurons. Nano Lett. 2007, 7, 513–519. [Google Scholar] [CrossRef] [PubMed]
- Shapiro, M.G.; Homma, K.; Villarreal, S.; Richter, C.P.; Bezanilla, F. Infrared light excites cells by changing their electrical capacitance. Nat. Commun. 2012, 3, 736. [Google Scholar] [CrossRef]
- Wells, J.; Kao, C.; Mariappan, K.; Albea, J.; Jansen, E.D.; Konrad, P.; Mahadevan-Jansen, A. Optical stimulation of neural tissue in vivo. Opt. Lett. 2005, 30, 504–506. [Google Scholar] [CrossRef]
- Bazard, P.; Frisina, R.D.; Walton, J.P.; Bhethanabotla, V.R. Nanoparticle-based Plasmonic Transduction for Modulation of Electrically Excitable Cells. Sci. Rep. 2017, 7, 7803. [Google Scholar] [CrossRef] [PubMed]
- Leibowitz, A.; Boyko, M.; Shapira, Y.; Zlotnik, A. Blood Glutamate Scavenging: Insight into Neuroprotection. Int. J. Mol. Sci. 2012, 13, 10041–10066. [Google Scholar] [CrossRef]
- Campos, F.; Sobrino, T.; Ramos-Cabrer, P.; Argibay, B.; Agulla, J.; Pérez-Mato, M.; Rodríguez-González, R.; Brea, D.; Castillo, J. Neuroprotection by glutamate oxaloacetate transaminase in ischemic stroke: An experimental study. J. Cereb. Blood Flow Metab. 2011, 31, 1378–1386. [Google Scholar] [CrossRef]
- Guerriero, R.M.; Giza, C.C.; Rotenberg, A. Glutamate and GABA Imbalance Following Traumatic Brain Injury. Curr. Neurol. Neurosci. Rep. 2015, 15. [Google Scholar] [CrossRef] [PubMed]
- Walton, H.; Dodd, P. Glutamate–glutamine cycling in Alzheimer’s disease. Neurochem. Int. 2007, 50, 1052–1066. [Google Scholar] [CrossRef]
- Claussen, J.C.; Artiles, M.S.; McLamore, E.S.; Mohanty, S.; Shi, J.; Rickus, J.L.; Fisher, T.S.; Porterfield, D.M. Electrochemical glutamate biosensing with nanocube and nanosphere augmented single-walled carbon nanotube networks: A comparative study. J. Mater. Chem. 2011, 21. [Google Scholar] [CrossRef]
- Moon, J.-M.; Thapliyal, N.; Hussain, K.K.; Goyal, R.N.; Shim, Y.-B. Conducting polymer-based electrochemical biosensors for neurotransmitters: A review. Biosens. Bioelectron. 2018, 102, 540–552. [Google Scholar] [CrossRef] [PubMed]
- Burmeister, J.J.; Gerhardt, G.A. Self-Referencing Ceramic-Based Multisite Microelectrodes for the Detection and Elimination of Interferences from the Measurement ofl-Glutamate and Other Analytes. Anal. Chem. 2001, 73, 1037–1042. [Google Scholar] [CrossRef] [PubMed]
- Durairaj, S.; Sidhureddy, B.; Cirone, J.; Chen, A. Nanomaterials-Based Electrochemical Sensors for In Vitro and In Vivo Analyses of Neurotransmitters. Appl. Sci. 2018, 8, 1504. [Google Scholar] [CrossRef]
- Schultz, J.; Uddin, Z.; Singh, G.; Howlader, M.M.R. Glutamate sensing in biofluids: Recent advances and research challenges of electrochemical sensors. Analyst 2020, 145, 321–347. [Google Scholar] [CrossRef]
- Zhu, C.; Yang, G.; Li, H.; Du, D.; Lin, Y. Electrochemical Sensors and Biosensors Based on Nanomaterials and Nanostructures. Anal. Chem. 2014, 87, 230–249. [Google Scholar] [CrossRef]
- Hwang, M.T.; Wang, Z.; Ping, J.; Ban, D.K.; Shiah, Z.C.; Antonschmidt, L.; Lee, J.; Liu, Y.; Karkisaval, A.G.; Johnson, A.T.C.; et al. DNA Nanotweezers and Graphene Transistor Enable Label-Free Genotyping. Adv. Mater. 2018. [Google Scholar] [CrossRef] [PubMed]
- Hwang, M.T.; Landon, P.B.; Lee, J.; Choi, D.; Mo, A.H.; Glinsky, G.; Lal, R. Highly specific SNP detection using 2D graphene electronics and DNA strand displacement. Proc. Natl. Acad. Sci. USA 2016, 113, 7088–7093. [Google Scholar] [CrossRef] [PubMed]
- Ban, D.K.; Liu, Y.; Wang, Z.; Ramachandran, S.; Sarkar, N.; Shi, Z.; Liu, W.; Karkisaval, A.; Martinez-Loran, E.; Zhang, F.; et al. Direct DNA Methylation Profiling with Electric Biosensor. ACS Nano 2020, 14, 6743–6751. [Google Scholar] [CrossRef] [PubMed]
- Jones, A.D., 3rd; Mi, G.; Webster, T.J. A Status Report on FDA Approval of Medical Devices Containing Nanostructured Materials. Trends Biotechnol. 2019, 37, 117–120. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Webster, T.J. Nanotechnology and nanomaterials: Promises for improved tissue regeneration. Nano Today 2009, 4, 66–80. [Google Scholar] [CrossRef]
- Khang, D.; Kim, S.Y.; Liu-Snyder, P.; Palmore, G.T.; Durbin, S.M.; Webster, T.J. Enhanced fibronectin adsorption on carbon nanotube/poly(carbonate) urethane: Independent role of surface nano-roughness and associated surface energy. Biomaterials 2007, 28, 4756–4768. [Google Scholar] [CrossRef]
- Alcaraz, J.P.; Cinquin, P.; Martin, D.K. Tackling the Concept of Symbiotic Implantable Medical Devices with Nanobiotechnologies. Biotechnol. J. 2018, 13, e1800102. [Google Scholar] [CrossRef]
- Hoogerheide, D.P.; Noskov, S.Y.; Jacobs, D.; Bergdoll, L.; Silin, V.; Worcester, D.L.; Abramson, J.; Nanda, H.; Rostovtseva, T.K.; Bezrukov, S.M. Structural features and lipid binding domain of tubulin on biomimetic mitochondrial membranes. Proc. Natl. Acad. Sci. USA 2017, 114, E3622–E3631. [Google Scholar] [CrossRef] [PubMed]
- Jadhav, V.; Hoogerheide, D.P.; Korlach, J.; Wanunu, M. Porous Zero-Mode Waveguides for Picogram-Level DNA Capture. Nano Lett. 2019, 19, 921–929. [Google Scholar] [CrossRef] [PubMed]
- Kotelevets, L.; Chastre, E.; Caron, J.; Mougin, J.; Bastian, G.; Pineau, A.; Walker, F.; Lehy, T.; Desmaele, D.; Couvreur, P. A Squalene-Based Nanomedicine for Oral Treatment of Colon Cancer. Cancer Res. 2017, 77, 2964–2975. [Google Scholar] [CrossRef]
- Homayun, B.; Lin, X.; Choi, H.J. Challenges and Recent Progress in Oral Drug Delivery Systems for Biopharmaceuticals. Pharmaceutics 2019, 11, 129. [Google Scholar] [CrossRef] [PubMed]
- Quarni, W.; Dutta, R.; Green, R.; Katiri, S.; Patel, B.; Mohapatra, S.S.; Mohapatra, S. Mithramycin A Inhibits Colorectal Cancer Growth by Targeting Cancer Stem Cells. Sci. Rep. 2019, 9, 15202. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.J.; Li, L.; Liu, X.J.; Li, Y.; Zhao, C.Y.; Wang, R.Q.; Zhen, Y.S. Mithramycin-loaded mPEG-PLGA nanoparticles exert potent antitumor efficacy against pancreatic carcinoma. Int. J. Nanomed. 2017, 12, 5255–5269. [Google Scholar] [CrossRef]
- Horst, D.; Kriegl, L.; Engel, J.; Kirchner, T.; Jung, A. CD133 expression is an independent prognostic marker for low survival in colorectal cancer. Br. J. Cancer 2008, 99, 1285–1289. [Google Scholar] [CrossRef] [PubMed]
- McHugh, K.J.; Nguyen, T.D.; Linehan, A.R.; Yang, D.; Behrens, A.M.; Rose, S.; Tochka, Z.L.; Tzeng, S.Y.; Norman, J.J.; Anselmo, A.C.; et al. Fabrication of fillable microparticles and other complex 3D microstructures. Science 2017, 357, 1138–1142. [Google Scholar] [CrossRef] [PubMed]
- Curry, E.J.; Le, T.T.; Das, R.; Ke, K.; Santorella, E.M.; Paul, D.; Chorsi, M.T.; Tran, K.T.M.; Baroody, J.; Borges, E.R.; et al. Biodegradable nanofiber-based piezoelectric transducer. Proc. Natl. Acad. Sci. USA 2020, 117, 214–220. [Google Scholar] [CrossRef] [PubMed]
- Curry, E.J.; Ke, K.; Chorsi, M.T.; Wrobel, K.S.; Miller, A.N., 3rd; Patel, A.; Kim, I.; Feng, J.; Yue, L.; Wu, Q.; et al. Biodegradable Piezoelectric Force Sensor. Proc. Natl. Acad. Sci. USA 2018, 115, 909–914. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.D.; Tran, K. Core-Shell Microneedle Platform for Transdermal and Pulsatile Drug/Vaccine Delivery and Method of Manufacturing the Same. U.S. Patent 16/293,588, 5 September 2019. [Google Scholar]
- Marshall, S.; Sahm, L.J.; Moore, A.C. The success of microneedle-mediated vaccine delivery into skin. Hum. Vaccines Immunother. 2016, 12, 2975–2983. [Google Scholar] [CrossRef]
- Gala, R.P.; Zaman, R.U.; D’Souza, M.J.; Zughaier, S.M. Novel Whole-Cell Inactivated Neisseria Gonorrhoeae Microparticles as Vaccine Formulation in Microneedle-Based Transdermal Immunization. Vaccines 2018, 6, 60. [Google Scholar] [CrossRef]
- Gala, R.P.; Popescu, C.; Knipp, G.T.; McCain, R.R.; Ubale, R.V.; Addo, R.; Bhowmik, T.; Kulczar, C.D.; D’Souza, M.J. Physicochemical and Preclinical Evaluation of a Novel Buccal Measles Vaccine. AAPS PharmSciTech 2017, 18, 283–292. [Google Scholar] [CrossRef] [PubMed]
- Lowry, T.W.; Kusi-Appiah, A.; Guan, J.J.; Van Winkle, D.H.; Davidson, M.W.; Lenhert, S. Materials Integration by Nanointaglio. Adv. Mater. Interfaces 2014, 1, 1300127. [Google Scholar] [CrossRef]
- Kusi-Appiah, A.E.; Lowry, T.W.; Darrow, E.M.; Wilson, K.A.; Chadwick, B.P.; Davidson, M.W.; Lenhert, S. Quantitative dose-response curves from subcellular lipid multilayer microarrays. Lab Chip 2015, 15, 3397–3404. [Google Scholar] [CrossRef]
- Lowry, T.W.; Hariri, H.; Prommapan, P.; Kusi-Appiah, A.; Vafai, N.; Bienkiewicz, E.A.; Van Winkle, D.H.; Stagg, S.M.; Lenhert, S. Quantification of Protein-Induced Membrane Remodeling Kinetics In Vitro with Lipid Multilayer Gratings. Small 2016, 12, 506–515. [Google Scholar] [CrossRef][Green Version]
- Mert, S.; Culha, M. Surface-enhanced Raman scattering-based detection of cancerous renal cells. Appl. Spectrosc. 2014, 68, 617–624. [Google Scholar] [CrossRef]
- Aydin, O.; Altas, M.; Kahraman, M.; Bayrak, O.F.; Culha, M. Differentiation of healthy brain tissue and tumors using surface-enhanced Raman scattering. Appl. Spectrosc. 2009, 63, 1095–1100. [Google Scholar] [CrossRef] [PubMed]
- Kuku, G.; Altunbek, M.; Culha, M. Surface-Enhanced Raman Scattering for Label-Free Living Single Cell Analysis. Anal. Chem. 2017, 89, 11160–11166. [Google Scholar] [CrossRef]
- Raman4Clinics. Available online: https://www.raman4clinics.eu/ (accessed on 8 May 2020).
- Culha, M. Raman spectroscopy for cancer diagnosis: How far have we come? Bioanalysis 2015, 7, 2813–2824. [Google Scholar] [CrossRef] [PubMed]
- Blackie, E.J.; Le Ru, E.C.; Etchegoin, P.G. Single-molecule surface-enhanced Raman spectroscopy of nonresonant molecules. J. Am. Chem. Soc. 2009, 131, 14466–14472. [Google Scholar] [CrossRef] [PubMed]
- Fleischmann, M.; Hendra, P.; McQuillan, A. Raman Spectra of Pyridine Adsorbed at a Silver Electrode. Chem. Phys. Lett. 1974, 26, 163–166. [Google Scholar] [CrossRef]
- Jeanmaire, D.L.; Van Duyne, R.P. Surface raman spectroelectrochemistry: Part I. Heterocyclic, aromatic, and aliphatic amines adsorbed on the anodized silver electrode. J. Electroanal. Chem. Interf. Electrochem. 1977, 84, 1–20. [Google Scholar] [CrossRef]
- Langhans, S.A. Three-Dimensional in Vitro Cell Culture Models in Drug Discovery and Drug Repositioning. Front. Pharmacol. 2018, 9, 6. [Google Scholar] [CrossRef]
- Antoni, D.; Burckel, H.; Josset, E.; Noel, G. Three-dimensional cell culture: A breakthrough in vivo. Int. J. Mol. Sci. 2015, 16, 5517–5527. [Google Scholar] [CrossRef]
- McKee, C.; Chaudhry, G.R. Advances and challenges in stem cell culture. Colloids Surf. B Biointerfaces 2017, 159, 62–77. [Google Scholar] [CrossRef]
- Wang, T.; Green, R.; Guldiken, R.; Mohapatra, S.; Mohapatra, S. Multiple-layer guided surface acoustic wave (SAW)-based pH sensing in longitudinal FiSS-tumoroid cultures. Biosens. Bioelectron. 2019, 124–125, 244–252. [Google Scholar] [CrossRef]
- Wang, T.; Murphy, R.; Wang, J.; Mohapatra, S.S.; Mohapatra, S.; Guldiken, R. Perturbation Analysis of a Multiple Layer Guided Love Wave Sensor in a Viscoelastic Environment. Sensors 2019, 19, 1749. [Google Scholar] [CrossRef]
- Wang, T.; Green, R.; Guldiken, R.; Wang, J.; Mohapatra, S.; Mohapatra, S.S. Finite Element Analysis for Surface Acoustic Wave Device Characteristic Properties and Sensitivity. Sensors 2019, 19, 1749. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Green, R.; Nair, R.R.; Howell, M.; Mohapatra, S.; Guldiken, R.; Mohapatra, S.S. Surface Acoustic Waves (SAW)-Based Biosensing for Quantification of Cell Growth in 2D and 3D Cultures. Sensors 2015, 15, 32045–32055. [Google Scholar] [CrossRef] [PubMed]
- Girard, Y.K.; Wang, C.; Ravi, S.; Howell, M.C.; Mallela, J.; Alibrahim, M.; Green, R.; Hellermann, G.; Mohapatra, S.S.; Mohapatra, S. A 3D fibrous scaffold inducing tumoroids: A platform for anticancer drug development. PLoS ONE 2013, 8, e75345. [Google Scholar] [CrossRef]
- Green, R.; Howell, M.; Khalil, R.; Nair, R.; Yan, J.; Foran, E.; Katiri, S.; Banerjee, J.; Singh, M.; Bharadwaj, S.; et al. Actinomycin D and Telmisartan Combination Targets Lung Cancer Stem Cells Through the Wnt/Beta Catenin Pathway. Sci. Rep. 2019, 9, 18177. [Google Scholar] [CrossRef] [PubMed]
- Kondiah, P.J.; Choonara, Y.E.; Kondiah, P.P.D.; Kumar, P.; Marimuthu, T.; du Toit, L.C.; Pillay, V. Development of an injectable pseudo-bone thermo-gel for application in small bone fractures. Int. J. Pharm. 2017, 520, 39–48. [Google Scholar] [CrossRef] [PubMed]
- Siyawamwaya, M.; du Toit, L.C.; Kumar, P.; Choonara, Y.E.; Kondiah, P.; Pillay, V. 3D printed, controlled release, tritherapeutic tablet matrix for advanced anti-HIV-1 drug delivery. Eur. J. Pharm. Biopharm. 2019, 138, 99–110. [Google Scholar] [CrossRef] [PubMed]
- Kondiah, P.J.; Kondiah, P.P.D.; Choonara, Y.E.; Marimuthu, T.; Pillay, V. A 3D Bioprinted Pseudo-Bone Drug Delivery Scaffold for Bone Tissue Engineering. Pharmaceutics 2020, 12, 166. [Google Scholar] [CrossRef]
- Kondiah, P.P.; Choonara, Y.E.; Kondiah, P.J.; Marimuthu, T.; du Toit, L.C.; Kumar, P.; Pillay, V. Recent Progress in 3D-printed Polymeric Scaffolds for Bone Tissue Engineering. In Advanced 3D-Printed Systems and Nanosystems for Drug Delivery and Tissue Engineering; du Toit, L.C., Pradeep, K., Choonara, Y.E., Pillay, V., Eds.; Elsevier: Amsterdam, The Netherlands, 2020. [Google Scholar]
- McLamore, E.S.; Palit Austin Datta, S.; Morgan, V.; Cavallaro, N.; Kiker, G.; Jenkins, D.M.; Rong, Y.; Gomes, C.; Claussen, J.; Vanegas, D.; et al. SNAPS: Sensor Analytics Point Solutions for Detection and Decision Support Systems. Sensors 2019, 19, 4935. [Google Scholar] [CrossRef]
- Garland, N.T.; McLamore, E.S.; Cavallaro, N.D.; Mendivelso-Perez, D.; Smith, E.A.; Jing, D.; Claussen, J.C. Flexible Laser-Induced Graphene for Nitrogen Sensing in Soil. ACS Appl. Mater. Interfaces 2018, 10, 39124–39133. [Google Scholar] [CrossRef]
- Jenkins, D.M.; Lee, B.E.; Jun, S.; Reyes-De-Corcuera, J.; McLamore, E.S. ABE-Stat, a Fully Open-Source and Versatile Wireless Potentiostat Project Including Electrochemical Impedance Spectroscopy. J. Electrochem. Soc. 2019, 166, B3056–B3065. [Google Scholar] [CrossRef]
- 2018 Edition of the Drinking Water Standards and Health Advisories Table; US Environmental Protection Agency: Washington, DC, USA, 2018.
- Vélez-Torres, I.; Vanegas, D.C.; McLamore, E.S.; Hurtado, D. Mercury Pollution and Artisanal Gold Mining in Alto Cauca, Colombia: Woman’s Perception of Health and Environmental Impacts. J. Environ. Dev. 2018, 27, 415–444. [Google Scholar] [CrossRef]
- Martin-Yerga, D.; Gonzalez-Garcia, M.B.; Costa-Garcia, A. Electrochemical determination of mercury: A review. Talanta 2013, 116, 1091–1104. [Google Scholar] [CrossRef] [PubMed]
- Morgan, V.; Casso-Hartmann, L.; Bahamon-Pinzon, D.; McCourt, K.; Hjort, R.G.; Bahramzadeh, S.; Velez-Torres, I.; McLamore, E.; Gomes, C.; Alocilja, E.C.; et al. Sensor-as-a-Service: Convergence of Sensor Analytic Point Solutions (SNAPS) and Pay-A-Penny-Per-Use (PAPPU) Paradigm as a Catalyst for Democratization of Healthcare in Underserved Communities. Diagnostics 2020, 10, 22. [Google Scholar] [CrossRef] [PubMed]
- Bergeson, L.L. Opportunities and Challenges for Health, Safety, and the Environment: The Regulatory Void. In Nanotechnology: Delivering on the Promise Volume 1; Cheng, H.N., Doemeny, L., Geraci, C.L., Schmidt, D.G., Eds.; American Chemical Society: Washington, DC, USA, 2016; pp. 97–102. [Google Scholar] [CrossRef]
- Philbert, M. Nanomaterials: Promise in Balance with Safety. In Nanotechnology: Delivering on the Promise Volume 1; Cheng, H.N., Doemeny, L., Geraci, C.L., Schmidt, D.G., Eds.; American Chemical Society: Washington, DC, USA, 2016; pp. 89–95. [Google Scholar] [CrossRef]
- Medley, T.L. Regulations: Facilitating Advancement or Serving as a Barrier—A Shared Responsibility. In Nanotechnology: Delivering on the Promise Volume 1; Cheng, H.N., Doemeny, L., Geraci, C.L., Schmidt, D.G., Eds.; American Chemical Society: Washington, DC, USA, 2016; pp. 75–84. [Google Scholar] [CrossRef]
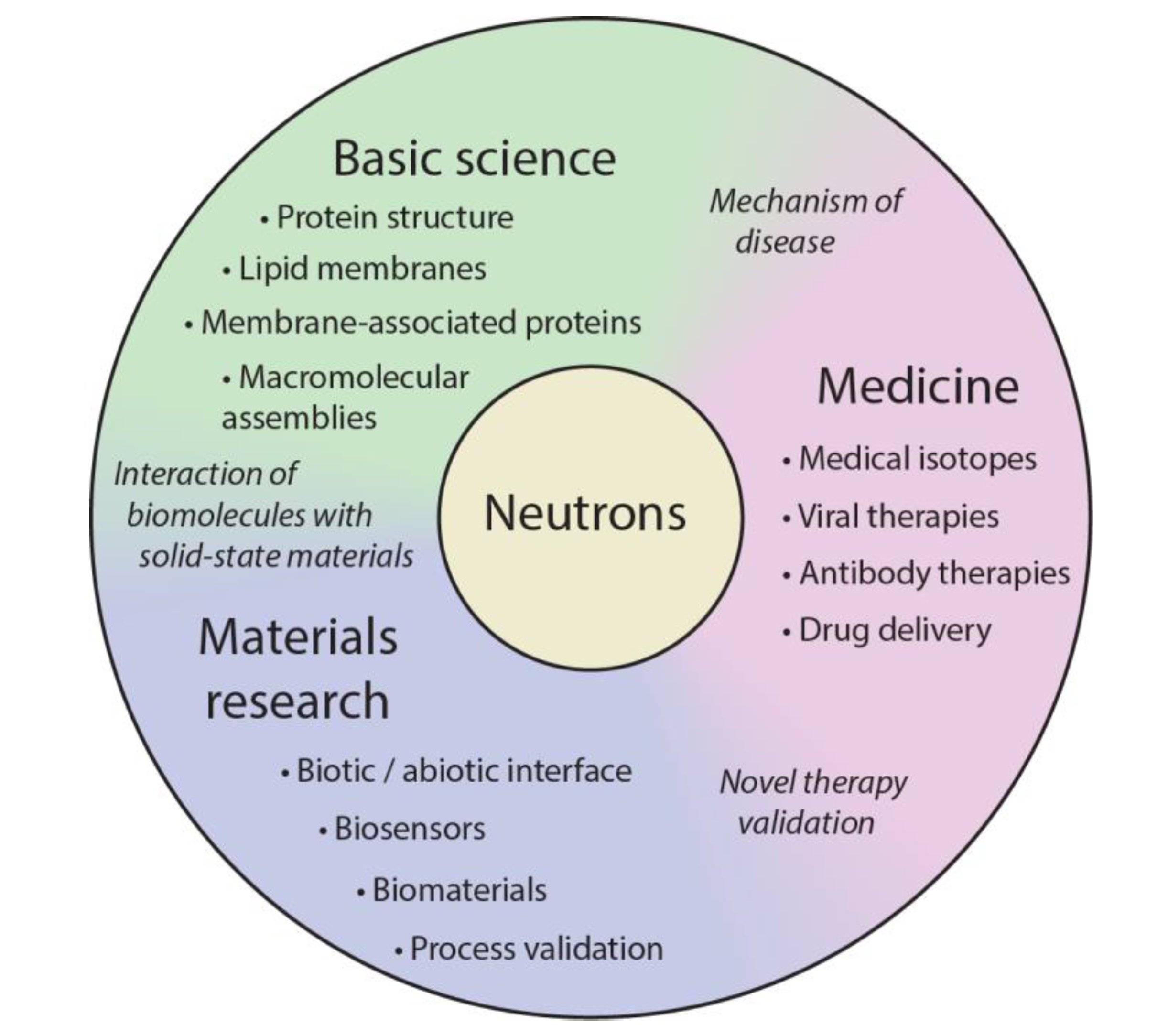
| Technique | Sample Form | Length (Time) Scale | Information |
|---|---|---|---|
| Small angle neutron scattering (SANS) | Solubilized | 1 nm–10,000 nm | Structure |
| Neutron reflectometry (NR) | Thin film | 1 nm–1,000 nm | Structure |
| Neutron macromolecular crystallography (NMX) | Crystallized | 0.1 nm resolution | Atomic structure |
| Neutron spin echo (NSE) | Solubilized | 0.1 nm–100 nm (0.01 ns–1000 ns) | Collective dynamics |
| Inelastic neutron scattering | Powder | 0.5 nm–5 nm (0.01 ns–5 ns) | Diffusive dynamics |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mohapatra, S.S.; Frisina, R.D.; Mohapatra, S.; Sneed, K.B.; Markoutsa, E.; Wang, T.; Dutta, R.; Damnjanovic, R.; Phan, M.-H.; Denmark, D.J.; et al. Advances in Translational Nanotechnology: Challenges and Opportunities. Appl. Sci. 2020, 10, 4881. https://doi.org/10.3390/app10144881
Mohapatra SS, Frisina RD, Mohapatra S, Sneed KB, Markoutsa E, Wang T, Dutta R, Damnjanovic R, Phan M-H, Denmark DJ, et al. Advances in Translational Nanotechnology: Challenges and Opportunities. Applied Sciences. 2020; 10(14):4881. https://doi.org/10.3390/app10144881
Chicago/Turabian StyleMohapatra, Shyam S., Robert D. Frisina, Subhra Mohapatra, Kevin B. Sneed, Eleni Markoutsa, Tao Wang, Rinku Dutta, Ratka Damnjanovic, Manh-Huong Phan, Daniel J. Denmark, and et al. 2020. "Advances in Translational Nanotechnology: Challenges and Opportunities" Applied Sciences 10, no. 14: 4881. https://doi.org/10.3390/app10144881
APA StyleMohapatra, S. S., Frisina, R. D., Mohapatra, S., Sneed, K. B., Markoutsa, E., Wang, T., Dutta, R., Damnjanovic, R., Phan, M.-H., Denmark, D. J., Biswal, M. R., McGill, A. R., Green, R., Howell, M., Ghosh, P., Gonzalez, A., Ahmed, N. T., Borresen, B., Farmer, M., ... Martin, D. K. (2020). Advances in Translational Nanotechnology: Challenges and Opportunities. Applied Sciences, 10(14), 4881. https://doi.org/10.3390/app10144881














