Featured Application
This work was focused in the generation of six yeast lines able to produce resveratrol from p-coumaric acid. Industrial use of these strains may be contemplated.
Abstract
Resveratrol is a nutraceutical with relevant benefits to human health. This investigation reports on the generation and evaluation of six recombinant yeast lines that produce resveratrol from p-coumaric acid. The yeast lines contained a single p-coumaric acid-Co-A ligase from Plagiochasma appendiculatum combined with the stilbene synthases from Parthenocissus henryana, Polygonum cuspidatum, Morus alba var. atropurpurea, Rheum tataricum, Vitis vinifera and Arachis hypogaea. Codon optimized versions of these sequences were inserted in an expression vector flanked by the constitutive PGK and GPD promoters before expression in Saccharomyces cerevisiae. Batch fermentation (60 h) revealed that yeast lines had different capacities (p < 0.01) to produce resveratrol. Slightly acidic pH (6) and concentrations <100 mg L−1 p-coumaric acid improved resveratrol yields. Among the six lines, those containing the stilbene synthases (STS) from P. cuspidatum and M. alba produced up to 39 mg L−1 using 70 mg L−1 p-coumaric acid. On the other hand, lines expressing STS from V. vinifera, A. hypogaea and R. tataricum generated resveratrol faster than other lines but accumulated lower amounts at the end of the batch period (27–30 mg L−1). The simultaneous consumption of ethanol and p-coumaric acid corroborates the role of ethanol as a carbon source involved in the conversion of p-coumaric acid into resveratrol.
1. Introduction
Resveratrol (3,4′,5-trihydroxystilbene) is a natural product with relevant nutraceutical activity [1]. The content of resveratrol in red wines (1–3 mg L−1) and peanuts (0.5–2.5 mg 100 g−1) is relatively high. However, continuous consumption of these foods remains controversial due to the coexistence of elevated amounts of ethanol and assimilable carbohydrates [2]. Rigorous in vitro and in vivo studies have revealed the beneficial effects of resveratrol, which are linked to antioxidant, antiinflammatory, anti-platelet aggregation, anti-atherogenic, oestrogen-like, immunomodulatory and chemopreventive activities [1]. Resveratrol has an evident role as a regulator of tumor initiation, promotion and progression [1]. Based on dose–response effects, some studies supported an anti-aging effect which was reported from microorganisms to mammals [3]. Due to these premises, resveratrol is considered as a promising additive for functional foods.
Thus far, several engineering approaches have been performed in order to scale the production of trans-resveratrol under controlled conditions. Significant advances in the heterologous production of the stilbene have been achieved in bacterial, yeast and plant models [4,5,6,7,8,9,10]. The biosynthesis of resveratrol is comprised of three or four steps which depend on the specific amino acid used as initial precursor (Figure 1).
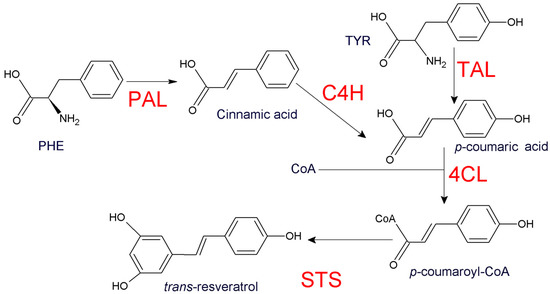
Figure 1.
Biosynthesis of resveratrol in higher plants. Keys enzymes are highlighted in red. Phenylalanine ammonia lyase (PAL), tyrosine ammonia lyase (TAL), cinnamate 4 hydroxylase (C4H), p-coumaric-acid coenzyme A ligase (4CL) and stilbene synthases (STS).
Phenylalanine is transformed into p-coumaric acid by phenylalanine ammonia lyase (PAL) and cinnamate 4 hydroxylase (C4H). However, an alternative pathway from tyrosine involves the enzymatic activity of tyrosine ammonia lyase (TAL) to produce p-coumaric acid which is subsequently converted into p-coumaroyl-CoA by p-coumaric-acid coenzyme A ligase (4CL). p-coumaroyl-CoA is finally transformed into trans-resveratrol by stilbene synthases (STS) [11]. Considering that plant models have the biochemical machinery to produce p-coumaroyl-CoA, the insertion of a single STS under constitutive promoters have resulted in the accumulation of resveratrol in transgenic plants [5,9,12]. Contrarily, resveratrol production in microorganisms requires exhaustive strategies which may include simultaneous insertion of PAL, TAL, C4H or chimeric genes containing C4H:STS [13]. Recent improvements on resveratrol yields were achieved from diverse methods. For example, by overexpressing electron carriers such as AtATR2 from Arabidopsis and CYB5 from S. cerevisiae to optimize the activity of C4H [7]. Other strategies included the overexpression or repression of enzymes involved in the transformation amino acidic precursors or malonate assimilating pathway genes, in order to increase initial precursors destined to the generation of resveratrol [10]. To the best of our knowledge, more than 20 different microbial strains have emerged from S. cerevisiae and E. coli as resveratrol producing systems [11,13]. Interestingly, some of those platforms were able to generate high amounts of resveratrol from basic substrates, such as glucose, fructose or ethanol [6,7]. Despite these substrates being readily available, other intermediates such as p-coumaric acid are still visualized as a non-expensive and more focalized alternatives [14]. The amphipathic character of p-coumaric acid and its specific location in resveratrol biosynthesis could be considered as two advantages for its use as a substrate in feeding experiments [14].
Coincidently, recent microbial strains that produce resveratrol contain similar combinations of coding sequences isolated from plant, bacterial, fungal or plant species, whereas the last step has been mainly confined to the STS activity of Vitis vinifera and Arachis hypogaea [11,13]. Currently, comparative studies on the efficacy of different STS in yeast systems are not available. Due to this fact, this study was focused in the construction and evaluation of recombinant strains of S. cerevisiae containing six codon optimized STS and its co-expression with the 4CL from the liverwort Plagiochasma appendiculatum which were evaluated for the production of resveratrol under batch fermentation.
2. Materials and Methods
2.1. Yeast Strain and DNA Sequences
The resveratrol pathway was engineered in Saccharomyces cerevisiae W303 [MATα leu2-3, 112 ura3-1 trp1-1 his3-11, 15 ade2-1]. Six yeast lines were generated by the insertion of codon optimized sequences (GeneScript Co., Piscataway, NJ, USA) of 4CL1 from Plagiochasma appendiculatum (Pa4CL1; accession: KJ944317) and STS from the angiosperms Parthenocissus henryana (PhStS; accession: AY094615), Polygonum cuspidatum (PcPKS5; accession: EU647245) Morus alba var. atropurpurea (MaSTS3; [15]), Rheum tataricum (RtSTS; accession: AF508150), Vitis vinifera (VvVST1; accession: NM001281010) and Arachis hypogaea (AhSTS; accession: EF620775). Optimized sequences (Supplementary Sequences S1–S7) were initially cloned in pUC57 and multiplied into Escherichia coli TOP10F’.
2.2. Genetic Construction and Heterologous Expression
S. cerevisiae W303 was basically engineered in accordance with Shin et al. [14] with slight modifications. pESC-TRP (Stratagene Co., San Diego, CA, USA) was used as an expression vector after punctual modifications. PGK promoter (containing the sites BamHI-EcoRI) and GPD promoter (containing the sites BamHI and ApaI) were amplified from the genomic DNA of S. cerevisiae using the yeast DNA extraction kit, high-fidelity DNA polymerase kit and DNA ligase from Thermo Scientific™. Oligonucleotides and PCR conditions were those described in Table 1. The resulting amplicons and pESC-TRP were digested using the corresponding nucleases before insertion into the multiple cloning site of pESC-TRP. This strategy led to the replacement of Gal10 and Gal1 promoters by constitutive PGK and GPD promoters to generate pESC-TRP-PGPD-PPGK expression vector [14]. Pa4CL1 and STS were screened for their compatible restriction sites using NEBcutter V2.0. Molecular cloning of the coding sequences was done into pESC-TRP-PGPD-PPGK coupling the restriction sites described in Table 1. Six constructions were created and named as pESC-Pa4CL1-PhStS, pESC-Pa4CL1- PcPKS5, pESC- Pa4CL1-MaSTS3, pESC- Pa4CL1-RtSTS, pESC-Pa4CL1-VvVST1 and pESC-Pa4CL1-AhSTS. These constructions contained the 4CL1 from P. appendiculatum combined with PhStS, PcPKS5, MaSTS3, RtSTS, VvVST1 and AhSTS, respectively. The correct orientation of these constructions was verified using an ABI PRISM 3700 sequencer (ABI, Foster City, CA, USA). The expression vectors were introduced into S. cerevisiae W303 by the lithium acetate protocol [16]. Plates containing selective medium formulated with 2% glucose, 0.67% yeast nitrogen base (without amino acids), 0.19% yeast synthetic drop-out supplement without tryptophan, and 2% agar were used to select recombinant cells of S. cerevesiae. The insertion of genetic material was corroborated by the amplification of STS by colony PCR using the oligonucleotides described in Table 1.

Table 1.
Oligonucleotides used for the expression of recombinant 4CL1 from Plagiochasma appendiculatum and six STS from different plant sources.
2.3. Yeast Viability and Effect of pH on p-Coumaric Acid Uptake
Tolerance of recombinant strains to p-coumaric acid was observed by dose–response curves using resazurin (Sigma-Aldrich Co., St. Louis, MO, USA) as an indicator of cell viability in 96-well plates at 28 °C during 60 h using YPD medium (1% yeast extract, 2% peptone and 2% glucose) [17]. Resazurin is a redox dye that shows colorimetric and fluorometric changes associated to metabolic activity. Stabilization of resazurin is monitored by changes in blue color to fluorescent pink color [17]. These changes are produced when the indicator is reduced to resorufin by mitochondrial enzymes by accepting the electrons from cytochromes, NADH, FADH and NADPH [17]. For resazurin assays (100 µM), a constant amount of 500,000 cells mL−1 were constantly tested in each well and absorbance was quantified in a microplate reader (Powcam) at 595 nm. The effect of pH (4–8) on p-coumaric acid consumption was determined by adding NaOH (0.1N) and HCl (0.1N) to YPD medium. Feeding experiments were carried out with 50 mg L−1 of p-coumaric acid and resveratrol levels were measured at 60 h.
2.4. Batch Fermentation
Recombinant S. cerevisiae strains were pre-inoculated in liquid YPD (pH 6.0) for 4 h at 30 °C and 250 rpm to obtain an OD600 = 0.5 (4 × 108 cells mL−1). Afterwards, 1.5 L of YPD medium (pH 6.2) were adjusted to OD600 = 0.01 containing about 8.0 × 106 cells mL−1 of recombinant yeasts. The cells were incubated at 30 °C and 100 rpm in baffled glass flasks (2 L) containing the same medium added with p-coumaric acid (Sigma-Aldrich Co., St. Louis, MO, USA) (10–200 mg L−1). Incubations were kept in the dark. Wild type S. cerevisiae W303 was subjected to the same experimental process. Ten replicates were done for each strain assayed using the optimal concentration of p-coumaric acid (70 mg L−1). OD600 was determined with an ELISA plate reader (SmartReader™ 96) in order to calculate cell proliferation. Dry cell mass was calculated as previously reported [14]. Batch parameters were monitored during a period of 60 h. The levels of glucose and ethanol in the studied samples were measured by HPLC (Hewlett Packard 1050 system coupled to 1047A refractive index detector) using a ORH-801 column, under the analytical conditions descried by Lefebvre et al. [18]. Calibration curves were designed using authentic standards from Sigma-Aldrich Co. (St. Louis, MO, USA) and J.T. Baker (Staines-upon-Thames, UK).
2.5. Extraction and Analysis of Resveratrol
Yeast cells were harvested by centrifugation at 3000× g for 5 min for immediate lysis using an ultrasonic disruptor (OMNI 400). Both free supernatant and lysates were extracted three times with an equivalent volume of diethyl ether (J.T. Baker). Mixtures of organic extracts and cell lysates were dried in a centrifuge concentrator (RVC 2-25 CD) for 20 min and resuspended in 1 mL of ethanol for HPLC analysis. Samples were kept at 4 °C in the dark to avoid oxidative degradation and isomerization of trans-resveratrol. HPLC runs were done for the simultaneous detection of resveratrol and p-coumaric acid in accordance with Glavnik et al. [19] using an Hypersil ODS C18 column (5 µm, 250 × 4.6 mm, 5 µm) at 306 nm. Retention time and calibration curves were established with authentic standards of trans-resveratrol and p-coumaric from Sigma-Aldrich Co.
2.6. Statistical Analysis
ANOVA-Tukey tests were carried out with SPSS version 17.0.2 to determine statistically significant differences among treatments at p < 0.01.
3. Results and Discussion
3.1. Tolerance of Yeast Strains to p-Coumaric Acid
Previous works suggested that p-coumaric acid can be used as a substrate for resveratrol production in engineered microorganisms expressing 4CL and STS [14,20]. However, these studies also suggested the potential toxicity of this phenolic compound on Escherichia coli and S. cerevisiae. In order to explore the antimicrobial effect of p-coumaric acid on recombinant lines, the broth microdilution method using rezasurin as an indicator of cell viability was performed (Figure 2). According to these results, all the strains had minimum inhibitory concentrations (MIC) over 100 mg L−1 p-coumaric acid, similar MIC values were reported for some strains of E. coli [21]. Interestingly, MIC values for all the assayed strains had statistically significant differences (p < 0.01), which may suggest that the genetic material contained in those strains influenced the resistance to p-coumaric acid. This resistance could be associated to the enzymatic activity of Pa4CL1 and STS inserted in the recombinant strains [22]. Interestingly, the lowest MIC observed was for wild type W303 (p < 0.01), which supports the idea that the insertion of genetic material may be favoring p-coumaric acid uptake.
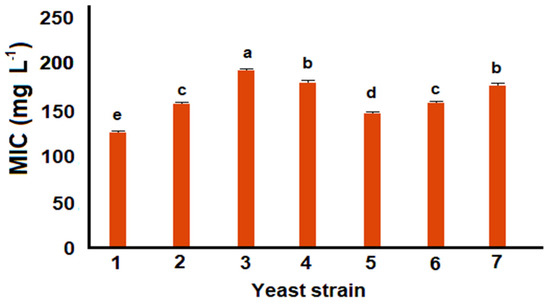
Figure 2.
Minimum inhibitory concentration (MIC) for wild and recombinant strains of S. cerevisiae treated with p-coumaric acid. Diverse letters indicate statistically significant differences (p < 0.01).
3.2. Conversion of p-Coumaric Acid into Resveratrol under Batch Fermentation Conditions
The conversion of p-coumaric acid into resveratrol (Figure 3) was monitored in each strain at different concentrations through fermented batch conditions (Figure 4). No insights of resveratrol glycosides were observed under our analytical conditions. After 60 h of batch fermentation, the consumption of p-coumaric acid by the yeast strains was completed (Figure 4). As expected, all the recombinant strains produced resveratrol after the addition of p-coumaric acid as a substrate (Figure 3A). The wild type W3030 strain did not convert p-coumaric acid into resveratrol (Figure 3B). Interestingly, the concentrations of p-coumaric acid added to the batch cultures were totally consumed but not completely converted into resveratrol (Figure 4). This biochemical tendency has been previously reported for recombinant S. cerevisiae strains that use p-coumaric acid as a precursor [14].
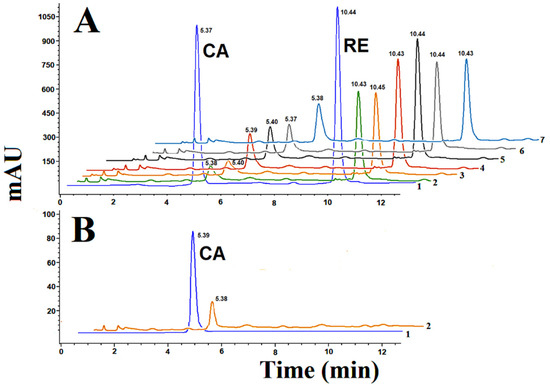
Figure 3.
Synthesis of resveratrol (RE) from p-coumaric acid (CA) by recombinant strains of S. cerevisiae. (A) Mixture of authentic standards of p-coumaric acid and trans-resveratrol (1), biotransformation products of pESC-Pa4CL1-PhStS (2), biotransformation products of pESC-Pa4CL1-PcPKS5 (3), biotransformation products of pESC-Pa4CL1-MaSTS3 (4), biotransformation products of pESC-Pa4CL1-RtSTS (5), biotransformation products of pESC-Pa4CL1-VvVST1 (6), biotransformation products of pESC-Pa4CL1-AhSTS (7). (B) Authentic standard of p-coumaric acid (CA) and biotransformation of p-coumaric by wild type W303 (2). Samples collected after 30 h of batch fermentation. Retention times are indicated for each peak.
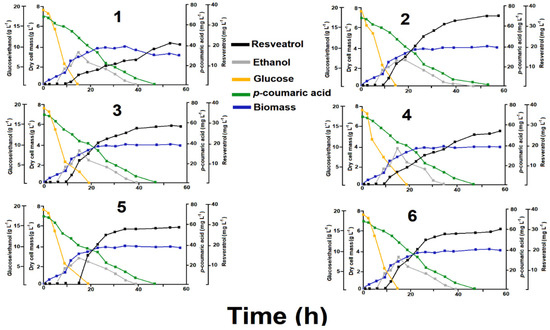
Figure 4.
Changes in the levels of glucose, ethanol, p-coumaric acid, resveratrol and dry cell mass under fermented batch conditions. Kinetics for the yeast lines pESC-Pa4CL1-PhStS (1), pESC-Pa4CL1-PcPKS5 (2), pESC-Pa4CL1-MaSTS3 (3), pESC-Pa4CL1-RtSTS (4), pESC-Pa4CL1-VvVST1 (5), pESC-Pa4CL1-AhSTS (6).
3.3. Effect of pH on p-Coumaric Acid Uptake
According to our results, pH produced evident changes in the transformation of p-coumaric acid into resveratrol (Figure 5). Resveratrol production was evidently different in the strains evaluated. pH 7–8 caused a considerable decrease in the levels of resveratrol, whereas acidic pH values (4–5) produced a favorable effect. In all the studied cases, pH 6 was the optimal value for the biotransformation process. The effect of alkaline pH was more evident in pESC-Pa4CL1-VvVST1 and pESC-Pa4CL1-AhSTS than in other recombinant lines. This effect should be associated to the beneficial effect of slightly acidic media on the growth of S. cerevisiae [23]. Also, H+ gradient may increase the permeability of carboxylic acids like p-coumaric acid [23].
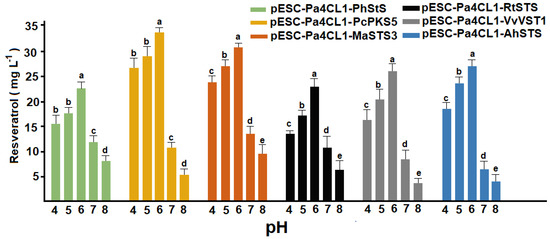
Figure 5.
Effect of pH on the conversion of p-coumaric acid into resveratrol. Diverse letters indicate statistically significant differences (p < 0.01). Error bars represent standard deviation of ten independent experiments (n = 10).
Dose–response curves performed under batch fermentation suggested that 70 mg L−1 p-coumaric acid were efficiently converted into resveratrol (Figure 6). Despite MIC values for recombinant strains were over 150 mg L−1, resveratrol yields were affected from 80 mg L−1 p-coumaric acid in batch fermentation and no levels of resveratrol were detected in reactions containing over 100 mg L−1 p-coumaric acid. These strains containing codon optimized STS were able to metabolize 4.3 more amount of substrate and quadruplicate the production of resveratrol in comparison with other recombinant microorganisms already reported [14,20]. Between 20% and 27% of p-coumaric acid was transformed into resveratrol.
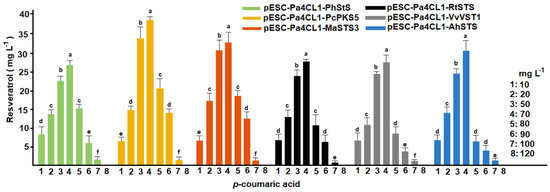
Figure 6.
Conversion of p-coumaric acid into resveratrol by recombinant strains of S. cerevisiae containing six stilbene synthases growing at pH 6. Different letters indicate statistically significant differences (p < 0.01). Error bars represent standard deviation of ten independent experiments (n = 10).
Interestingly, a different capacity to produce resveratrol was observed in each strain evaluated. Under the assayed conditions, pESC-Pa4CL1-PhStS had a similar activity to pESC-Pa4CL1-VvVST1, whereas a comparable activity was observed between pESC-Pa4CL1-MaSTS3 and pESC-Pa4CL1-AhSTS to produce resveratrol (Table 2). Among the six strains assayed, pESC-Pa4CL1-PcPKS5, pESC-Pa4CL1-MaSTS3 generated more resveratrol levels after the addition of 70 mg L−1 p-coumaric acid. Despite the enzymatic activity of individual STS should be strongly involved in this results, other intrinsic factors that influence the synthesis and regulation of heterologous enzymes in S. cerevisiae should be considered [24]. Thus, at least an additional heterologous system should be used to corroborate the results obtained in this work.

Table 2.
Production of resveratrol at two concentrations of p-coumaric acid (50 and 70 mg L−1) after 60 h batch fermentation.
Under batch culture, the recombinant strains exhibited similar parameters (Figure 4). The depletion of glucose levels was inverse to the development of cell growth (3.6 ± 0.2 g L−1) and ethanol production (6.7 ± 0.9 g L−1), which demonstrated the efficient replication of recombinant cells (Figure 4). Interestingly, an inverse tendency was observed between glucose consumption and resveratrol accumulation. This fact suggests that resveratrol synthesis begins after glucose reduction as previously reported [14]. Once glucose is almost or totally consumed, the cells uses p-coumaric acid to produce resveratrol (Figure 4). Remarkably, ethanol produced by glucose oxidation and exogenous p-coumaric acid were simultaneously used by recombinant yeasts, suggesting that ethanol is probably channeled as an energetic substrate for the conversion of p-coumaric acid into resveratrol.
Despite a similar growth pattern being observed for all the strains, resveratrol yields had evident differences. Among the six lines studied, pESC-Pa4CL1-PcPKS5 and pESC-Pa4CL1-MaSTS3 were the most effective for accumulating resveratrol at the end of the batch period (Figure 4). In this context, thresholds of resveratrol production varied in time for each strain assayed. For pESC-Pa4CL1-PhStS and pESC-Pa4CL1-RtSTS, maximum levels were detected between 45 and 50 h, whereas an asymptotic tendency was observed for pESC-Pa4CL1-PcPKS5 (from 35 h), pESC-Pa4CL1-MaSTS3 (from 35 h), pESC-Pa4CL1-VvVST1 (from 30 h), pESC-Pa4CL1-AhSTS (from 30 h). These results suggest that lines containing the STS from Vitis vinifera and Arachis hypogaea generate resveratrol faster than other strains, but they accumulate less amounts at the end of the batch period. This finding should be related with substrate affinity (Km) and saturation points (Vmax) reported by the STS expressed in these recombinant lines [22,25]. These qualities should be of the interest to produce resveratrol at industrial level.
Currently, resveratrol production in heterologous systems has achieved significant advances [4,5,6,7,8,9,10]. Due to the low number of reactions involved in resveratrol biosynthesis, several orthologs of PAL, TAL, C4H, 4CL and STS have been used to engineer a wide spectrum of microorganisms that produce substantial amounts of resveatrol. To the best of our knowledge, this is the first work reporting the use of a 4CL from a liverwort (Plagiochasma appendiculatum) to produce resveratrol in a yeast system [26]. According to our results, the use of codon optimized sequences and the experimental conditions described for batch fermentation led to quadruplicate the levels of resveratrol when compared with previous works [14,20]. In addition, specific pH and light protection had a clear influence on resveratrol yields.
4. Conclusions
Six recombinant yeast lines containing codon optimized sequences of enzymes involved in resveratrol biosynthesis were obtained. Among these lines, pESC-Pa4CL1-PcPKS5 and pESC-Pa4CL1-MaSTS3 generated higher resveratrol levels than the other recombinant strains. The use of optimized sequences, modifications in pH and the batch fermentation conditions reported in this investigation, substantially increased resveratrol yields starting from p-coumaric acid as an initial substrate.
Supplementary Materials
The following are available online at https://www.mdpi.com/2076-3417/10/14/4847/s1, Sequences S1–S7: Codon optimized sequences used in this work.
Author Contributions
Conceptualization, N.V.-R., A.R., O.R.-A. and E.R.-R.; methodology, N.V.-R., A.R., E.R.-R. and G.L.-C.; software, J.L.V.-C. and G.L.-C.; validation, N.V.-R., A.R., O.R.-A. and J.L.V.-C.; formal analysis, N.V.-R., A.R. and O.R.-A.; resources, E.R.-R., A.R. and O.R.-A.; Original-draft preparation, N.V.-R. and O.R.-A.; writing—review and editing, N.V.-R. and O.R.-A.; visualization, O.R.-A. and E.R.-R.; supervision, E.R.-R.; project administration, E.R.-R.; funding acquisition, O.R.-A., G.L.-C. and J.L.V.-C. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the program Cátedras-CONACyT-project 578 and to the program PRODEP 2020 from the Secretary of Public Education (SEP).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Elshaer, M.; Chen, Y.; Wang, X.J.; Tang, X. Resveratrol: An overview of its anti-cancer mechanisms. Life Sci. 2018, 207, 340–349. [Google Scholar] [CrossRef] [PubMed]
- de la Lastra, C.A.; Villegas, I. Resveratrol as an antioxidant and pro-oxidant agent: Mechanisms and clinical implications. Biochem. Soc. Trans. 2007, 35, 1156–1160. [Google Scholar] [CrossRef] [PubMed]
- Bhullar, K.S.; Hubbard, B.P. Lifespan and healthspan extension by resveratrol. Biochim. Biophys. Acta Mol. Basis Dis. 2015, 1852, 1209–1218. [Google Scholar] [CrossRef] [PubMed]
- Samappito, S.; Page, J.E.; Schmidt, J.; De-Eknamkul, W.; Kutchan, T.M. Aromatic and pyrone polyketides synthesized by a stilbene synthase from Rheum tataricum. Phytochemistry 2003, 62, 313–323. [Google Scholar] [CrossRef]
- Pan, L.P.; Yu, S.Y.; Chen, C.J.; Li, H.; Wu, Y.L.; Li, H.H. Cloning a peanut resveratrol synthase gene and its expression in purple sweet potato. Plant Cell Rep. 2012, 31, 121–131. [Google Scholar] [CrossRef]
- Li, M.; Kildegaard, K.R.; Chen, Y.; Rodriguez, A.; Borodina, I.; Nielsen, J. De novo production of resveratrol from glucose or ethanol by engineered Saccharomyces cerevisiae. Metab. Eng. 2015, 32, 1–11. [Google Scholar] [CrossRef]
- Li, M.; Schneider, K.; Kristensen, M.; Borodina, I.; Nielsen, J. Engineering yeast for highlevel production of stilbenoid antioxidants. Sci. Rep. 2015, 6, 36827. [Google Scholar] [CrossRef]
- Lim, C.G.; Fowler, Z.L.; Hueller, T.; Schaffer, S.; Koffas, M.A. High-yield resveratrol production in engineered Escherichia coli. Appl. Environ. Microbiol. 2015, 77, 3451–3460. [Google Scholar] [CrossRef]
- Zheng, S.; Zhao, S.; Li, Z.; Wang, Q.; Yao, F.; Yang, L.; Pan, J.; Liu, J. Evaluating the Effect of expressing a peanut resveratrol synthase gene in rice. PLoS ONE 2015, 10, e0136013. [Google Scholar] [CrossRef]
- Camacho-Zaragoza, J.M.; Hernández-Chávez, G.; Moreno-Avitia, F.M.; Ramírez-Iñiguez, R.; Martínez, A.; Gosset, G. Engineering of a microbial coculture of Escherichia coli strains for the biosynthesis of resveratrol. Microb. Cell Fact. 2016, 15, 163. [Google Scholar] [CrossRef]
- Jeandet, P.; Delaunois, B.; Aziz, A.; Donnez, D.; Vasserot, Y.; Cordelier, S.; Courot, E. Metabolic engineering of yeast and plants for the production of the biologically active hydroxystilbene, resveratrol. J. Biomed. Biotechnol. 2012, 579089. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Hu, Y.; Wang, X.; Zhong, J.; Lin, Z. High Content of resveratrol in lettuce transformed with a stilbene synthase gene of Parthenocissus henryana. J. Agric. Food Chem. 2006, 54, 8082–8085. [Google Scholar] [CrossRef] [PubMed]
- Thapa, S.B.; Pandey, R.P.; Park, Y.; Sohng, J.K. Biotechnological advances in resveratrol production and its chemical diversity. Molecules 2019, 24, 2571. [Google Scholar] [CrossRef] [PubMed]
- Shin, S.Y.; Han, N.M.; Park, Y.C.; Kim, M.D.; Seo, J.H. Production of resveratrol from p-coumaric acid in recombinant Saccharomyces cerevisiae expressing 4-coumarate:coenzyme A ligase and stilbene synthase genes. Enzyme Microb. Technol. 2011, 48, 48–53. [Google Scholar] [CrossRef]
- Wang, C.; Zhi, S.; Liu, C.; Xu, F.; Zhao, A.; Wang, X.; Ren, Y.; Li, Z.; Yu, M. Characterization of stilbene synthase genes in mulberry (Morus atropurpurea) and metabolic engineering for the production of resveratrol in Escherichia coli. J. Agric. Food Chem. 2017, 65, 1659–1668. [Google Scholar] [CrossRef]
- Rose, M.D.; Winston, F.; Hieter, P. Methods in Yeast Genetics: A Laboratory Course Manual, 1th ed.; Cold Spring Harbor Press: New York, NY, USA, 1990. [Google Scholar] [CrossRef]
- Sarker, S.D.; Nahar, L.; Kumarasamy, Y. Microtiter plate-based antibacterial assay incorporating resazurin as an indicator of cell growth, and its application in the in vitro antibacterial screening of phytochemicals. Methods 2007, 42, 321–324. [Google Scholar] [CrossRef]
- Lefebvre, D.; Gabriel, V.; Vayssier, Y.; Fontagné-Faucher, C. Simultaneous HPLC determination of sugars, organic acids and ethanol in sourdough process. LWT-Food Sci. Technol. 2002, 355, 407–414. [Google Scholar] [CrossRef]
- Glavnik, V.; Simonovska, B.; Albreht, A.; Vovk, I. TLC and HPLC screening of p-coumaric acid, trans-resveratrol, and pterostilbene in bacterial cultures, food supplements, and wine. J. Planar Chromat. 2012, 25, 251–258. [Google Scholar] [CrossRef]
- Watts, K.T.; Lee, P.C.; Schmidt-Dannert, C. Biosynthesis of plant-specific stilbene polyketides in metabolically engineered Escherichia coli. BMC Biotechnol. 2006, 6, 22. [Google Scholar] [CrossRef]
- Lou, Z.; Wang, H.; Rao, S.; Sun, J.; Ma, C.; Li, J. p-Coumaric acid kills bacteria through dual damage mechanisms. Food Control 2012, 25, 550–554. [Google Scholar] [CrossRef]
- Guo, Y.W.; Guo, H.L.; Li, X.; Huang, L.L.; Zhang, B.N.; Pang, X.B.; Liu, B.Y.; Ma, L.Q.; Wang, H. Two type III polyketide synthases from Polygonum cuspidatum: Gene structure, evolutionary route and metabolites. Plant Biotechnol. Rep. 2013, 7, 371–381. [Google Scholar] [CrossRef]
- Benvidi, A.; Dadras, A.; Abbasi, S.; Tezerjani, M.D.; Rezaeinasab, M.; Tabaraki, R.; Namazian, M. Experimental and computational study of the pK a of coumaric acid derivatives. J. Chin. Chem. Soc. 2019, 66, 589–593. [Google Scholar] [CrossRef]
- Han, M.; Yu, X. Enhanced expression of heterologous proteins in yeast cells via the modification of N-glycosylation sites. Bioengineered 2015, 6, 115–118. [Google Scholar] [CrossRef]
- Tropf, S.; Lanz, T.; Rensing, S.A.; Schröder, J.; Schröder, G. Evidence that stilbene synthases have developed from chalcone synthases several times in the course of evolution. J. Mol. Evol. 1994, 38, 610–618. [Google Scholar] [CrossRef]
- Gao, S.; Yu, H.N.; Xu, R.X.; Cheng, A.X.; Lou, H.X. Cloning and functional characterization of a 4-coumarate CoA ligase from liverwort Plagiochasma appendiculatum. Phytochemstry 2015, 111, 48–58. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).