An Innovative Low-Cost Equipment for Electro-Concentration of Microalgal Biomass
Abstract
:Featured Application
Abstract
1. Introduction
2. Materials and Methods
2.1. Strain Culture
2.2. Response-Surface Methodology for Variable Evaluation
- maximum efficiency of cell concentration.
- minimum working volume of 500 mL and maximum of 2000 mL.
- Easy cleaning and maintenance.
- Final cost of less than 300 USD.
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
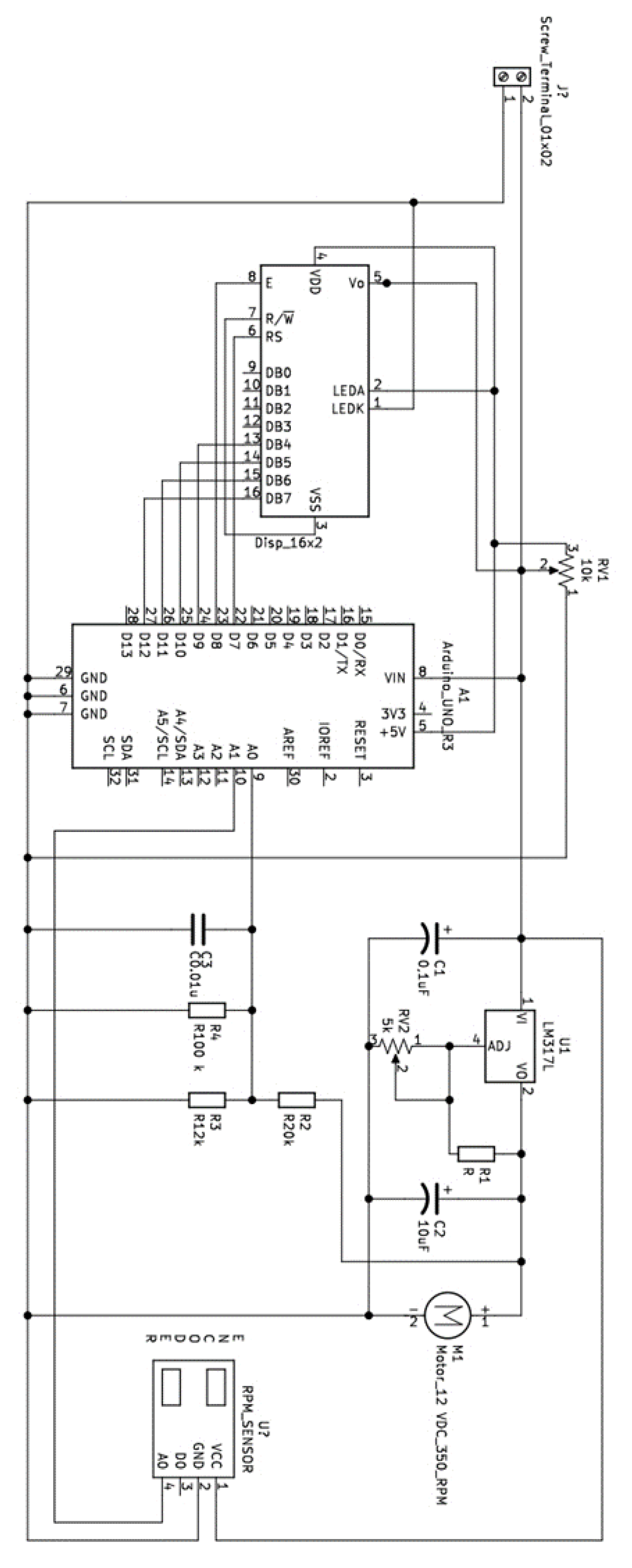
Appendix B

Appendix C

Appendix D
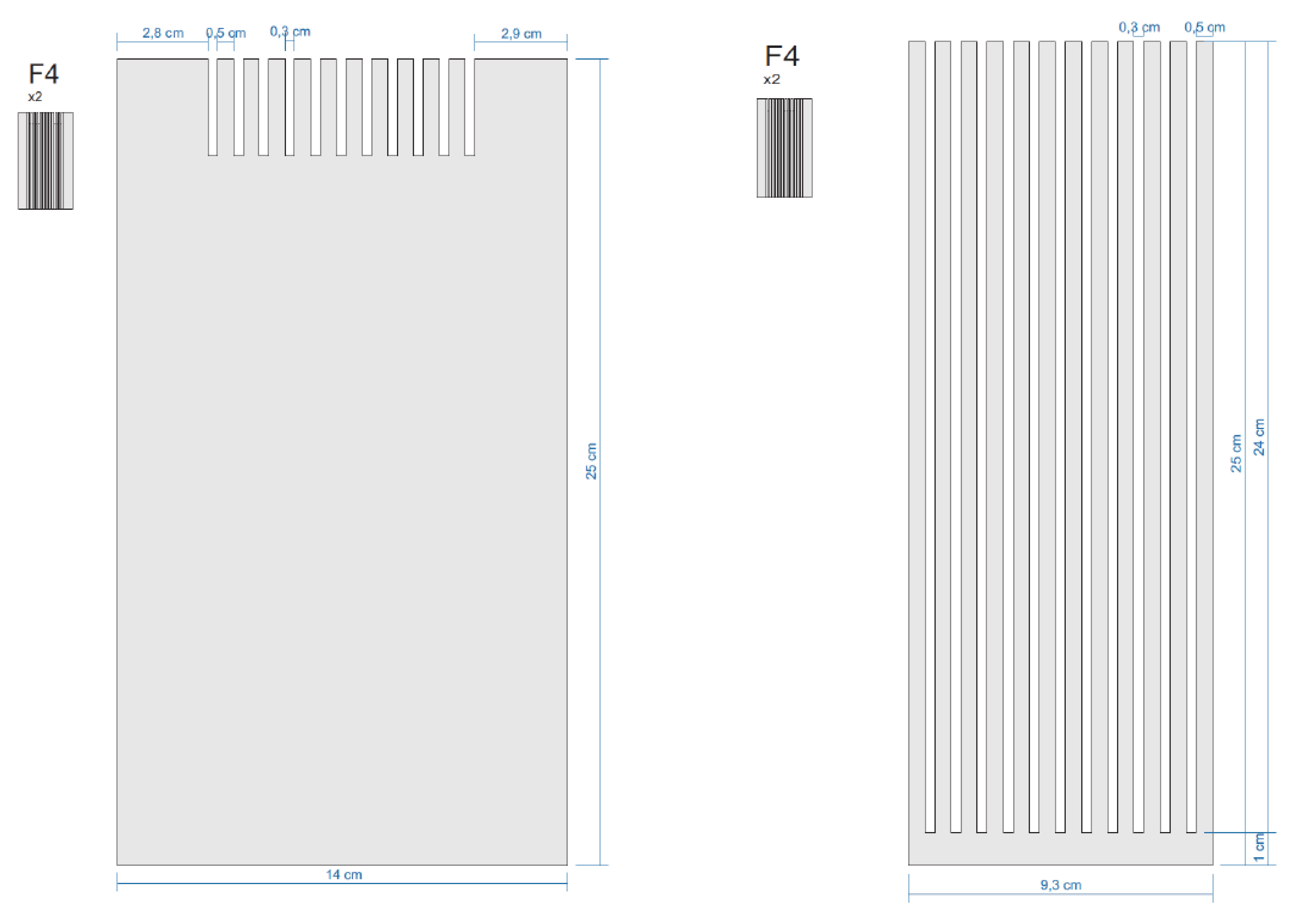
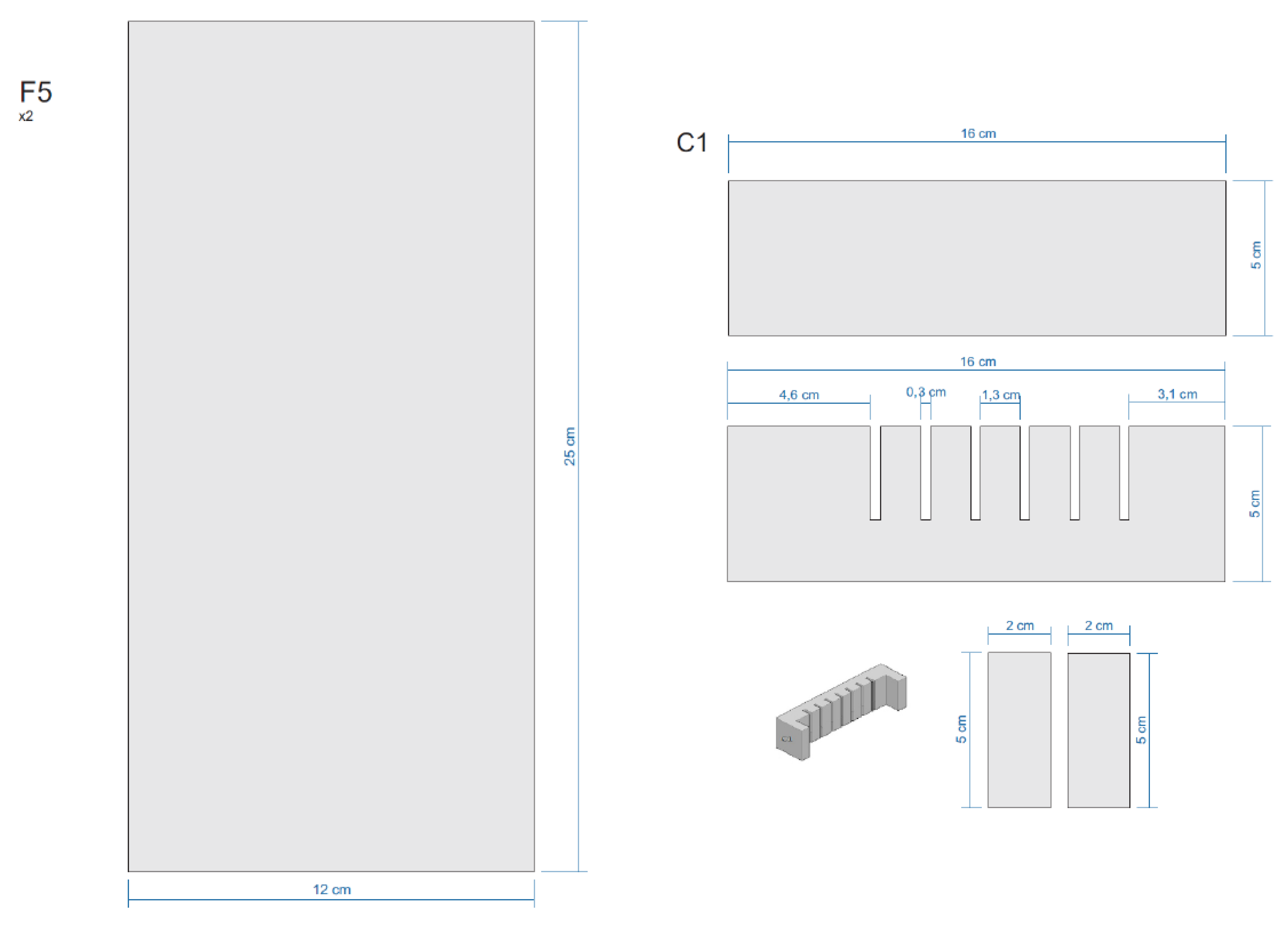
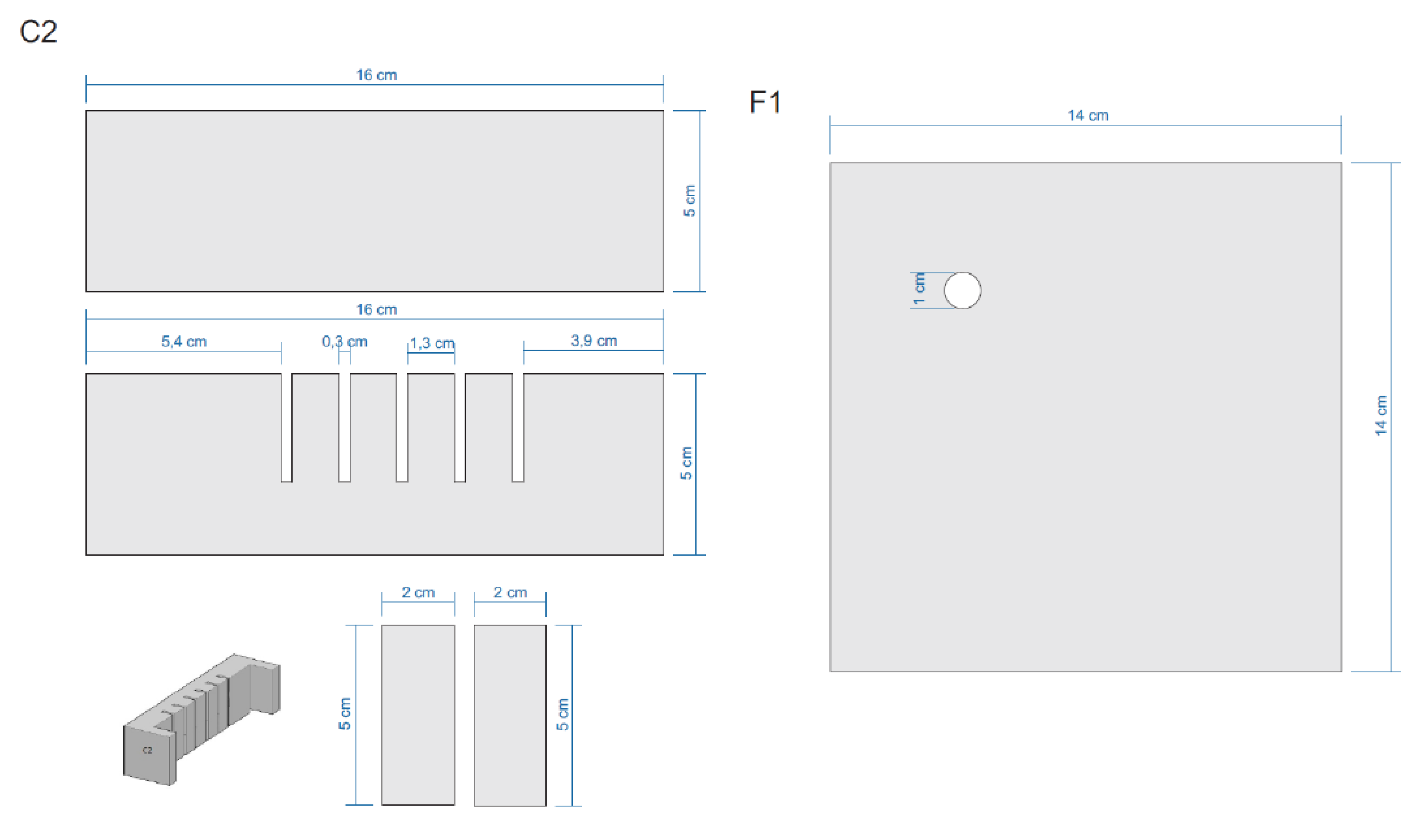
Appendix E
| Reference Description | Article | Quantity | Unit Cost (USD) | Total Price (USD) | Source of Materials | |||
|---|---|---|---|---|---|---|---|---|
| Electroflotation | Acrylic | 1.5 | m² | 55.84 | m² | 83.76 | Local supplier | |
| Wire caliber18 | 2 | m | 1.01 | m | 2.02 | Local supplier | ||
| Connectors banana plug | 2 | ud | 0.30 | ud | 0.60 | Local supplier | ||
| Cutting and assembly | 34.72 | 34.72 | Local supplier | |||||
| Subtotal | 121.10 | |||||||
| mixing | Arduino® NANO | 1 | ud | 3.47 | ud | 3.47 | shorturl.at/hEU09 | |
| LCD 16 × 2 | 1 | ud | 6.99 | ud | 6.99 | shorturl.at/gyCYZ | ||
| Neodymium magnet | 2 | ud | 8.99 | ud | 8.99 | shorturl.at/yzBLM | ||
| L298N | 1 | ud | 3.76 | ud | 3.76 | shorturl.at/npRX9 | ||
| Potentiometer 10k | 1 | ud | 8.99 | ud | 8.99 | shorturl.at/afryO | ||
| Motor Mh7 300 RPMs | 1 | ud | 15.99 | ud | 15.99 | shorturl.at/jvBJO | ||
| Subtotal | 48.19 | |||||||
| Accessories | Magnetic stir bar | 1 | ud | 3.47 | ud | 3.47 | Local supplier | |
| Aluminum sheets | 0.3234 | m2 | 33.27 | m2 | 10.75 | Local supplier | ||
| Aluminum sheets cut | 11 | ud | 0.10 | ud | 1.10 | Local supplier | ||
| 0–50 V Power Supply Stabilizer Module 15 A 750 W | 1 | ud | 48.03 | ud | 75.00 | shorturl.at/GOT27 | ||
| Subtotal | 90.32 | |||||||
| TOTAL | 259.61 USD | |||||||
References
- Lal, A.; Ghosh, S.; Das, D. Improvement in electrically induced biomass harvesting of Chlorella sp. MJ 11/11 for bulk biomass production. J. Appl. Phycol. 2018, 30, 979–993. [Google Scholar] [CrossRef]
- Ummalyma, S.B.; Mathew, A.K.; Pandey, A.; Sukumaran, R.K. Harvesting of microalgal biomass: Efficient method for flocculation through pH modulation. Bioresour. Technol. 2016, 213, 216–221. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Q.; Pang, Q.; Pan, X.; Chika, A.O.; Wang, L.; Shi, J.; Jia, L.; Chen, C.; Gao, Y. Facile sand enhanced electro-flocculation for cost-efficient harvesting of Dunaliella salina. Bioresour. Technol. 2015, 187, 326–330. [Google Scholar] [CrossRef]
- Misra, R.; Guldhe, A.; Singh, P.; Rawat, I.; Stenström, T.A.; Bux, F. Evaluation of operating conditions for sustainable harvesting of microalgal biomass applying electrochemical method using non sacrificial electrodes. Bioresour. Technol. 2015, 176, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Golzary, A.; Imanian, S.; Abdoli, M.A.; Khodadadi, A.; Karbassi, A. A cost-effective strategy for marine microalgae separation by electro-coagulation-flotation process aimed at bio-crude oil production: Optimization and evaluation study. Sep. Purif. Tech. 2015, 147, 156–165. [Google Scholar] [CrossRef]
- Lee, A.K.; Lewis, D.M.; Ashman, P.J. Harvesting of marine microalgae by electroflocculation: The energetics, plant design, and economics. Appl. Energy 2013, 108, 45–53. [Google Scholar] [CrossRef]
- Marrone, B.L.; Lacey, R.E.; Anderson, D.B.; Bonner, J.; Coons, J.; Dale, T.; Downes, C.M.; Fernando, S.; Fuller, C.; Goodall, B.; et al. Review of the harvesting and extraction program within the National Alliance for Advanced Biofuels and Bioproducts. Algal. Res. 2017, 33, 470–485. [Google Scholar] [CrossRef]
- Shi, W.; Zhu, L.; Chen, Q.; Lu, J.; Pan, G.; Hu, L.; Yi, Q. Synergy of flocculation and flotation for microalgae harvesting using aluminium electrolysis. Bioresour. Technol. 2017, 233, 127–133. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Đukić, A.; Firak, M. Hydrogen production using alkaline electrolyzer and photovoltaic (PV) module. Int. J. Hydrog. Energy 2011, 36, 7799–7806. [Google Scholar] [CrossRef]
- Mollaha, M.Y.A.; Morkovsky, P.; Gomes, J.A.G.; Kesmez, M.; Parga, J.; Cocke, D.L. Fundamentals, present and future perspectives of electrocoagulation. J. Hazard. Mater. 2004, 114, 199–210. [Google Scholar] [CrossRef]
- Emamjomeh, M.M.; Sivakumar, M. An empirical model for defluoridation by batch monopolar electrocoagulation/flotation (ECF) process. J. Hazard. Mater. 2006, 131, 118–125. [Google Scholar] [CrossRef]
- Gamage, N.P.; Rimer, J.D.; Chellam, S. Improvements in permeate flux by aluminum electroflotation pretreatment during microfiltration of surface water. J. Membr. Sci. 2012, 411–412, 45–53. [Google Scholar] [CrossRef]
- Castellaños-Estupiñan, M.A.; Sánchez-Galvis, E.M.; García-Martínez, J.B.; Barajas-Ferreira, C.; Zuorro, A.; Barajas-Solano, A.F. Design of an electroflotation system for the concentration and harvesting of freshwater microalgae. Chem. Eng. Trans. 2018, 64, 1–6. [Google Scholar]
- Andersen, R.A.; Berges, J.A.; Harrison, P.J.; Watanabe, M.M. Appendix A—Recipes for Freshwater and Seawater Media. In Algal Culturing Techniques; Andersen, R.A., Ed.; Elsevier Academic Press: Burlington, MA, USA, 2005; pp. 429–538. [Google Scholar]
- Zenouzi, A.; Ghobadian, B.; Hejazi, M.A.; Rahnemoon, P. Harvesting of microalgae Dunaliella salina using electroflocculation. J. Agric. Sci. Technol. 2013, 15, 879–888. [Google Scholar]
- Zhou, W.; Gao, L.; Cheng, W.; Chen, L.; Wang, J.; Wang, H.; Zhang, W.; Liu, T. Electro-flotation of Chlorella sp. assisted with flocculation by chitosan. Algal. Res. 2016, 18, 7–14. [Google Scholar] [CrossRef]
- Liu, S.; Abu Hajar, H.A.; Riefler, G.; Stuart, B.J. Investigation of electrolytic flocculation for microalga: Scenedesmus sp. using aluminum and graphite electrodes. RSC. Adv. 2018, 8, 38808–38817. [Google Scholar] [CrossRef] [Green Version]
- Bleeke, F.; Quante, G.; Winckelmann, D.; Klöck, G. Effect of voltage and electrode material on electroflocculation of Scenedesmus acuminatus. Bioresour. Bioprocess. 2015, 2, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Gao, S.; Du, M.; Tian, J.; Yang, J.; Yang, J.; Ma, F.; Nan, J. Effects of chloride ions on electro-coagulation-flotation process with aluminum electrodes for algae removal. J. Hazard. Mater. 2010, 182, 827–834. [Google Scholar] [CrossRef]
- Gao, S.; Yang, J.; Tian, J.; Ma, F.; Tu, G.; Du, M. Electro-coagulation-flotation process for algae removal. J. Hazard. Mater. 2010, 177, 336–343. [Google Scholar] [CrossRef]
- Chatsungnoen, T.; Chisti, Y. Flocculation and electroflocculation for algal biomass recovery. In Biofuels from Algae, 2nd ed.; Pandey, A., Chang, S., Soccol, C., Lee, D., Chisti, Y., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; Volume 1, pp. 257–286. [Google Scholar]
- Maffei, G.; Bracciale, M.P.; Broggi, A.; Zuorro, A.; Santarelli, M.L.; Lavecchia, R. Effect of an enzymatic treatment with cellulase and mannanase on the structural properties of Nannochloropsis microalgae. Bioresour. Technol. 2018, 249, 592–598. [Google Scholar] [CrossRef]
- Lavecchia, R.; Medici, F.; Patterer, M.S.; Zuorro, A. Lead removal from water by adsorption on spent coffee grounds. Chem. Eng. Trans. 2016, 47, 295–300. [Google Scholar]
- Fidaleo, M.; Zuorro, A.; Lavecchia, R. Antimicrobial activity of some italian honeys against pathogenic bacteria. Chem. Eng. Trans. 2011, 47, 295–300. [Google Scholar]
- Rahmani, A.; Zerrouki, D.; Djafer, L.; Ayral, A. Hydrogen recovery from the photovoltaic electroflocculation-flotation process for harvesting Chlorella pyrenoidosa microalgae. Int. J. Hyd. Energy 2017, 42, 19591–19596. [Google Scholar] [CrossRef]
- Baierle, F.; John, D.; Souza, M.; Bjerk, T.; Moraes, M.; Hoeltz, M.; Rohlfej, A.; Camargo, M.; Corbellini, V.; Schneider, R. Biomass from microalgae separation by electroflotation with iron and aluminum spiral electrodes. Chem. Eng. J. 2015, 267, 274–281. [Google Scholar] [CrossRef]
- Vandamme, D.; Pontes, S.C.V.; Goiris, K.; Foubert, I.; Pinoy, L.J.J.; Muylaert, K. Evaluation of electro-coagulation–flocculation for harvesting marine and freshwater microalgae. Biotechnol. Bioeng. 2011, 108, 2320–2329. [Google Scholar] [CrossRef] [Green Version]
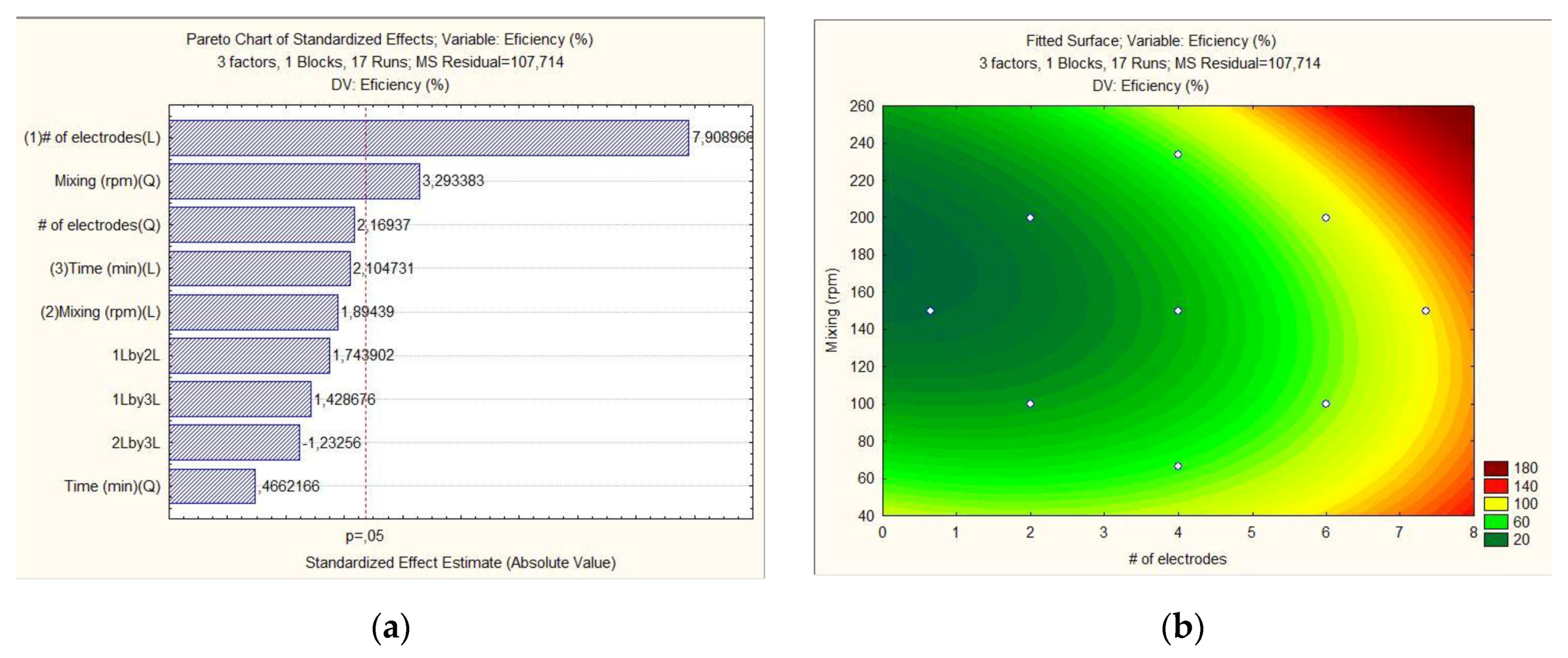
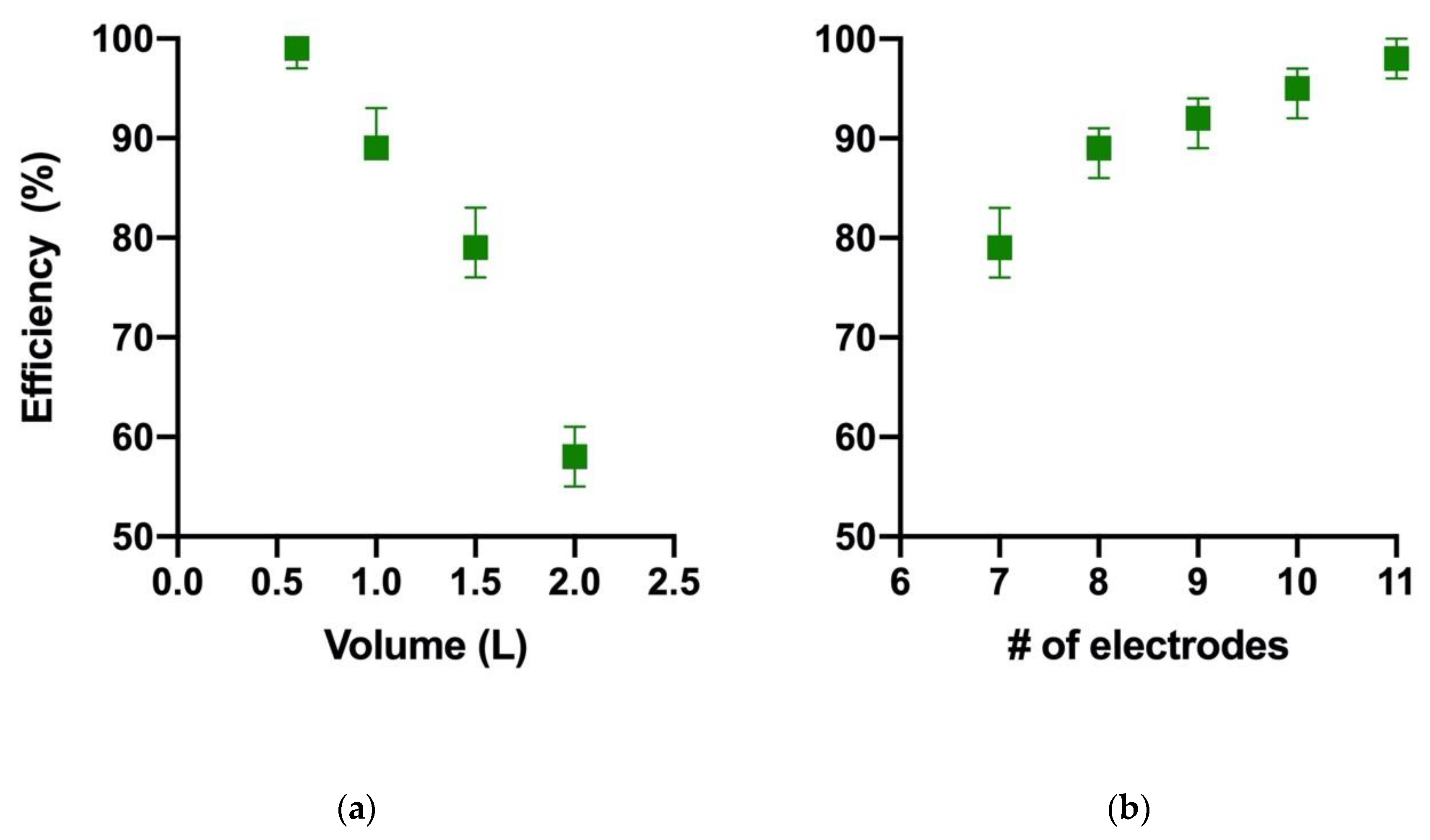

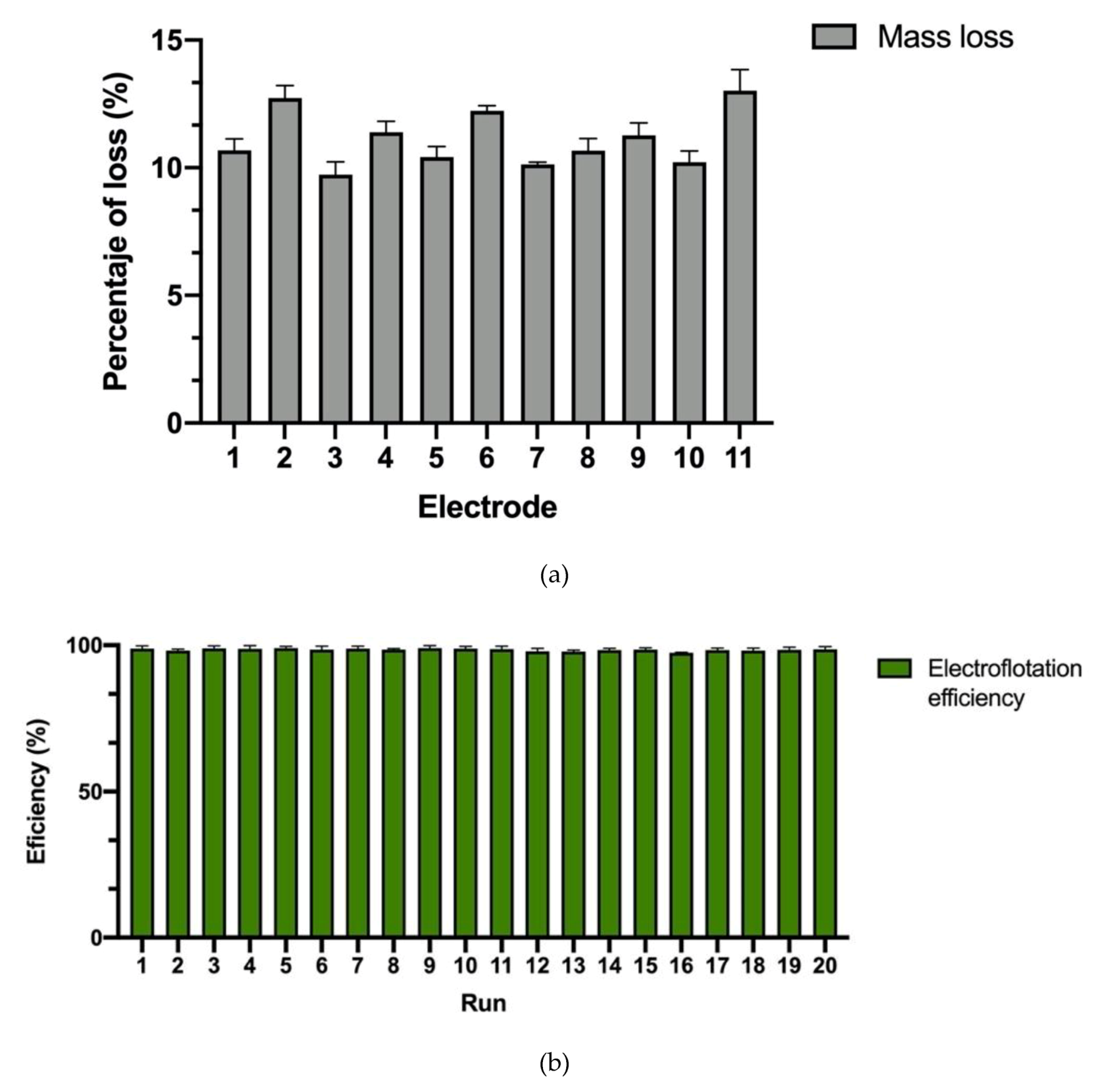
| Level | # of Electrodes | Mixing (rpm) | Time (min) |
|---|---|---|---|
| −1 | 2 | 100 | 10 |
| 0 | 4 | 150 | 15 |
| 1 | 6 | 200 | 20 |
| Experiment | # of Electrodes | Mixing (rpm) | Time (min) | Efficiency (%) | Temperature (°C) |
|---|---|---|---|---|---|
| 5 (C) | 4 | 150 | 15 | 32.07 | 35.27 |
| 1 | 2 | 100 | 10 | 33.67 | 32.72 |
| 16 | 4 | 150 | 23 | 38.71 | 43.55 |
| 3 | 6 | 100 | 20 | 81.27 | 35.60 |
| 15 | 4 | 150 | 6,6 | 15.48 | 28.61 |
| 8 | 6 | 100 | 10 | 48.85 | 29.21 |
| 13 | 4 | 66 | 15 | 38.55 | 31.53 |
| 17 (C) | 4 | 150 | 15 | 32.07 | 35.28 |
| 10 (C) | 4 | 150 | 15 | 32.07 | 35.28 |
| 12 | 7 | 150 | 15 | 83.76 | 36.22 |
| 11 | 1 | 150 | 15 | 0 | 25 |
| 4 | 6 | 200 | 10 | 79.83 | 28.39 |
| 2 | 2 | 200 | 20 | 28.13 | 58.54 |
| 9 | 6 | 200 | 20 | 89.92 | 31.77 |
| 7 | 2 | 200 | 10 | 34.79 | 42.20 |
| 14 | 4 | 234 | 15 | 64.99 | 29.86 |
| 6 | 2 | 100 | 20 | 40.64 | 36.67 |
| Strain | Electrode Material | Working Volume (L) | Biomass Concentration (g/L) | # of Electrodes | Mixing (rpm) | Time(min) | Efficiency (%) | Author |
|---|---|---|---|---|---|---|---|---|
| Chlorella sp. MJ 11/11 | Stainless steel | 0.4 | 1.5 | 2 | --- | 30 | 98 | [1] |
| Chlorella sp. 0217 | Graphite coupled with chitosan | 1 | 0.5 | 2 | 30 | 3 | 90 | [16] |
| Chlorella sp. (PTCC 6010) | Al | 1.28 | --- | 4 | 100 (high), 30 (low) | 1 (high), 15 (low) | 96.8 | [5] |
| Chlorella pyrenoidosa | Al, Zn, Cu, Fe. and non-sacrificial carbon electrode | 0.6 | 2.2 ± 0.15 | 3 (2 cathodes, 1 anode) | --- | 5 | 95.83 | [25] |
| Chlorella vulgaris | Al | 0.4 | --- | 2 | --- | 8 | 100 | [8] |
| Chlorella vulgaris UTEX 1803 | Al and Cu | 0.5 | 0.9 | 2 | 200 | 25 | 97–88 | [13] |
| Chlorella vulgaris | Al or Fe (Anode), and IrO2/TiO2 (cathode) | 1 | 0.5 | 2 | --- | 30 | 88 | [26] |
| Desmodesmus subspicatus | Al or Fe Spiral electrode | --- | --- | --- | --- | 20 | 95.4 | [27] |
| Dunaliella bardawil 30861 | Al coupled with sand | 0.2 | 0.88 | 2 | 150 | 3 | 97.16 | [3] |
| Dunaliella salina | Al | 0.3 | --- | 2 | 100 | 7 | 98.9 | [15] |
| Microcystis aeruginosa | Al | 1 | --- | 2 | 200 | 45 | 100 | [19] |
| Microcystis aeruginosa | Al and Fe | 1 | --- | 2 | 200 | 50 | 100 | [20] |
| Phaeodactylum tricornutum | Al or Fe (Anode), and IrO2/TiO2 (cathode) | 1 | 0.5 | 2 | --- | 20 | 85 | [26] |
| Scenedesmus sp. | Al and graphite | 0.34 | 0.25 | 2 | --- | 20 | 98.5–92 | [17] |
| Scenedesmus acuminatus | Mg, Al, Zn, Cu, Fe, and brass | 0.09 | --- | 2 | 100 | 7.3–30.9 | 90 | [18] |
| Scenedesmus obliquus FR751179.1 | Graphite mixed with Al2(SO4)3 | 0.9 | 2.4 | 3 (2 cathodes, 1 anode) | --- | 60 | 83 | [4] |
| Tetraselmis sp. | Al | 4.8 | --- | 2 | --- | 15 | --- | [6] |
| Scenedesmus sp. UFPS_002 | Al | 2 | 1.2 | 11 | 150 | 15 | 100 | This paper |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sanchez-Galvis, E.M.; Cardenas-Gutierrez, I.Y.; Contreras-Ropero, J.E.; García-Martínez, J.B.; Barajas-Solano, A.F.; Zuorro, A. An Innovative Low-Cost Equipment for Electro-Concentration of Microalgal Biomass. Appl. Sci. 2020, 10, 4841. https://doi.org/10.3390/app10144841
Sanchez-Galvis EM, Cardenas-Gutierrez IY, Contreras-Ropero JE, García-Martínez JB, Barajas-Solano AF, Zuorro A. An Innovative Low-Cost Equipment for Electro-Concentration of Microalgal Biomass. Applied Sciences. 2020; 10(14):4841. https://doi.org/10.3390/app10144841
Chicago/Turabian StyleSanchez-Galvis, Edwar M., Ingri Y. Cardenas-Gutierrez, Jefferson E. Contreras-Ropero, Janet B. García-Martínez, Andrés F. Barajas-Solano, and Antonio Zuorro. 2020. "An Innovative Low-Cost Equipment for Electro-Concentration of Microalgal Biomass" Applied Sciences 10, no. 14: 4841. https://doi.org/10.3390/app10144841
APA StyleSanchez-Galvis, E. M., Cardenas-Gutierrez, I. Y., Contreras-Ropero, J. E., García-Martínez, J. B., Barajas-Solano, A. F., & Zuorro, A. (2020). An Innovative Low-Cost Equipment for Electro-Concentration of Microalgal Biomass. Applied Sciences, 10(14), 4841. https://doi.org/10.3390/app10144841








