Featured Application
This paper features the design and construction of low-cost equipment for the harvesting of microalgal and cyanobacterial biomass under laboratory conditions.
Abstract
Microalgal harvesting is one of the most challenging processes in the development of algal research and development. Several methods, such as centrifugation, flocculation and filtration, are available at the laboratory scale. However, the requirement for expensive pieces of equipment and the possibility of biomass contamination are recurring gaps that hinder the development of microalgae R&D (research and development) in different parts of the world. Recently, electroflotation has been proved to be a suitable method for the harvesting of different species of microalgae and cyanobacteria. To this day, there are no companies that sell laboratory-scale electroflotation equipment; this is mainly due to the gap in the knowledge of which factors (time, mixing rate, number of electrodes and others) will affect the efficiency of concentration without reducing the biomass quality. This paper aims to build an innovative, low-cost electroflotation system for under 300 USD (United States dollar) with cheap and resistant materials. To achieve our goal, we tested the interaction of three variables (time, mixing rate and amount of electrodes). Results showed that an efficiency closer to 100% could be achieved in under 20 min using > 10 electrodes and 150 rpm (round per minute). We hope this innovative approach can be used by different researchers to improve our knowledge of the concentration and harvesting of algae and cyanobacteria.
1. Introduction
The R&D (research and development) on microalgal usage has expanded tremendously over the last two decades. From biofuels to nutraceuticals, this microorganism has led an industrial expansion of novel products for different markets worldwide. Microalgae cells are tiny, usually ranging from one to ten micrometers with a low specific gravity (1–1.1 g/L) [1], and when produced in large-scale reactors (such as open ponds or Photobioreactors), they tend to be highly diluted (on the order of 1 to 2 g/L) [2,3].
Given its nature, the most troublesome step of microalgae research and production is the concentration and dewatering of produced biomass. This process is a labor-intensive and time-intensive step, which separates the microalgal biomass from water for effective downstream processing [4]. Algae concentration and separation from the exhausted media demands large amounts of energy [5]; therefore, extended operation times are required to concentrate significant amounts of biomass.
In a broad view, in the last ten years, over 1100 research papers focused on algal production and metabolite extraction have been published. To the author’s knowledge, the vast majority of these papers used centrifuges for algae concentration. Other technologies for algal concentration, such as flocculation (auto-, bio- or microbial flocculation), flotation, filtration, etc., exist; however, flocs collected may contain a certain amount of flocculant (organic or inorganic), which in turn may contaminate the final biomass, thus reducing the suitability of produced biomass for some purposes [6].
In 2018, the National Alliance for Advanced Biofuels and Bioproducts (NAABB) in its final report [7] recommended that electroflotation was the most efficient and sustainable method for algae concentration and dewatering. There are three established electrolytic methods: electrocoagulation, electroflotation and electroflocculation. This physical/chemical process is founded on the principle of the movement of electrically charged particles in an electric field [4] and the in situ generation of flocculants during metal electrolysis [8].
Briefly, an electric current is applied to the solution between two electrodes; then, metal ions are released from the sacrificial anode through electrolytic oxidation. At the same time, oxygen and hydrogen microbubbles generated at the anode and cathode flow through the suspension [9]. Metal ions react with the pollutants, forming flocs, which in turn can be lifted to the surface by the microbubbles or sediment in the lower part of the reactor [6,10,11] and can be easily removed from the system’s surface [12].
In 2018 [13], we proposed an Arduino-based magnetic stirrer for the harvesting of biomass through electroflotation. In this study, we found that a short distance between electrodes, medium mixing rates (200 rpm (round per minute)) and 50 W could remove up to 100% of algal biomass from 500 mL of culture media. Other authors have studied potential variables such as voltage, pH, time, current intensity, electrode material, temperature and submerged area of electrodes. Each one of those experiments employed inexpensive materials and equipment found in most laboratories around the world (glass beakers, magnetic stirrer, power supply and lab stands) [1,3,4,5,6,8,13]. Despite its simplicity, there is no available equipment for the concentration and harvesting of algal biomass through electroflotation. This may occur because there is no consensus on which are the most critical variables for biomass concentration, which makes it challenging to build equipment that can be used for different species of both microalgae and cyanobacteria.
The aim of this project is to design and build efficient, low-cost (<300 USD (United States dollar)) electroflotation equipment for the concentration and dewatering of algal biomass. To achieve the above, the interaction of three key factors (number of electrodes, mixing rate and time) was employed.
2. Materials and Methods
2.1. Strain Culture
Scenedesmus sp. UFPS_002 was obtained for the INNOValgae collection (Universidad Francisco de Paula Santander, Cúcuta, Colombia). The strain was grown in 2000 mL tubular glass reactors with a culture volume of 1300 mL containing Bold Basal Medium [14]. The alga was mixed through the injection of air with 1% (v/v) CO2 at a flow rate of 0.78 L/min and a light:dark cycle of 12:12 h at 120 µmol/(m2s).
2.2. Response-Surface Methodology for Variable Evaluation
The interaction between three critical variables in the process was evaluated using a 33 (3 factors, 3 levels) nonfactorial response-surface design with two central points on STATISTICA 7.0 software (7.0, Statsoft, Tulsa, OK, United States, 2004) (Table 1).

Table 1.
Variables for electroflotation of algal biomass.
Each of the experiments was performed using 300 mL of 30-day-old algae culture in a 600 mL beaker. Aluminum electrodes (13 cm long, 5 cm wide), with a distance of 5 mm between electrodes and an electric current of 50 W (50 V, 1 amp), were employed. To avoid deviations by electrode degradation, every single experiment was performed using new electrodes. All the samples were mixed using an Arduino-based magnetic stirrer described by [13].
The efficiency of cell concentration was determined by optical density (550 nm) of the culture. Each experiment was measured five times (original and four replicates). The efficiency was obtained by replacing the values obtained in Equation (1).
Once the factors affecting the process were obtained, the stability of the method was evaluated by increasing the reaction volume to 2000 mL. In this stage, the electrodes were reused up to 20 cycles.
At the end of every cycle, the electrodes were washed with deionized water, dried in an oven (100 °C, 24 h) and stored in a desiccator until a constant weight was obtained. After the process, the weight of each electrode was recorded, and it was used again until 20 cycles were achieved.
From the results, a system was designed to fit the needs of a microalgae-culture laboratory. The following parameters were taken into account:
- maximum efficiency of cell concentration.
- minimum working volume of 500 mL and maximum of 2000 mL.
- Easy cleaning and maintenance.
- Final cost of less than 300 USD.
3. Results
From all the experiments, it was possible to retrieve the concentrated biomass. However, there were significant differences between the experiments. Table 2 presents the results for biomass concentration and temperature of the media. There was a considerable increase in temperature (>35 °C) in those experiments with efficiencies below 40%. This increase in the temperature of the medium can have adverse effects on the stability and quality of the biomass. However, Test 5 showed efficiency values below 20%, but with a temperature below 30 °C; this is due to the short time of exposure to the process in the experiment (6.6 min). According to [15], the distance between electrodes affects the overall energy consumption in the process. Our findings showed that not only the distance but the number of electrodes used could increase energy consumption (fewer electrodes, higher energy consumption).

Table 2.
The efficiency of algae concentration and temperature of media.
The experimental data concerning the number of electrodes, mixing rate and time on the concentration efficiency of the alga was fitted to two different models: linear (L) and quadratic (Q). The Pareto analysis (Figure 1a) illustrates that (p = 0.05) the number of electrodes and agitation were the variables that most affected the concentration process. These results are consistent with the results presented by [16], where they demonstrated that the number of electrodes and agitation were critical variables for increasing efficiency since they allowed for increasing the active area of contact with the media and decreasing the electrical consumption.
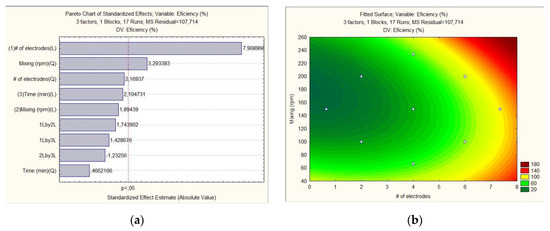
Figure 1.
Pareto analysis of variables (a) and surface response between the most critical variables, mixing and number of electrodes (b).
By analyzing the interaction between mixing and the number of electrodes (Figure 1b), Equation (2) was obtained, where X is the number of electrodes and Y is the mixing. These operating conditions are consistent with the results obtained by [16], where mixing had a direct relationship with the time of concentration since at speeds between 150 and 210 rpm, the biomass is aggregated in less time, with an efficiency of 90%. However, they recommended the use of agitation close to 150 rpm to save energy and maintain higher efficiencies.
The verification of the proposed operating conditions for the efficient concentration of the biomass was tested using different volumes of culture media (0.6, 1, 1.5 and 2 L). Results are presented in Figure 2a.
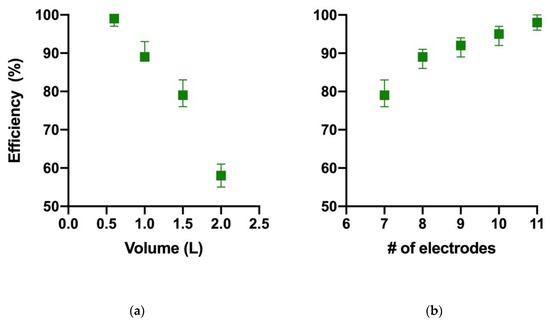
Figure 2.
Efficiency of concentration using different volumes of algae culture (a) and different numbers of electrodes (b) at 150 rpm and 6 electrodes.
As the volume of algae increased, the efficiency was substantially reduced. Another factor that can affect this is the geometry of the reactor in which the experiments were carried out. However, neither of these two variables (volume and geometry) has been previously reported in the literature.
Loss of efficiency due to increased volume is a problem that can hamper the viability of electroflotation for algae harvesting. Therefore, to demonstrate its stability, we carried out a couple of experiments, increasing the number of electrodes (with volume constant at 2 L) until obtaining the desired efficiency. Figure 2b shows the results for these experiments.
The verification of the proposed operating conditions for the efficient concentration of the biomass was tested.
The new operating conditions (11 electrodes, 150 rpm, 20 min) were tested, and a piece of innovative equipment was designed and built for the project. This new equipment has a built-in, Arduino-based magnetic stirrer at the bottom, with a working volume of 2L (Figure 3). The design of each section, the size of the electrodes and the electrical blueprint of the magnetic stirrer can be found in Appendix A, Appendix B, Appendix C. The stability of the electrodes was evaluated during 20 concentration cycles using 2 L of Scenedesmus sp.

Figure 3.
The concentration of biomass using the new equipment for electroflotation.
After 15 min, the process reached an efficiency of 100%, with no increase in the temperature of the media (Figure 3). This process was repeated 20 times. According to the results presented in Figure 4, it was possible to determine that, after 20 cycles, the electrodes could lose up to 15% (w/w) of their mass. These results allowed for inferring that, on average, each electrode could lose 0.57% (w/w) of its weight for each liter processed. However, the efficiency of each of the cycles was above 95% (Figure 4), with no reduction in its effectiveness. The above demonstrates that electroflotation is a stable, repeatable process and that the electrodes can withstand several cycles without the need to replace them in short periods. This equipment had a cost of 260 USD. All the parts and where to buy them can be found in Appendix D.
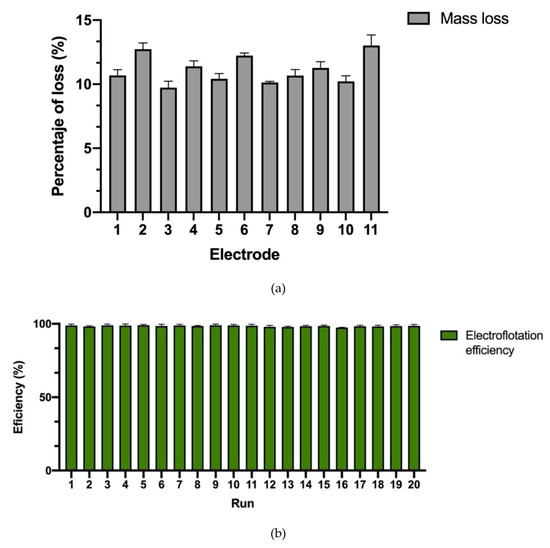
Figure 4.
Mass loss of the electrodes (a) and efficiency of electroflotation by the reuse of electrodes (b).
4. Discussion
For the proper concentration of algal biomass, mixing of the media is required to improve cell–electrode interaction, which in turn increases the floc formation [16]. Other authors, such as [17], found that this variable did not affect the efficiency of algal harvesting. In the present work, we found that mixing affected the concentration of algal biomass positively, without a high increase in temperature. The only variable that did not affect it (either positively or negatively) was time. However, longer times (>30 min) could eventually increase energy consumption.
Through the development of this work, an electrical current of 50 W was used. [16,18,19,20] found that at a higher voltage (>30 V), the time was reduced (<10 min). This occurs due to an increase in the number of free ions from the sacrificial electrode, which accelerates the shift of cell-surface charges, allowing for faster flocculation.
Another variable that directly affected the efficiency was the distance between the electrodes. All the experiments performed employed a length of 5 mm, which, according to [16], is an optimal distance to increase the efficiency of the process and reduce the time. According to [21], distances less than 10 mm affect the formation of gas bubbles around the electrode, and more considerable distances can negatively affect the overall efficiency and increase energy consumption. Optimization by Response Surface Methodology can lead to the best results [22,23,24]
Another crucial factor is the electrode material. Table 3 presents a short review of different materials employed for the harvesting of algae and cyanobacteria. The most common material is aluminum. Other materials, such as graphite, require the addition of electrolytes or chemical flocculants, such as Al2(SO4)3 or chitosan, to achieve higher efficiencies (>92%) [4,16].

Table 3.
Electrode materials evaluated for the harvesting of microalgae and cyanobacteria.
5. Conclusions
The present work explored the interaction between critical variables (mixing, number of electrodes and time) for the efficient concentration and harvesting of microalgal biomass through an electroflotation process. Results showed that time could be significantly reduced (from 30 to 15 min) as long as the ten or more electrodes were active and medium mixing rates (150 rpm) were used. From the data, the innovative equipment had a lower-medium cost (260 USD), with cheap and resistant materials that anyone can build. This new configuration proves that the electrodes can be reused several times, which in turn reduces the cost of the concentration for up to 2 L of algal suspension with a concentration of 1.1 g/L of algal biomass. We hope this innovative approach can be used by different researchers to improve our knowledge of the concentration and harvesting of algae and cyanobacteria.
Author Contributions
Conceptualization, J.E.C.-R., I.Y.C.-G. and A.Z.; data curation, A.F.B.-S.; formal analysis, J.B.G.-M. and A.Z.; funding acquisition, E.M.S.-G. and A.F.B.-S.; investigation, J.E.C.-R., I.Y.C.-G. and E.M.S.-G.; methodology, J.B.G.-M., A.F.B.-S. and A.Z.; project administration, A.F.B-S.; resources, J.E.C.-R., I.Y.C.-G. and E.M.S.-G.; software, J.B.G.-M. and E.M.S.-G.; supervision, A.F.B.-S. and A.Z.; validation, J.E.C.-R. and I.Y.C.-G.; visualization, A.F.B.-S. and A.Z.; writing—original draft, A.F.B.-S. and A.Z.; writing—review and editing, J.B.G.-M., A.F.B.-S. and A.Z. All authors have read and agreed to the published version of the manuscript.
Funding
The Gen Foundation funded this research with the “Isolation of thermotolerant algae as a novel source of food colorants” project, as did the Universidad Francisco de Paula Santander Internal Research funding (FINU 27-2019). The Universidad de Santander (UDES) funded the APC.
Acknowledgments
We would like to express our sincere gratitude to Eng. M.A. Castellanos-Estupiñan for the design and construction of aluminum electrodes; to the Universidad de Santander (UDES) for covering the expenses of scientific editing and the APC; to the Universidad Francisco de Paula Santander for providing the equipment for this research; and to the Colombian Ministry of Science, Technology and Innovation (MINCIENCIAS) for the support for national PhD doctorates through the Francisco José de Caldas scholarship program and the young researchers’ “Jovenes Investigadores e Innovadores” scholarship program.
Conflicts of Interest
The authors declare no conflict of interest.
Appendix A
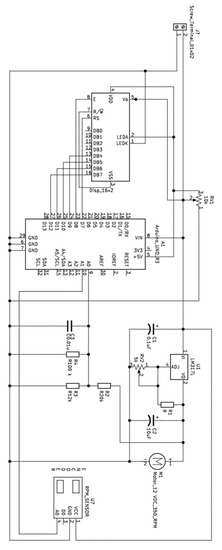
Figure A1.
Magnetic Stirrer with Speed Control.
Appendix B

Figure A2.
Electrode Design. Note: Each electrode was built using 0.1 cm thick aluminum plates.
Appendix C
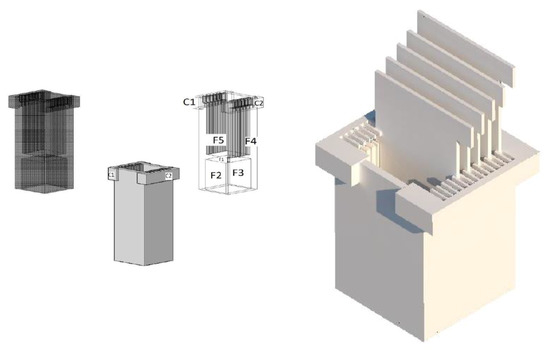
Figure A3.
Assembly of the Equipment with the Electrodes.
Appendix D

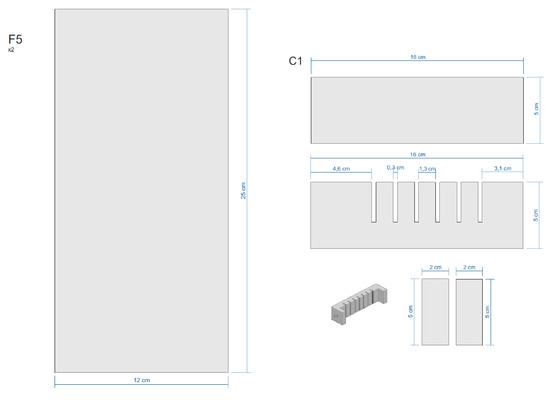
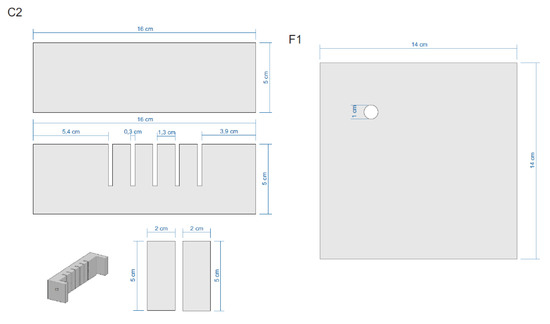
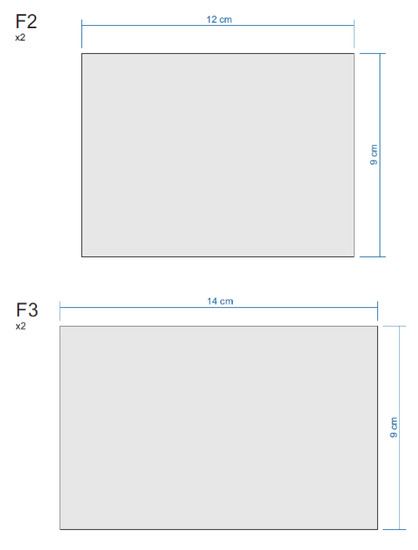
Figure A4.
Sections of the Equipment.
Appendix E

Table A1.
Cost and Source of Materials for the Construction of the Equipment.
Table A1.
Cost and Source of Materials for the Construction of the Equipment.
| Reference Description | Article | Quantity | Unit Cost (USD) | Total Price (USD) | Source of Materials | |||
|---|---|---|---|---|---|---|---|---|
| Electroflotation | Acrylic | 1.5 | m² | 55.84 | m² | 83.76 | Local supplier | |
| Wire caliber18 | 2 | m | 1.01 | m | 2.02 | Local supplier | ||
| Connectors banana plug | 2 | ud | 0.30 | ud | 0.60 | Local supplier | ||
| Cutting and assembly | 34.72 | 34.72 | Local supplier | |||||
| Subtotal | 121.10 | |||||||
| mixing | Arduino® NANO | 1 | ud | 3.47 | ud | 3.47 | shorturl.at/hEU09 | |
| LCD 16 × 2 | 1 | ud | 6.99 | ud | 6.99 | shorturl.at/gyCYZ | ||
| Neodymium magnet | 2 | ud | 8.99 | ud | 8.99 | shorturl.at/yzBLM | ||
| L298N | 1 | ud | 3.76 | ud | 3.76 | shorturl.at/npRX9 | ||
| Potentiometer 10k | 1 | ud | 8.99 | ud | 8.99 | shorturl.at/afryO | ||
| Motor Mh7 300 RPMs | 1 | ud | 15.99 | ud | 15.99 | shorturl.at/jvBJO | ||
| Subtotal | 48.19 | |||||||
| Accessories | Magnetic stir bar | 1 | ud | 3.47 | ud | 3.47 | Local supplier | |
| Aluminum sheets | 0.3234 | m2 | 33.27 | m2 | 10.75 | Local supplier | ||
| Aluminum sheets cut | 11 | ud | 0.10 | ud | 1.10 | Local supplier | ||
| 0–50 V Power Supply Stabilizer Module 15 A 750 W | 1 | ud | 48.03 | ud | 75.00 | shorturl.at/GOT27 | ||
| Subtotal | 90.32 | |||||||
| TOTAL | 259.61 USD | |||||||
References
- Lal, A.; Ghosh, S.; Das, D. Improvement in electrically induced biomass harvesting of Chlorella sp. MJ 11/11 for bulk biomass production. J. Appl. Phycol. 2018, 30, 979–993. [Google Scholar] [CrossRef]
- Ummalyma, S.B.; Mathew, A.K.; Pandey, A.; Sukumaran, R.K. Harvesting of microalgal biomass: Efficient method for flocculation through pH modulation. Bioresour. Technol. 2016, 213, 216–221. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Q.; Pang, Q.; Pan, X.; Chika, A.O.; Wang, L.; Shi, J.; Jia, L.; Chen, C.; Gao, Y. Facile sand enhanced electro-flocculation for cost-efficient harvesting of Dunaliella salina. Bioresour. Technol. 2015, 187, 326–330. [Google Scholar] [CrossRef]
- Misra, R.; Guldhe, A.; Singh, P.; Rawat, I.; Stenström, T.A.; Bux, F. Evaluation of operating conditions for sustainable harvesting of microalgal biomass applying electrochemical method using non sacrificial electrodes. Bioresour. Technol. 2015, 176, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Golzary, A.; Imanian, S.; Abdoli, M.A.; Khodadadi, A.; Karbassi, A. A cost-effective strategy for marine microalgae separation by electro-coagulation-flotation process aimed at bio-crude oil production: Optimization and evaluation study. Sep. Purif. Tech. 2015, 147, 156–165. [Google Scholar] [CrossRef]
- Lee, A.K.; Lewis, D.M.; Ashman, P.J. Harvesting of marine microalgae by electroflocculation: The energetics, plant design, and economics. Appl. Energy 2013, 108, 45–53. [Google Scholar] [CrossRef]
- Marrone, B.L.; Lacey, R.E.; Anderson, D.B.; Bonner, J.; Coons, J.; Dale, T.; Downes, C.M.; Fernando, S.; Fuller, C.; Goodall, B.; et al. Review of the harvesting and extraction program within the National Alliance for Advanced Biofuels and Bioproducts. Algal. Res. 2017, 33, 470–485. [Google Scholar] [CrossRef]
- Shi, W.; Zhu, L.; Chen, Q.; Lu, J.; Pan, G.; Hu, L.; Yi, Q. Synergy of flocculation and flotation for microalgae harvesting using aluminium electrolysis. Bioresour. Technol. 2017, 233, 127–133. [Google Scholar] [CrossRef] [PubMed]
- Đukić, A.; Firak, M. Hydrogen production using alkaline electrolyzer and photovoltaic (PV) module. Int. J. Hydrog. Energy 2011, 36, 7799–7806. [Google Scholar] [CrossRef]
- Mollaha, M.Y.A.; Morkovsky, P.; Gomes, J.A.G.; Kesmez, M.; Parga, J.; Cocke, D.L. Fundamentals, present and future perspectives of electrocoagulation. J. Hazard. Mater. 2004, 114, 199–210. [Google Scholar] [CrossRef]
- Emamjomeh, M.M.; Sivakumar, M. An empirical model for defluoridation by batch monopolar electrocoagulation/flotation (ECF) process. J. Hazard. Mater. 2006, 131, 118–125. [Google Scholar] [CrossRef]
- Gamage, N.P.; Rimer, J.D.; Chellam, S. Improvements in permeate flux by aluminum electroflotation pretreatment during microfiltration of surface water. J. Membr. Sci. 2012, 411–412, 45–53. [Google Scholar] [CrossRef]
- Castellaños-Estupiñan, M.A.; Sánchez-Galvis, E.M.; García-Martínez, J.B.; Barajas-Ferreira, C.; Zuorro, A.; Barajas-Solano, A.F. Design of an electroflotation system for the concentration and harvesting of freshwater microalgae. Chem. Eng. Trans. 2018, 64, 1–6. [Google Scholar]
- Andersen, R.A.; Berges, J.A.; Harrison, P.J.; Watanabe, M.M. Appendix A—Recipes for Freshwater and Seawater Media. In Algal Culturing Techniques; Andersen, R.A., Ed.; Elsevier Academic Press: Burlington, MA, USA, 2005; pp. 429–538. [Google Scholar]
- Zenouzi, A.; Ghobadian, B.; Hejazi, M.A.; Rahnemoon, P. Harvesting of microalgae Dunaliella salina using electroflocculation. J. Agric. Sci. Technol. 2013, 15, 879–888. [Google Scholar]
- Zhou, W.; Gao, L.; Cheng, W.; Chen, L.; Wang, J.; Wang, H.; Zhang, W.; Liu, T. Electro-flotation of Chlorella sp. assisted with flocculation by chitosan. Algal. Res. 2016, 18, 7–14. [Google Scholar] [CrossRef]
- Liu, S.; Abu Hajar, H.A.; Riefler, G.; Stuart, B.J. Investigation of electrolytic flocculation for microalga: Scenedesmus sp. using aluminum and graphite electrodes. RSC. Adv. 2018, 8, 38808–38817. [Google Scholar] [CrossRef]
- Bleeke, F.; Quante, G.; Winckelmann, D.; Klöck, G. Effect of voltage and electrode material on electroflocculation of Scenedesmus acuminatus. Bioresour. Bioprocess. 2015, 2, 1–8. [Google Scholar] [CrossRef]
- Gao, S.; Du, M.; Tian, J.; Yang, J.; Yang, J.; Ma, F.; Nan, J. Effects of chloride ions on electro-coagulation-flotation process with aluminum electrodes for algae removal. J. Hazard. Mater. 2010, 182, 827–834. [Google Scholar] [CrossRef]
- Gao, S.; Yang, J.; Tian, J.; Ma, F.; Tu, G.; Du, M. Electro-coagulation-flotation process for algae removal. J. Hazard. Mater. 2010, 177, 336–343. [Google Scholar] [CrossRef]
- Chatsungnoen, T.; Chisti, Y. Flocculation and electroflocculation for algal biomass recovery. In Biofuels from Algae, 2nd ed.; Pandey, A., Chang, S., Soccol, C., Lee, D., Chisti, Y., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; Volume 1, pp. 257–286. [Google Scholar]
- Maffei, G.; Bracciale, M.P.; Broggi, A.; Zuorro, A.; Santarelli, M.L.; Lavecchia, R. Effect of an enzymatic treatment with cellulase and mannanase on the structural properties of Nannochloropsis microalgae. Bioresour. Technol. 2018, 249, 592–598. [Google Scholar] [CrossRef]
- Lavecchia, R.; Medici, F.; Patterer, M.S.; Zuorro, A. Lead removal from water by adsorption on spent coffee grounds. Chem. Eng. Trans. 2016, 47, 295–300. [Google Scholar]
- Fidaleo, M.; Zuorro, A.; Lavecchia, R. Antimicrobial activity of some italian honeys against pathogenic bacteria. Chem. Eng. Trans. 2011, 47, 295–300. [Google Scholar]
- Rahmani, A.; Zerrouki, D.; Djafer, L.; Ayral, A. Hydrogen recovery from the photovoltaic electroflocculation-flotation process for harvesting Chlorella pyrenoidosa microalgae. Int. J. Hyd. Energy 2017, 42, 19591–19596. [Google Scholar] [CrossRef]
- Baierle, F.; John, D.; Souza, M.; Bjerk, T.; Moraes, M.; Hoeltz, M.; Rohlfej, A.; Camargo, M.; Corbellini, V.; Schneider, R. Biomass from microalgae separation by electroflotation with iron and aluminum spiral electrodes. Chem. Eng. J. 2015, 267, 274–281. [Google Scholar] [CrossRef]
- Vandamme, D.; Pontes, S.C.V.; Goiris, K.; Foubert, I.; Pinoy, L.J.J.; Muylaert, K. Evaluation of electro-coagulation–flocculation for harvesting marine and freshwater microalgae. Biotechnol. Bioeng. 2011, 108, 2320–2329. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).