The Effect of Extended Ball-Milling upon Three-Dimensional and Two-Dimensional Perovskite Crystals Properties
Abstract
1. Introduction
2. Materials and Methods
2.1. Synthesis of Perovskites
2.2. High-Energy Ball-Milling Processes
2.3. Samples Characterization
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Grancini, G.; Nazeeruddin, M.K. Dimensional tailoring of hybrid perovskites for photovoltaics. Nat. Rev. Mater. 2019, 4, 4–22. [Google Scholar] [CrossRef]
- Fu, Y.; Zhu, H.; Schrader, A.W.; Liang, D.; Ding, Q.; Joshi, P.; Hwang, L.; Zhu, X.Y.; Jin, S. Nanowire Lasers of Formamidinium Lead Halide Perovskites and Their Stabilized Alloys with Improved Stability. Nano Lett. 2016, 16, 1000–1008. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Mei, J.; Wang, Y.; Zhang, L.; Zhao, H.; Zhao, D. Fast Photoconductive Responses in Organometal Halide Perovskite Photodetectors. ACS Appl. Mater. Interfaces 2016, 8, 2840–2846. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.; Han, J.S.; Hong, K.; Kim, S.Y.; Jang, H.W. Organic–Inorganic Hybrid Halide Perovskites for Memories, Transistors, and Artificial Synapses. Adv. Mater. 2018, 30, 1–21. [Google Scholar] [CrossRef]
- Zuo, T.; He, X.; Hu, P.; Jiang, H. Organic-Inorganic Hybrid Perovskite Single Crystals: Crystallization, Molecular Structures, and Bandgap Engineering. ChemNanoMat 2019, 5, 278–289. [Google Scholar] [CrossRef]
- Colella, S.; Mazzeo, M.; Rizzo, A.; Gigli, G.; Listorti, A. The Bright Side of Perovskites. J. Phys. Chem. Lett. 2016, 7, 4322–4334. [Google Scholar] [CrossRef]
- Anni, M.; Cretì, A.; Zhang, Y.; De Giorgi, M.L.; Lomascolo, M. Investigation of the role of the environment on the photoluminescence and the exciton relaxation of CsPbBr3 nanocrystals thin films. Appl. Sci. 2020, 10, 2148. [Google Scholar] [CrossRef]
- Huang, Y.; Li, L.; Liu, Z.; Jiao, H.; He, Y.; Wang, X.; Zhu, R.; Wang, D.; Sun, J.; Chen, Q.; et al. The intrinsic properties of FA(1-:X)MAxPbI3 perovskite single crystals. J. Mater. Chem. A 2017, 5, 8537–8544. [Google Scholar] [CrossRef]
- Cheng, X.; Yang, S.; Cao, B.; Tao, X.; Chen, Z. Single Crystal Perovskite Solar Cells: Development and Perspectives. Adv. Funct. Mater. 2020, 30, 1–20. [Google Scholar] [CrossRef]
- Giuri, A.; Masi, S.; Listorti, A.; Gigli, G.; Colella, S.; Esposito Corcione, C.; Rizzo, A. Polymeric rheology modifier allows single-step coating of perovskite ink for highly efficient and stable solar cells. Nano Energy 2018, 54, 400–408. [Google Scholar] [CrossRef]
- Yamada, Y.; Yamada, T.; Phuong, L.Q.; Maruyama, N.; Nishimura, H.; Wakamiya, A.; Murata, Y.; Kanemitsu, Y. Dynamic Optical Properties of CH3NH3PbI3 Single Crystals As Revealed by One- and Two-Photon Excited Photoluminescence Measurements. J. Am. Chem. Soc. 2015, 137, 10456–10459. [Google Scholar] [CrossRef] [PubMed]
- Ding, J.; Yan, Q. Organic-Inorganic Hybrid Halide; Perovskite. Single 2017, 60, 1063–1078. [Google Scholar]
- Yamada, T.; Yamada, Y.; Kanemitsu, Y. Photon recycling in perovskite CH3NH3PbX3 (X = I, Br, Cl) bulk single crystals and polycrystalline films. J. Lumin. 2020, 220, 116987. [Google Scholar] [CrossRef]
- Masi, S.; Sestu, N.; Valenzano, V.; Higashino, T.; Imahori, H.; Saba, M.; Bongiovanni, G.; Armenise, V.; Milella, A.; Gigli, G.; et al. Simple Processing Additive-Driven 20% Efficiency for Inverted Planar Heterojunction Perovskite Solar Cells. ACS Appl. Mater. Interfaces 2020, 12, 18431–18436. [Google Scholar] [CrossRef]
- Yang, J.; Chen, S.; Xu, J.; Zhang, Q.; Liu, H.; Liu, Z.; Yuan, M. A review on improving the quality of Perovskite Films in Perovskite Solar Cells via the weak forces induced by additives. Appl. Sci. 2019, 9, 4393. [Google Scholar] [CrossRef]
- Shen, H.; Nan, R.; Jian, Z.; Li, X. Defect step controlled growth of perovskite MAPbBr3 single crystal. J. Mater. Sci. 2019, 54, 11596–11603. [Google Scholar] [CrossRef]
- Wang, Y.L.; Chang, S.; Chen, X.M.; Ren, Y.D.; Shi, L.F.; Liu, Y.H.; Zhong, H.Z. Rapid Growth of Halide Perovskite Single Crystals: From Methods to Optimization Control. Chin. J. Chem. 2019, 37, 616–629. [Google Scholar] [CrossRef]
- Pan, W.; Wei, H.; Yang, B. Development of Halide Perovskite Single Crystal for Radiation Detection Applications. Front. Chem. 2020, 8, 1–9. [Google Scholar] [CrossRef]
- Alberti, A.; Deretzis, I.; Mannino, G.; Smecca, E.; Giannazzo, F.; Listorti, A.; Colella, S.; Masi, S.; La Magna, A. Nitrogen Soaking Promotes Lattice Recovery in Polycrystalline Hybrid Perovskites. Adv. Energy Mater. 2019, 9, 1–12. [Google Scholar] [CrossRef]
- Chen, L.; Tan, Y.Y.; Chen, Z.X.; Wang, T.; Hu, S.; Nan, Z.A.; Xie, L.Q.; Hui, Y.; Huang, J.X.; Zhan, C.; et al. Toward Long-Term Stability: Single-Crystal Alloys of Cesium-Containing Mixed Cation and Mixed Halide Perovskite. J. Am. Chem. Soc. 2019, 141, 1665–1671. [Google Scholar] [CrossRef]
- Kim, H.; Byun, H.R.; Jeong, M.S. Synthesis and Characterization of Multiple-Cation Rb(MAFA)PbI 3 Perovskite Single Crystals. Sci. Rep. 2019, 9, 1–7. [Google Scholar] [CrossRef]
- Rong, S.; Xiao, Y.; Jiang, J.; Zeng, Q.; Li, Y. Strongly Enhanced Photoluminescence and Photoconductivity in Erbium-Doped MAPbBr 3 Single Crystals. J. Phys. Chem. C 2020, 124, 8992–8998. [Google Scholar] [CrossRef]
- Wu, C.; Guo, D.; Li, P.; Wang, S.; Liu, A.; Wu, F. A study on the effects of mixed organic cations on the structure and properties in lead halide perovskites. Phys. Chem. Chem. Phys. 2020, 22, 3105–3111. [Google Scholar] [CrossRef]
- Pisanu, A.; Coduri, M.; Morana, M.; Ciftci, Y.O.; Rizzo, A.; Listorti, A.; Gaboardi, M.; Bindi, L.; Queloz, V.I.E.; Milanese, C.; et al. Exploring the role of halide mixing in lead-free BZA2SnX4 two dimensional hybrid perovskites. J. Mater. Chem. A 2020, 8, 1875–1886. [Google Scholar] [CrossRef]
- Wang, C.; Ecker, B.R.; Wei, H.; Huang, J.; Gao, Y. Environmental Surface Stability of the MAPbBr3 Single Crystal. J. Phys. Chem. C 2018, 122, 3513–3522. [Google Scholar] [CrossRef]
- Chen, C.; Hu, X.; Lu, W.; Chang, S.; Shi, L.; Li, L.; Zhong, H.; Han, J.B. Elucidating the phase transitions and temperature-dependent photoluminescence of MAPbBr3 single crystal. J. Phys. D. Appl. Phys. 2018, 51. [Google Scholar] [CrossRef]
- Dimesso, L.; Wittich, C.; Mayer, T.; Jaegermann, W. Phase-change behavior of hot-pressed methylammonium lead bromide hybrid perovskites. J. Mater. Sci. 2019, 54, 2001–2015. [Google Scholar] [CrossRef]
- Jaffe, A.; Lin, Y.; Beavers, C.M.; Voss, J.; Mao, W.L.; Karunadasa, H.I. High-pressure single-crystal structures of 3D lead-halide hybrid perovskites and pressure effects on their electronic and optical properties. ACS Cent. Sci. 2016, 2, 201–209. [Google Scholar] [CrossRef]
- Sun, S.; Deng, Z.; Wu, Y.; Wei, F.; Halis Isikgor, F.; Brivio, F.; Gaultois, M.W.; Ouyang, J.; Bristowe, P.D.; Cheetham, A.K.; et al. Variable temperature and high-pressure crystal chemistry of perovskite formamidinium lead iodide: A single crystal X-ray diffraction and computational study. Chem. Commun. 2017, 53, 7537–7540. [Google Scholar] [CrossRef]
- McClintock, L.; Xiao, R.; Hou, Y.; Gibson, C.; Travaglini, H.C.; Abramovitch, D.; Tan, L.Z.; Senger, R.T.; Fu, Y.; Jin, S.; et al. Temperature and Gate Dependence of Carrier Diffusion in Single Crystal Methylammonium Lead Iodide Perovskite Microstructures. J. Phys. Chem. Lett. 2020, 11, 1000–1006. [Google Scholar] [CrossRef]
- Huang, W.; Yue, S.; Liu, Y.; Zhu, L.; Jin, P.; Wu, Q.; Zhang, Y.; Chen, Y.; Liu, K.; Liang, P.; et al. Observation of Unusual Optical Band Structure of CH3NH3PbI3 Perovskite Single Crystal. ACS Photonics 2018, 5, 1583–1590. [Google Scholar] [CrossRef]
- Liu, C.; Li, Z.; Yang, L.; Yao, X.; Li, H.; Liu, X.; Zhao, Y.; Zhu, P.; Cui, T.; Sun, C.; et al. Optical Behaviors of a Microsized Single-Crystal MAPbI3 Plate under High Pressure. J. Phys. Chem. C 2019. [Google Scholar] [CrossRef]
- Bonomi, S.; Tredici, I.; Albini, B.; Galinetto, P.; Rizzo, A.; Listorti, A.; Tamburini, U.A.; Malavasi, L. Ambient condition retention of band-gap tuning in MAPbI3 induced by high pressure quenching. Chem. Commun. 2018, 54, 13212–13215. [Google Scholar] [CrossRef] [PubMed]
- Colella, S.; Todaro, M.; Masi, S.; Listorti, A.; Altamura, D.; Caliandro, R.; Giannini, C.; Carignani, E.; Geppi, M.; Meggiolaro, D.; et al. Light-Induced Formation of Pb3+ Paramagnetic Species in Lead Halide Perovskites. ACS Energy Lett. 2018, 3, 1840–1847. [Google Scholar] [CrossRef]
- Elseman, A.M.; Rashad, M.M.; Hassan, A.M. Easily Attainable, Efficient Solar Cell with Mass Yield of Nanorod Single-Crystalline Organo-Metal Halide Perovskite Based on a Ball Milling Technique. ACS Sustain. Chem. Eng. 2016, 4, 4875–4886. [Google Scholar] [CrossRef]
- Protesescu, L.; Yakunin, S.; Nazarenko, O.; Dirin, D.N.; Kovalenko, M.V. Low-Cost Synthesis of Highly Luminescent Colloidal Lead Halide Perovskite Nanocrystals by Wet Ball Milling. ACS Appl. Nano Mater. 2018, 1, 1300–1308. [Google Scholar] [CrossRef]
- Zhang, L.; Xu, Z.; Cao, L.; Yao, X. Synthesis of BF-PT perovskite powders by high-energy ball milling. Mater. Lett. 2007, 61, 1130–1133. [Google Scholar] [CrossRef]
- Manukyan, K.V.; Yeghishyan, A.V.; Moskovskikh, D.O.; Kapaldo, J.; Mintairov, A.; Mukasyan, A.S. Mechanochemical synthesis of methylammonium lead iodide perovskite. J. Mater. Sci. 2016, 51, 9123–9130. [Google Scholar] [CrossRef]
- Jodlowski, A.D.; Yépez, A.; Luque, R.; Camacho, L.; de Miguel, G. Benign-by-Design Solventless Mechanochemical Synthesis of Three-, Two-, and One-Dimensional Hybrid Perovskites. Angew. Chem. Int. Ed. 2016, 55, 14972–14977. [Google Scholar] [CrossRef]
- Palazon, F.; El Ajjouri, Y.; Bolink, H.J. Making by Grinding: Mechanochemistry Boosts the Development of Halide Perovskites and Other Multinary Metal Halides. Adv. Energy Mater. 2019, 1902499, 1–13. [Google Scholar] [CrossRef]
- Rashad, M.M.; Elseman, A.M.; Hassan, A.M. Facile synthesis, characterization and structural evolution of nanorods single-crystalline (C4H9NH3)2PbI2X2 mixed halide organometal perovskite for solar cell application. Optik (Stuttg) 2016, 127, 9775–9787. [Google Scholar] [CrossRef]
- Palazon, F.; El Ajjouri, Y.; Sebastia-Luna, P.; Lauciello, S.; Manna, L.; Bolink, H.J. Mechanochemical synthesis of inorganic halide perovskites: Evolution of phase-purity, morphology, and photoluminescence. J. Mater. Chem. C 2019, 7, 11406–11410. [Google Scholar] [CrossRef]
- Mekkat, P.; Predeep, P. Hybrid perovskite single crystal with extended absorption edge and environmental stability: Towards a simple and easy synthesis procedure. Mater. Chem. Phys. 2020, 239, 122084. [Google Scholar] [CrossRef]
- Le Bail, A. Whole powder pattern decomposition methods and applications: A retrospection. Powder Diffr. 2005, 20, 316–326. [Google Scholar] [CrossRef]
- Mao, L.; Stoumpos, C.C.; Kanatzidis, M.G. Two-Dimensional Hybrid Halide Perovskites: Principles and Promises. J. Am. Chem. Soc. 2019, 141, 1171–1190. [Google Scholar] [CrossRef]
- Liu, Y.; Ye, H.; Zhang, Y.; Zhao, K.; Yang, Z.; Yuan, Y.; Wu, H.; Zhao, G.; Yang, Z.; Tang, J.; et al. Surface-Tension-Controlled Crystallization for High-Quality 2D Perovskite Single Crystals for Ultrahigh Photodetection. Matter 2019, 1, 465–480. [Google Scholar] [CrossRef]
- Postorino, P.; Malavasi, L. Pressure-Induced Effects in Organic-Inorganic Hybrid Perovskites. J. Phys. Chem. Lett. 2017, 8, 2613–2622. [Google Scholar] [CrossRef]
- Szafrański, M.; Katrusiak, A. Mechanism of Pressure-Induced Phase Transitions, Amorphization, and Absorption-Edge Shift in Photovoltaic Methylammonium Lead Iodide. J. Phys. Chem. Lett. 2016, 7, 3458–3466. [Google Scholar] [CrossRef]
- Singh, S.; Li, C.; Panzer, F.; Narasimhan, K.L.; Graeser, A.; Gujar, T.P.; Köhler, A.; Thelakkat, M.; Huettner, S.; Kabra, D. Effect of Thermal and Structural Disorder on the Electronic Structure of Hybrid Perovskite Semiconductor CH3NH3PbI3. J. Phys. Chem. Lett. 2016, 7, 3014–3021. [Google Scholar] [CrossRef]
- Shi, D.; Adinolfi, V.; Comin, R.; Yuan, M.; Alarousu, E.; Buin, A.; Chen, Y.; Hoogland, S.; Rothenberger, A.; Katsiev, K.; et al. Low trap-state density and long carrier diffusion in organolead trihalide perovskite single crystals. Science 2015, 347, 519–522. [Google Scholar] [CrossRef]
- Suárez-Forero, D.G.; Giuri, A.; De Giorgi, M.; Polimeno, L.; De Marco, L.; Todisco, F.; Gigli, G.; Dominici, L.; Ballarini, D.; Ardizzone, V.; et al. Quantum Nature of Light in Nonstoichiometric Bulk Perovskites. ACS Nano 2019, 13, 10711–10716. [Google Scholar] [CrossRef]
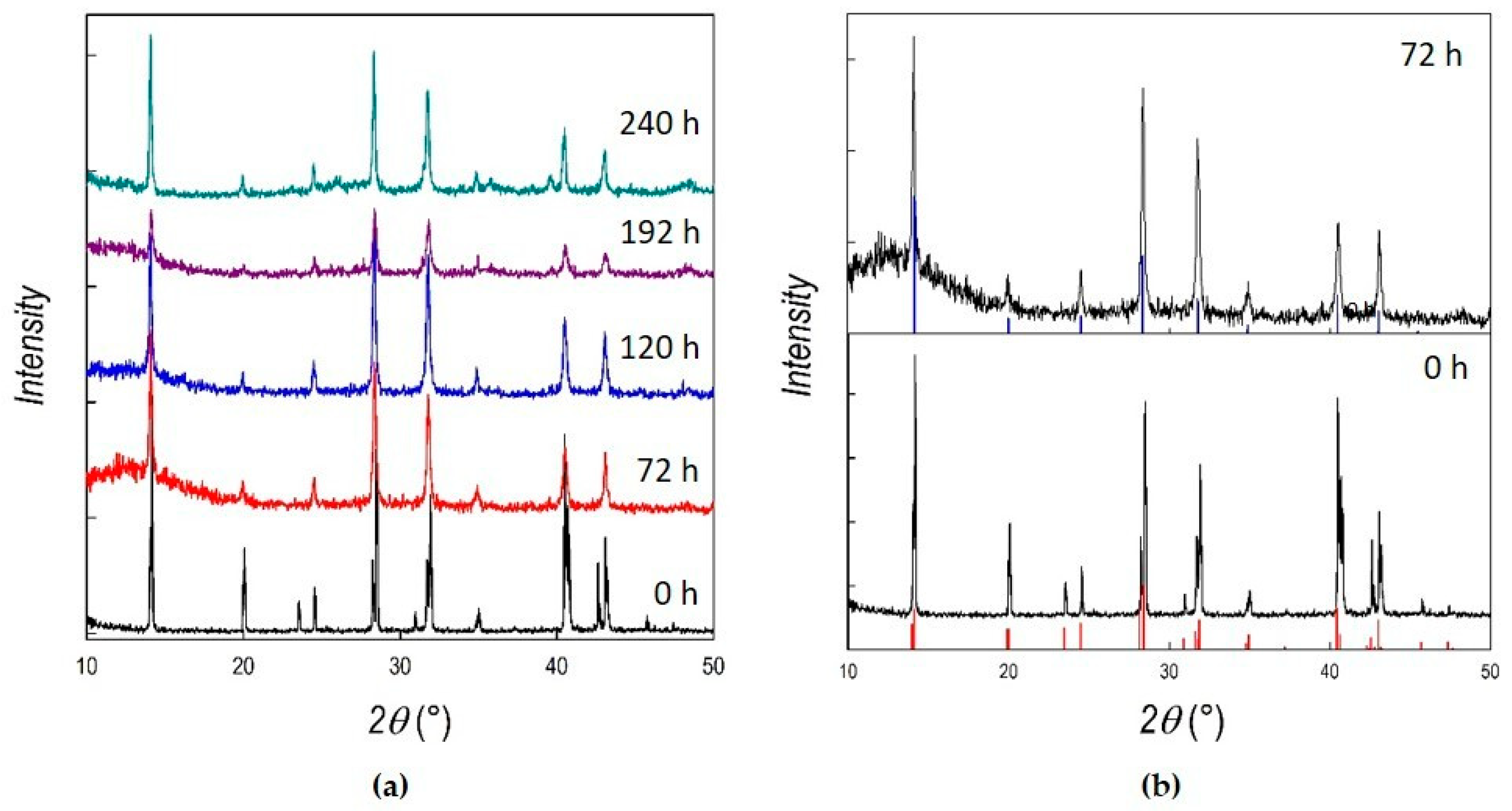


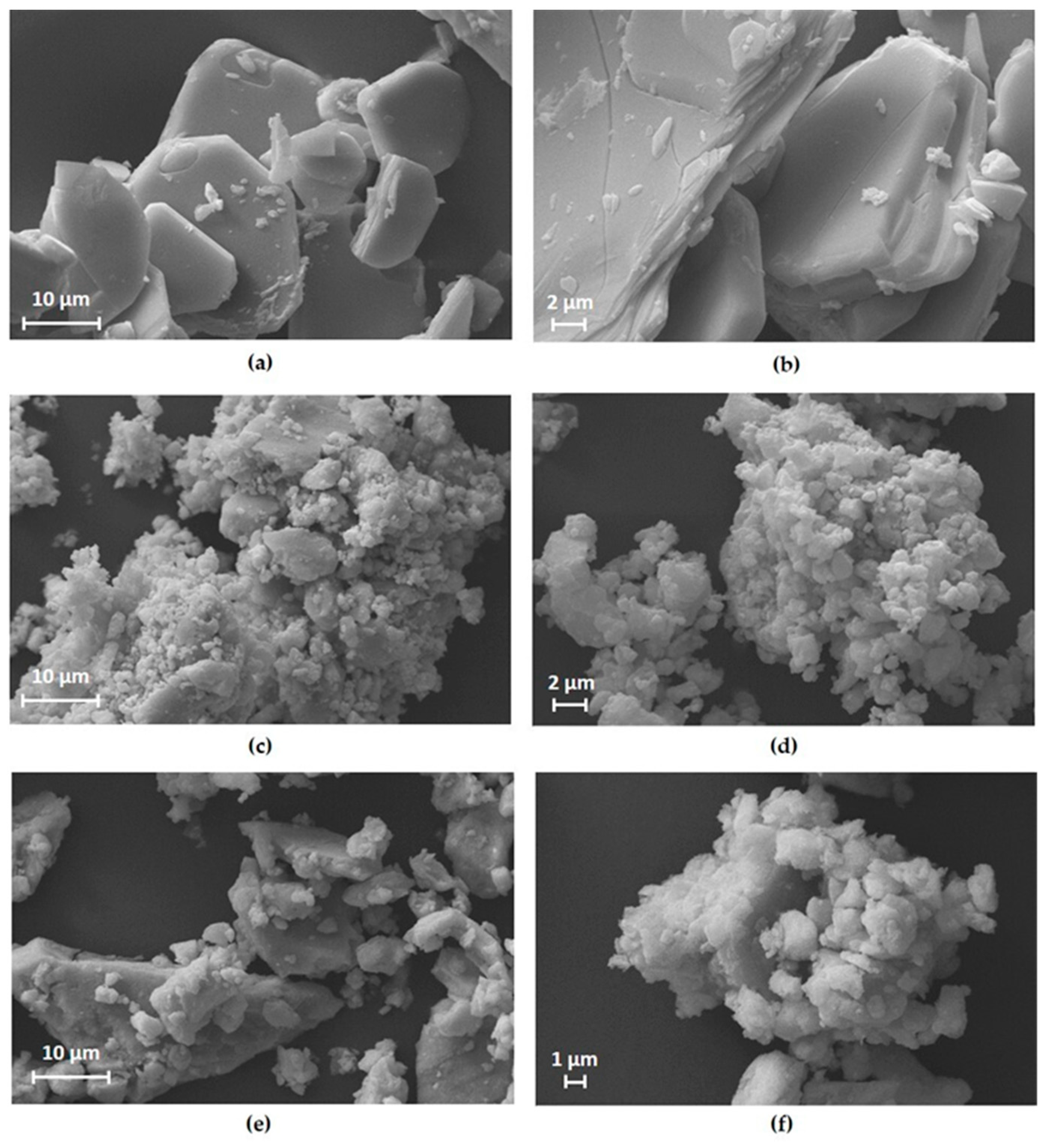
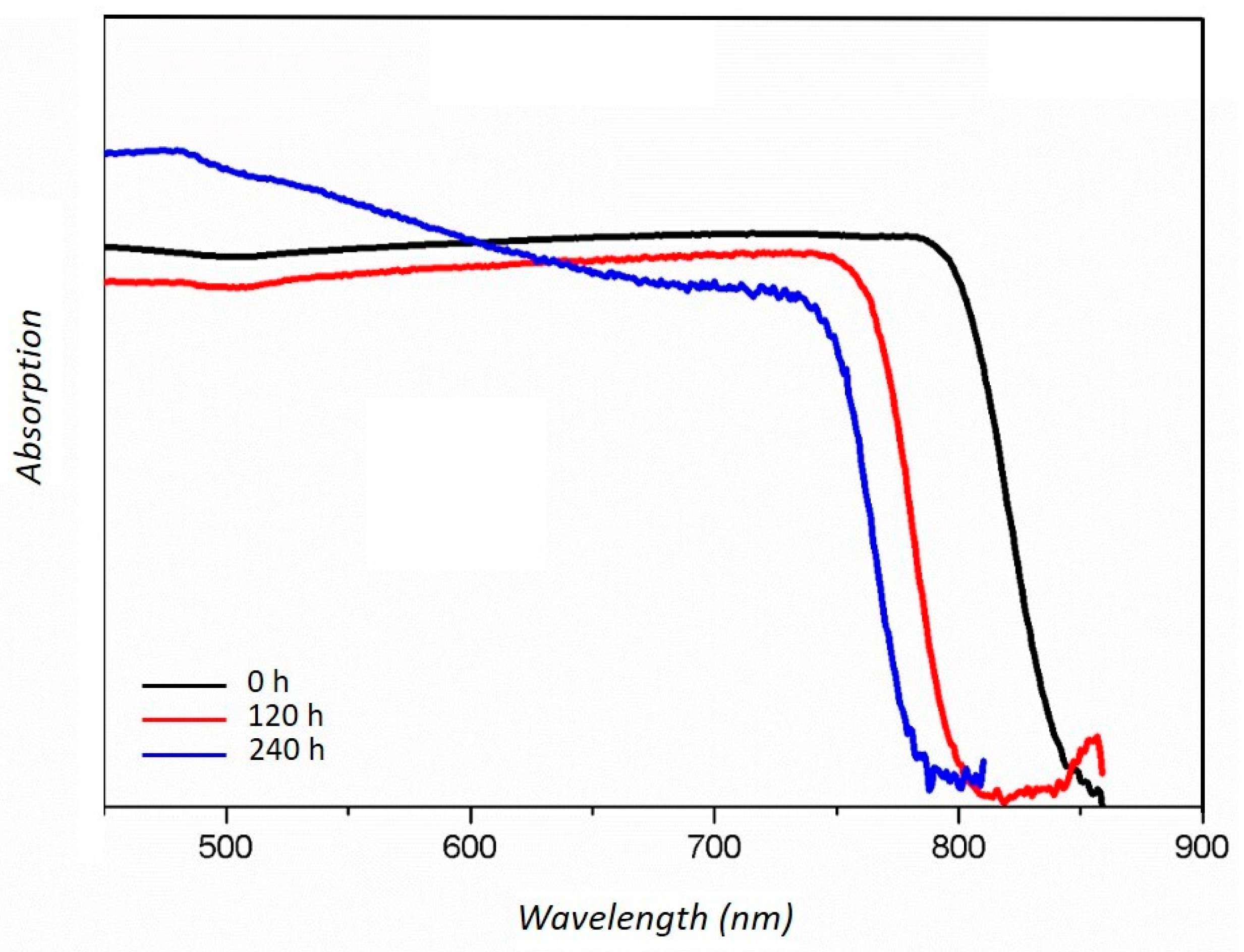
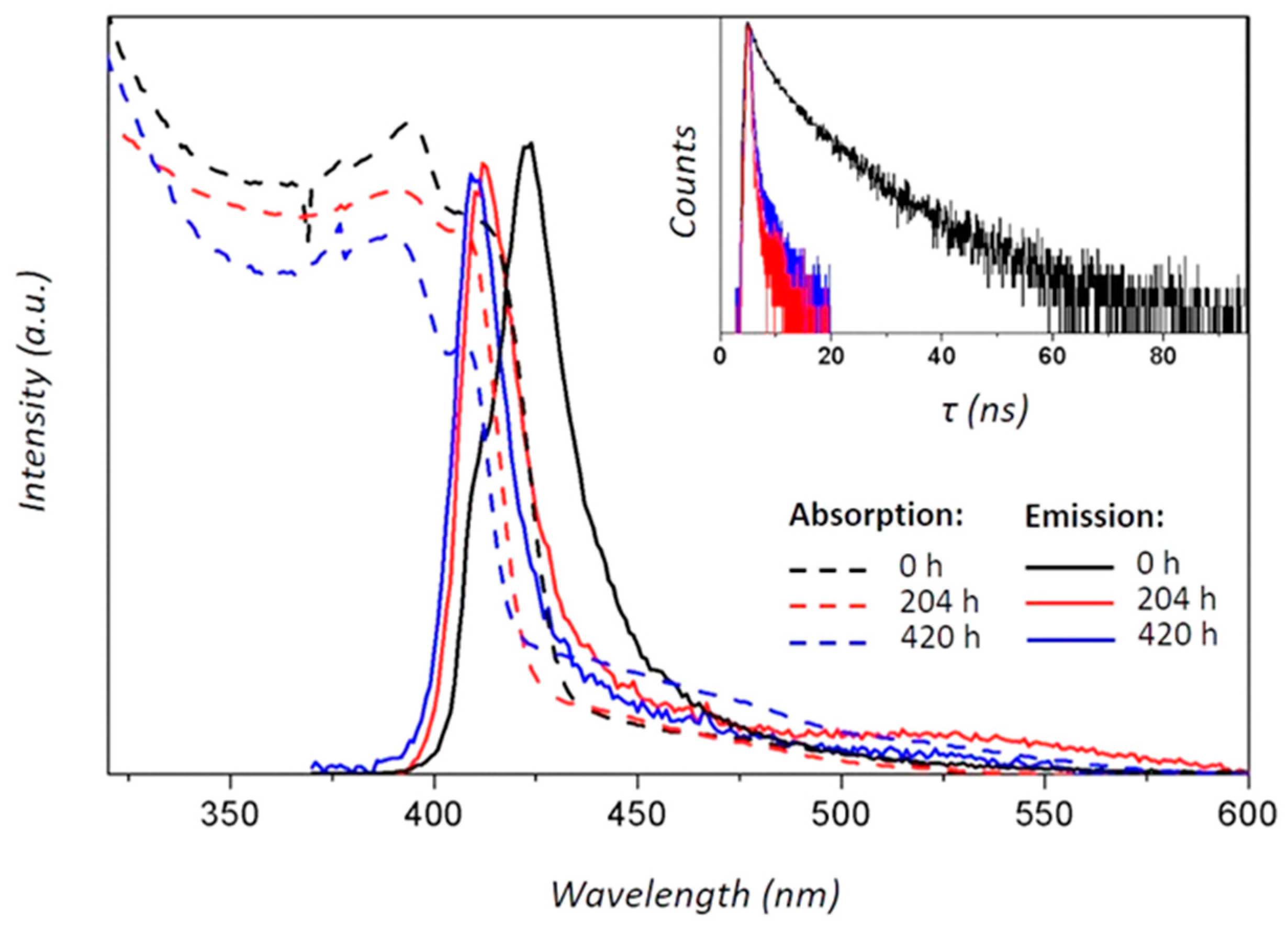
| Crystalline Phase | Ball-Milling Time (h) | A (Å) | C (Å) | Cell Volume (Å3) |
|---|---|---|---|---|
| Tetragonal | 0 | 8.868 ± 0.001 | 12.628 ± 0.001 | 248.300 |
| Cubic | 72 | 6.291 ± 0.001 | − | 248.977 |
| 120 | 6.293 ± 0.001 | − | 249.214 | |
| 192 | 6.295 ± 0.001 | − | 249.452 | |
| 240 | 6.301 ± 0.001 | − | 250.166 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bonomi, S.; Armenise, V.; Accorsi, G.; Colella, S.; Rizzo, A.; Fracassi, F.; Malavasi, L.; Listorti, A. The Effect of Extended Ball-Milling upon Three-Dimensional and Two-Dimensional Perovskite Crystals Properties. Appl. Sci. 2020, 10, 4775. https://doi.org/10.3390/app10144775
Bonomi S, Armenise V, Accorsi G, Colella S, Rizzo A, Fracassi F, Malavasi L, Listorti A. The Effect of Extended Ball-Milling upon Three-Dimensional and Two-Dimensional Perovskite Crystals Properties. Applied Sciences. 2020; 10(14):4775. https://doi.org/10.3390/app10144775
Chicago/Turabian StyleBonomi, Sara, Vincenza Armenise, Gianluca Accorsi, Silvia Colella, Aurora Rizzo, Francesco Fracassi, Lorenzo Malavasi, and Andrea Listorti. 2020. "The Effect of Extended Ball-Milling upon Three-Dimensional and Two-Dimensional Perovskite Crystals Properties" Applied Sciences 10, no. 14: 4775. https://doi.org/10.3390/app10144775
APA StyleBonomi, S., Armenise, V., Accorsi, G., Colella, S., Rizzo, A., Fracassi, F., Malavasi, L., & Listorti, A. (2020). The Effect of Extended Ball-Milling upon Three-Dimensional and Two-Dimensional Perovskite Crystals Properties. Applied Sciences, 10(14), 4775. https://doi.org/10.3390/app10144775









