Simultaneous Determination of Vitamin E and Vitamin K in Food Supplements Using Adsorptive Stripping Square-Wave Voltammetry at Glassy Carbon Electrode
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents and Chemicals
2.2. Pretreatment of Glassy Carbon Electrode
2.3. Apparatus
2.4. Sample Preparation for Voltammetric Analysis
2.5. Methods
2.5.1. Square-Wave Adsorptive Stripping Voltammetry
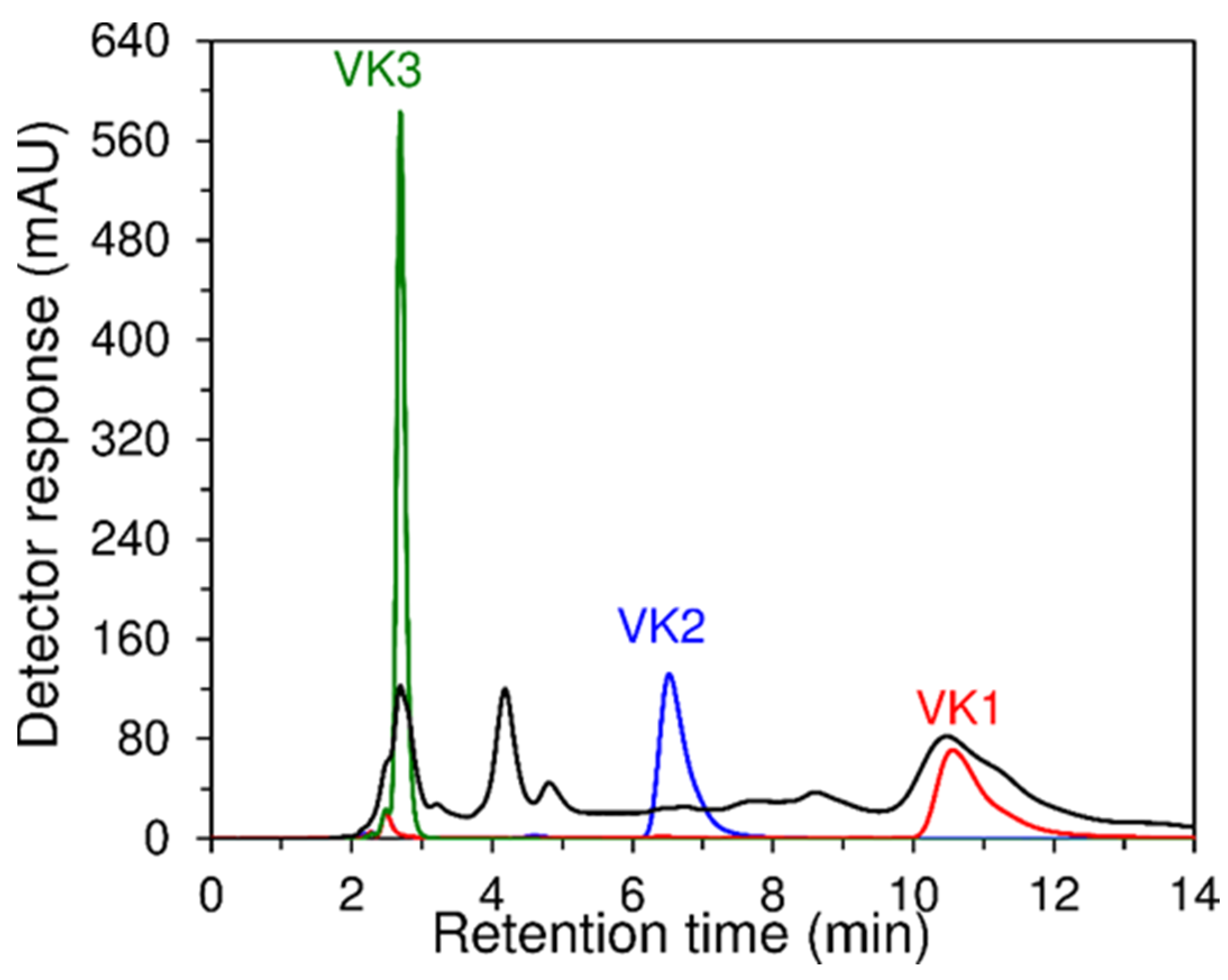
2.5.2. High-Performance Liquid Chromatography
2.6. Statistical Evaluation
3. Results and Discussion
3.1. Optimization of Adsorption
3.1.1. Effect of Organic Solvent Content
3.1.2. Effect of Ionic Strength
3.1.3. Speed of Stirring
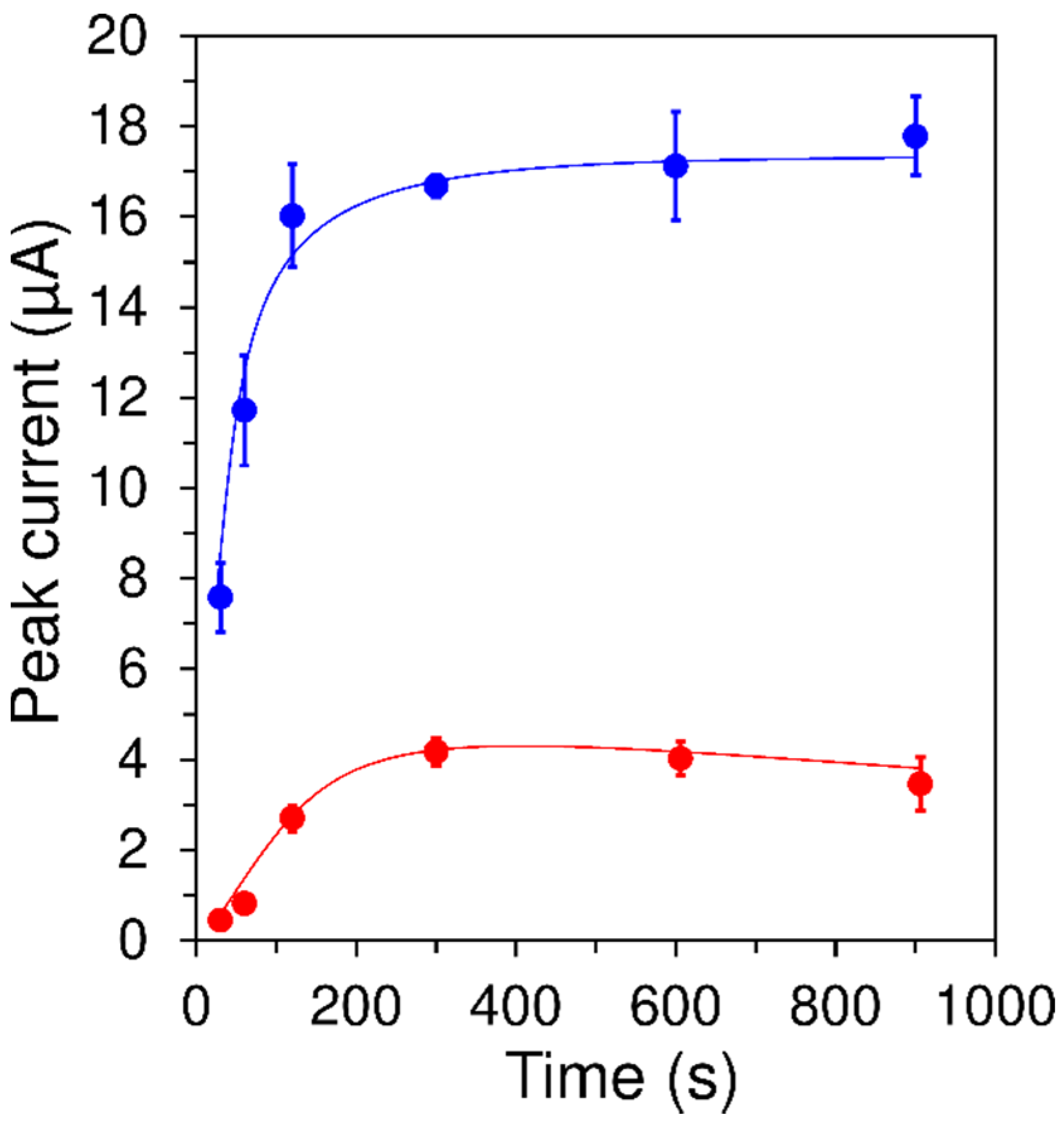
3.1.4. Accumulation Time
3.2. Optimization of Subsequent Voltammetric Detection
3.2.1. Effect of Detection Media
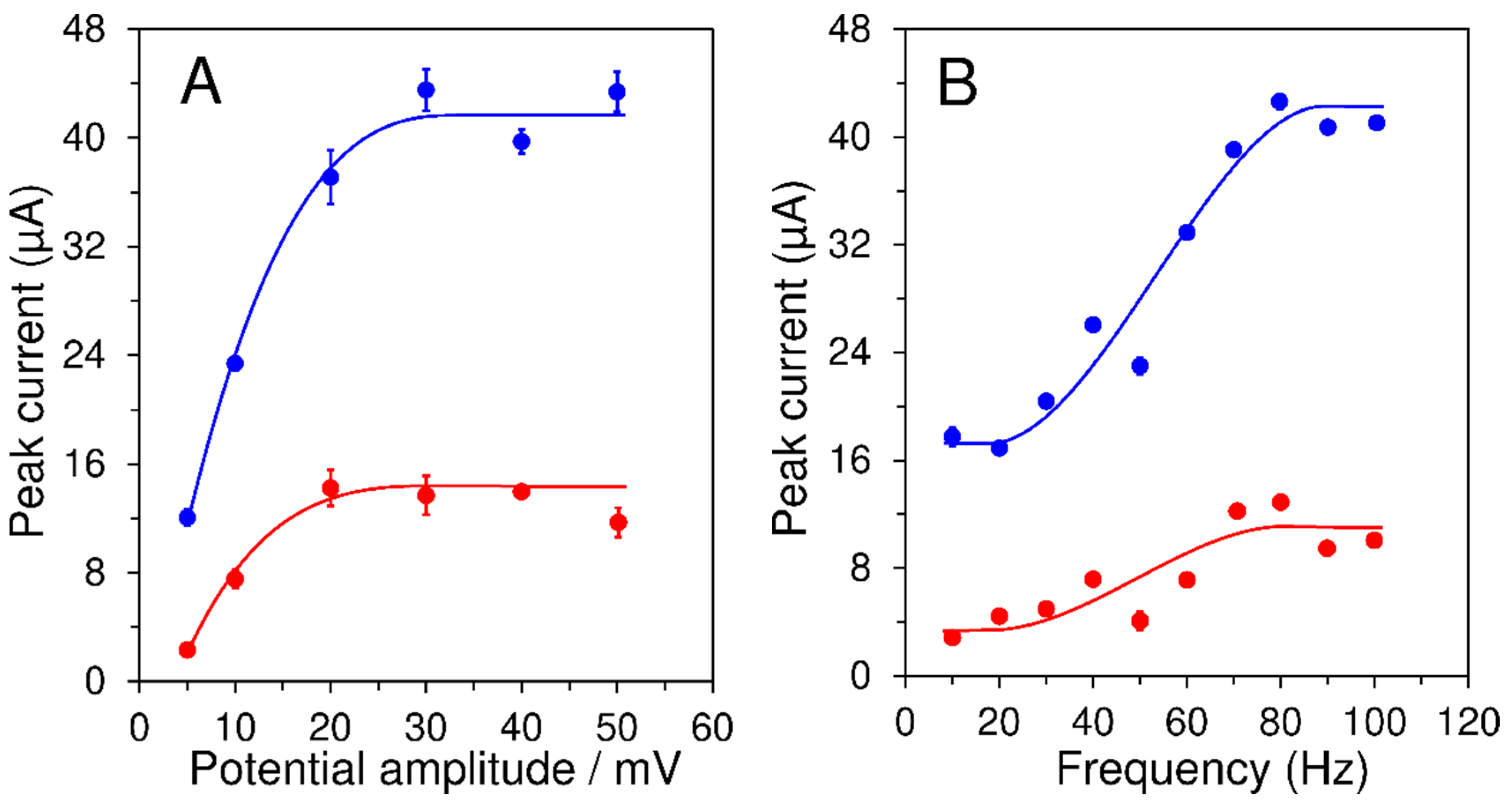
3.2.2. Effect of Square-Wave Voltammetry Working Parameters
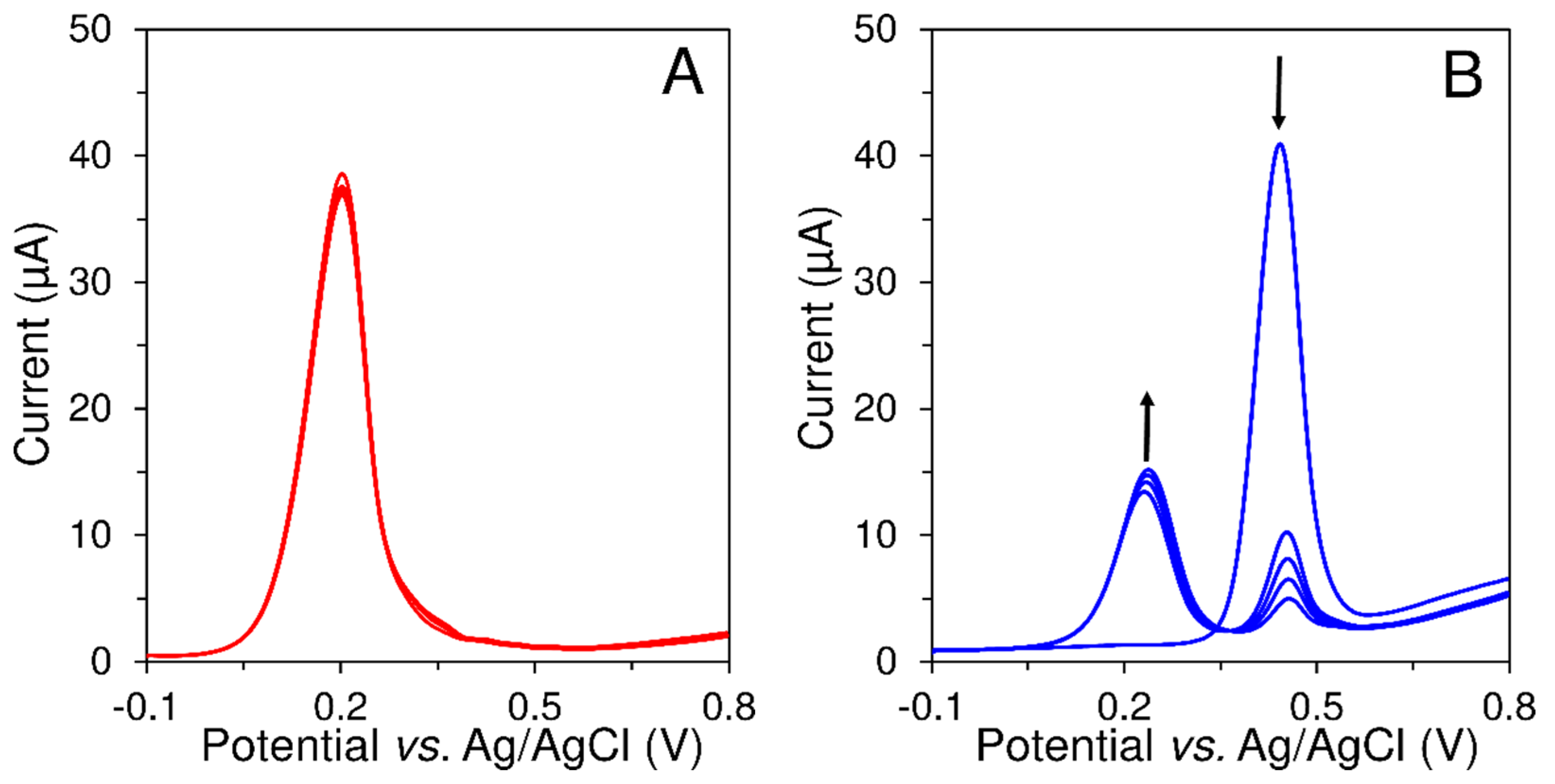
3.2.3. Memory Effect
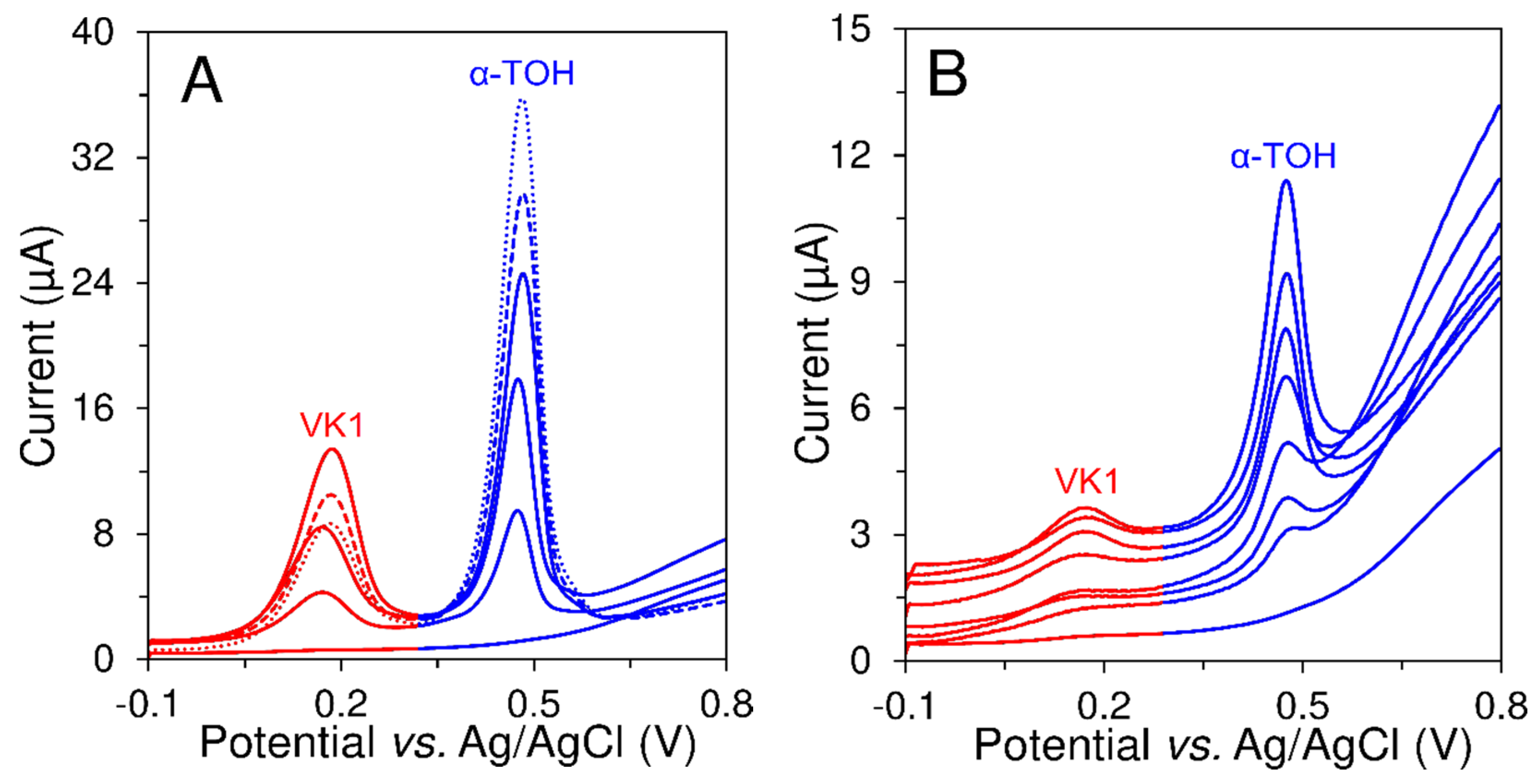
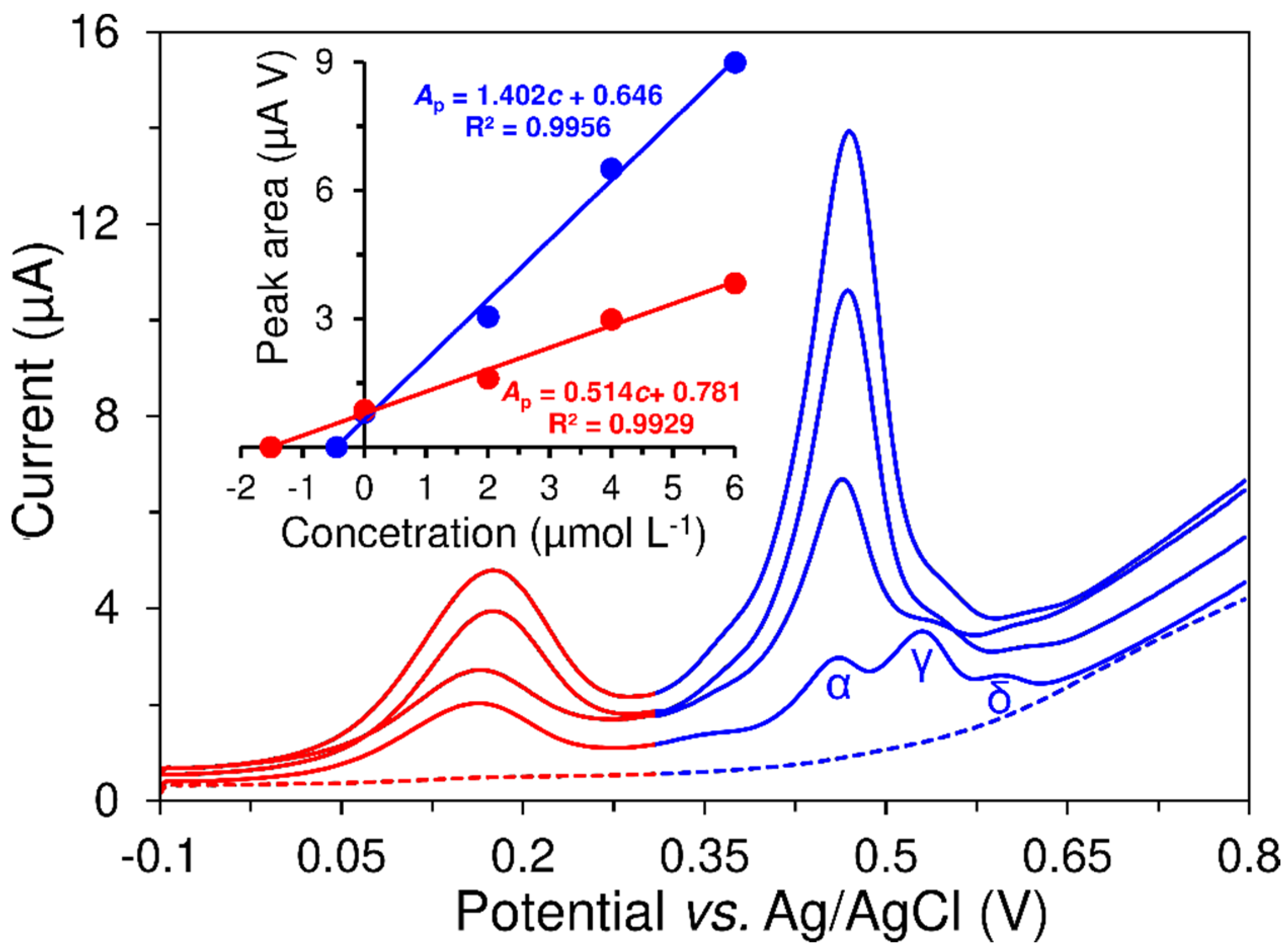
3.3. Analytical Performance of the Developed Voltammetric Method
3.4. Analysis of Food Supplements
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sýs, M.; Švecová, B.; Švancara, I.; Metelka, R. Determination of vitamin E in margarines and edible oils using square wave anodic stripping voltammetry with a glassy carbon paste electrode. Food Chem. 2017, 229, 621–627. [Google Scholar] [CrossRef] [PubMed]
- Sýs, M.; Jashari, G.; Švecová, B.; Arbneshi, T.; Metelka, R. Determination of vitamin K1 using square wave adsorptive stripping voltammetry at solid glassy carbon electrode. J. Electroanal. Chem. 2018, 821, 10–15. [Google Scholar] [CrossRef]
- AL-Eitan, L.N.; Alzoubi, K.H.; Al-Smadi, L.I.; Khabour, O.F. Vitamin E protects against cisplatin-induced genotoxicity in human lymphocytes. Toxicol. In Vitro 2020, 62, 104672. [Google Scholar] [CrossRef]
- Sýs, M.; Žabčíková, S.; Červenka, L.; Vytřas, K. Adsorptive stripping voltammetry in lipophilic vitamins abstract determination. Potravin. Slovak J. Food Sci. 2016, 10, 260–264. [Google Scholar] [CrossRef]
- Webster, R.D. Voltammetry of the liposoluble vitamins (A, D, E and K) in organic solvents. Chem. Rec. 2011, 12, 188–200. [Google Scholar] [CrossRef]
- Parvin, M.H.; Arjomandi, J.; Lee, J.Y. γ-Al2O3 nanoparticle catalyst mediated polyaniline gold electrode biosensor for vitamin E. Catal. Commun. 2018, 110, 59–63. [Google Scholar] [CrossRef]
- Lang, J.K.; Packer, L. Quantitative determination of vitamin E and oxidized and reduced coenzyme Q by high-performance liquid chromatography with in-line ultraviolet and electrochemical detection. J. Chromatogr. A 1987, 385, 109–117. [Google Scholar] [CrossRef]
- Ly, S.Y. Voltammetric analysis of DL-α-tocopherol with a paste electrode. J. Sci. 2008, 88, 1272–1276. [Google Scholar] [CrossRef]
- Richardson, D.P.; Astrup, A.; Cocaul, A.; Ellis, P. The nutritional and health benefits of almonds: A healthy food choice. Food Sci. Technol. Bull. Funct. Foods 2009, 6, 41–50. [Google Scholar] [CrossRef]
- Chetot, T.; Taufana, S.; Benoit, E.; Lattard, V. Vitamin K antagonist rodenticides display different teratogenic activity. Reprod. Toxicol. 2020, 93, 131–136. [Google Scholar] [CrossRef] [PubMed]
- De Oliveira, R.B.; Stinghen, A.E.M.; Massy, Z.A. Vitamin K role in mineral and bone disorder of chronic kidney disease. Clin. Chim. 2020, 502, 66–72. [Google Scholar] [CrossRef]
- Takeda, K.; Morita, A.; Ikenaka, Y.; Nakayama, S.M.M.; Ishizuka, M. Comparison of two reducing agents dithiothreitol and tris (3-hydroxypropyl) phosphine for in vitro kinetic assay of vitamin K epoxide reductase. Vet. Anim. Sci. 2020, 9. [Google Scholar] [CrossRef]
- Wakabayashi, H.; Onodera, K.; Yamato, S.; Shimada, K. Simultaneous determination of vitamin K analogs in human serum by sensitive and selective high-performance liquid chromatography with electrochemical detection. Nutrition 2003, 19, 661–665. [Google Scholar] [CrossRef]
- Schlievert, P.M.; Merriman, J.A.; Salgado-Pabón, W.; Mueller, E.A.; Spaulding, A.R.; Vu, B.G.; Chuang-Smith, O.N.; Kohler, P.L.; Kirby, J.R. Menaquinone analogs inhibit growth of bacterial pathogens. Antimicrob. Agents Chemother. 2013, 57, 5432–5437. [Google Scholar] [CrossRef]
- Akbari, A.; Jelodar, G.; Nazifi, S.; Sajedianfard, J. An overview of the characteristics and function of vitamin C in various tissues: Relying on its antioxidant function. Zahedan J. Res. Med. Sci. 2016, 18. [Google Scholar] [CrossRef]
- Brigelius-Flohe, R.; Traber, M.G. Vitamin E: Function and metabolism. FASEB J. 1999, 13, 1145–1155. [Google Scholar] [CrossRef] [PubMed]
- Browne, D.; McGuinness, B.; Woodside, J.V.; McKay, G.J. Vitamin E and Alzheimer’s disease: What do we know so far? Clin. Interv. Aging 2019, 14, 1303–1317. [Google Scholar] [CrossRef] [PubMed]
- Fryer, M.J. Vitamin E may slow kidney failure owing to oxidative stress. Redox Rep. 1997, 3, 259–261. [Google Scholar] [CrossRef] [PubMed]
- Girolami, A.; Ferrari, S.; Cosi, E.; Santarossa, C.; Randi, M.L. Vitamin K-dependent coagulation factors that may be responsible for both bleeding and thrombosis (FII, FVII, and FIX). Clin. Appl. Thromb./Hemost. 2018, 24, 42S–47S. [Google Scholar] [CrossRef]
- Girolami, A.; Ferrari, S.; Cosi, E.; Girolami, B.; Lombardi, A.M. Congenital prothrombin defects: They are not only associated with bleeding but also with thrombosis: A new classification is needed. Hematology 2017, 23, 105–110. [Google Scholar] [CrossRef]
- Bajaj, R.; Karthikeyan, G.; Sinha, N.; Lokhandwala, Y.; Rao, D.; Kaushik, S.K.; Jain, S.L.; Narayanan, S.; Seth, A.; Satyamurthi, I.; et al. CSI consensus statement on prosthetic valve follow up. Indian Heart J. 2012, 64, S3. [Google Scholar] [CrossRef]
- Zamarrefio, M.M.D.; Pérez, A.S.; Pérez, C.G.; MCndez, J.H. High-performance liquid chromatography with electrochemical detection for the simultaneous determination of vitamin A, D3 and E in milk. J. Chromatogr. A 1992, 623, 69–74. [Google Scholar] [CrossRef]
- Fauler, G.; Leis, H.J.; Schalamon, J.; Muntean, W.; Gleispach, H. Method for the determination of vitamin K1(20) in human plasma by stable isotope dilution/gas chromatography/mass spectrometry. J. Mass Spectrom. 1996, 31, 655–660. [Google Scholar] [CrossRef]
- Kamal-Eldin, A.; Görgen, S.; Pettersson, J.; Lampi, A.M. Normal-phase high-performance liquid chromatography of tocopherols and tocotrienols Comparison of different chromatographic columns. J. Chromatogr. A 2000, 881, 217–227. [Google Scholar] [CrossRef]
- McMurray, C.H.; Blanchflower, W.J. Application of a high-performance liquid chromatographic fluorescence method for the rapid determination of α-tocopherol in the plasma of cattle and pigs and its comparison with direct fluorescence and high-performance liquid chromatography—Ultraviolet detection methods. J. Chromatogr. A 1979, 178, 525–531. [Google Scholar] [CrossRef]
- Hart, J.P.; Wring, S.A.; Morgan, I.C. Pre-concentration of vitamin K1 (phylloquinone) at carbon paste electrodes and its determination in plasma by adsorptive stripping voltammetry. Analyst 1989, 114, 933–937. [Google Scholar] [CrossRef]
- Sýs, M.; Žabčíková, S.; Červenka, L.; Vytřas, K. Comparison of adsorptive with extractive stripping voltammetry in electrochemical determination of retinol. Potravinarstvo 2017, 11, 96–105. [Google Scholar] [CrossRef]
- Komorsky-Lovrić, Š.; Lovrić, M. Theory of square-wave stripping voltammetry with adsorptive accumulation. Fresenius’ Z. Für Anal. Chem. 1989, 335, 289–294. [Google Scholar] [CrossRef]
- Silva, P.L.; Bastos, E.L.; El Seoud, O.A. Solvation in binary mixtures of water and polar aprotic solvents: theoretical calculations of the concentrations of solvent−water hydrogen-bonded species and application to thermosolvatochromism of polarity probes. J. Phys. Chem. B 2007, 111, 6173–6180. [Google Scholar] [CrossRef]
- Donohue, M.D.; Aranovich, G.L. Classification of Gibbs adsorption isotherms. Adv. Colloid Interface Sci. 1998, 76, 137–152. [Google Scholar] [CrossRef]
- Yao, W.W.; Peng, H.M.; Webster, R.D. Electrochemistry of α-tocopherol (vitamin E) and α-tocopherol quinone films deposited on electrode surfaces in the presence and absence of lipid multilayers. J. Phys. Chem. C 2009, 113, 21805–21814. [Google Scholar] [CrossRef]
- Yang, J.E.; Yoon, J.H.; Won, M.S.; Shim, Y.B. Electrochemical and spectroelectrochemical behaviors of vitamin K1/lipid modified electrodes and the formation of radical anion in aqueous media. Bull. Korean Chem. Soc. 2010, 31, 3133–3138. [Google Scholar] [CrossRef]
- Vire, J.C.; Abo El Maali, N.; Patriarche, G.J.; Christian, G.D. Square-wave adsorptive stripping voltammetry of menadione (vitamin K3). Talanta 1988, 35, 997–1000. [Google Scholar] [CrossRef]
- Wang, L.Z.; Ma, C.S.; Zhang, X.L.; Xu, Y. Determination of vitamin K3 by cathodic stripping voltammetry. Microchem. J. 1994, 50, 101–105. [Google Scholar] [CrossRef]
- Sýs, M.; Metelka, R.; Stočes, M.; Vytřas, K. Electrochemical properties of a-tocopherol in aqueous electrolytes after its previous extraction into the glassy carbon paste from aqueous-acetonic mixture. Mon. Für Chem. Chem. Mon. 2016, 147, 31–38. [Google Scholar] [CrossRef]
- Hart, J.P.; Catterall, A. Electrosorption of vitamin K1 at mercury and its determination at submicrogram levels by differential pulse voltammetry at a hanging mercury electrode. Anal. Chim. Acta 1981, 128, 245–250. [Google Scholar] [CrossRef]
- Vire, J.C.; Lopez, V.; Patriarche, G.J.; Christian, G.D. Determination of vitamin K1 by adsorptive stripping square-wave voltammetry. Anal. Lett. 1988, 21, 2217–2225. [Google Scholar] [CrossRef]
- Lubeckyj, R.A.; Winkler-Moser, J.K.; Fhaner, M.J. Application of differential pulse voltammetry to determine the efficiency of stripping tocopherols from commercial fish oil. J. Am. Oil Chem. Soc. 2017, 94, 527–536. [Google Scholar] [CrossRef]
- Wilson, G.J.; Lin, C.Y.; Webster, R.D. Significant differences in the electrochemical Behavior of the α-, β-, γ-, δ-tocopherols (vitamin E). J. Phys. Chem. B 2006, 110, 11540–11548. [Google Scholar] [CrossRef]
- Lamon-Fava, S.; Sadowski, J.A.; Davidson, K.W.; O’Brien, M.E.; McNamara, J.R.; Schaefer, E.J. Plasma lipoproteins as carriers of phylloquinone (vitamin K1) in humans. Am. J. Clin. Nutr. 1998, 67, 1226–1231. [Google Scholar] [CrossRef]
| Electrode | Analyte | Method | Linear Range (mol L−1) | LOD (mol L−1) | Reference |
|---|---|---|---|---|---|
| HMDE | VK1 | DPP | 2.2 × 10−8–2.2 × 10−7 | [36] | |
| HMDE | VK1 | SWAdSV | 1.0 × 10−9–1.0 × 10−6 | [37] | |
| HMDE | VK3 | SWAdSV | 2.0 × 10−10–5.0 × 10−7 | 1.3 × 10−10 | [33] |
| CPE | VK1 | LSAdSV | 6.7 × 10−7–4.4 × 10−6 | 4.0 × 10−7 | [26] |
| GCE | VK1 | SWAdSV | 5.0 × 10−6–1.0 × 10−4 | 5.1 × 10−8 | [2] |
| GCE | VK1 | SWAdSV | 1.0 × 10−8–1.0 × 10−6 | 8.9 × 10−9 | [2] |
| GCE | VK1 | SWAdSV | 7.7 × 10−8–1.0 × 10−6 | 2.5 × 10−8 | This work |
| GCPE | VE (α-TOH) | SWASV | 5.0 × 10−7–4.0 × 10−5 | 1.0 × 10−7 | [1] |
| GCE | VE (α-TOH) | SWAdSV | 2.9 × 10−8–1.0 × 10−6 | 1.0 × 10−8 | This work |
| Food Supplement | SWAdSV | HPLC | Declared Content | |||
|---|---|---|---|---|---|---|
| VK | VE | VK | VE | VK | VE | |
| Model sample | 4.72 ± 0.28 | 42.86 ± 1.94 | 4.8 ± 0.13 | 45.3 ± 1.6 | 4.51 | 43.71 |
| Sample 1 | 0.11 ± 0.02 | – | 0.12 ± 0.01 | – | 0.10 | – |
| Sample 2 | – | 0.20 ± 0.02 | – | 0.21 ± 0.01 | – | 2.50 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kastrati, G.; Jashari, G.; Sýs, M.; Švecová, B.; Arbneshi, T.; Metelka, R.; Bílková, Z.; Korecká, L. Simultaneous Determination of Vitamin E and Vitamin K in Food Supplements Using Adsorptive Stripping Square-Wave Voltammetry at Glassy Carbon Electrode. Appl. Sci. 2020, 10, 4759. https://doi.org/10.3390/app10144759
Kastrati G, Jashari G, Sýs M, Švecová B, Arbneshi T, Metelka R, Bílková Z, Korecká L. Simultaneous Determination of Vitamin E and Vitamin K in Food Supplements Using Adsorptive Stripping Square-Wave Voltammetry at Glassy Carbon Electrode. Applied Sciences. 2020; 10(14):4759. https://doi.org/10.3390/app10144759
Chicago/Turabian StyleKastrati, Gylxhane, Granit Jashari, Milan Sýs, Blanka Švecová, Tahir Arbneshi, Radovan Metelka, Zuzana Bílková, and Lucie Korecká. 2020. "Simultaneous Determination of Vitamin E and Vitamin K in Food Supplements Using Adsorptive Stripping Square-Wave Voltammetry at Glassy Carbon Electrode" Applied Sciences 10, no. 14: 4759. https://doi.org/10.3390/app10144759
APA StyleKastrati, G., Jashari, G., Sýs, M., Švecová, B., Arbneshi, T., Metelka, R., Bílková, Z., & Korecká, L. (2020). Simultaneous Determination of Vitamin E and Vitamin K in Food Supplements Using Adsorptive Stripping Square-Wave Voltammetry at Glassy Carbon Electrode. Applied Sciences, 10(14), 4759. https://doi.org/10.3390/app10144759






