Weakly-Supervised Classification of HER2 Expression in Breast Cancer Haematoxylin and Eosin Stained Slides
Abstract
Featured Application
Abstract
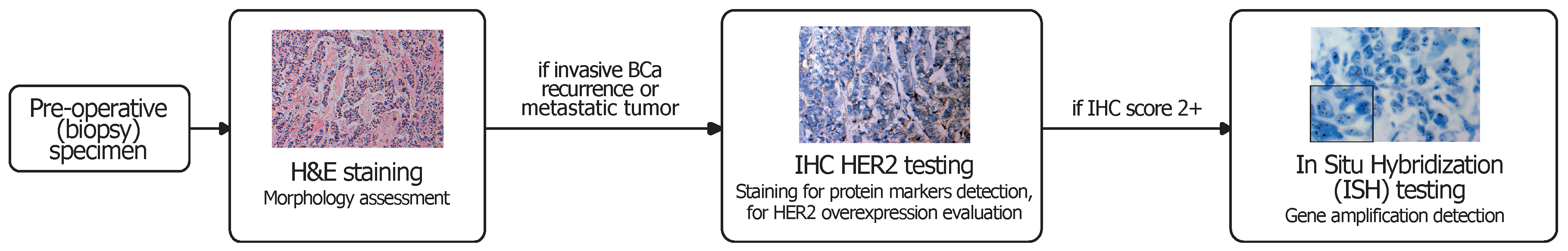
1. Introduction
- –
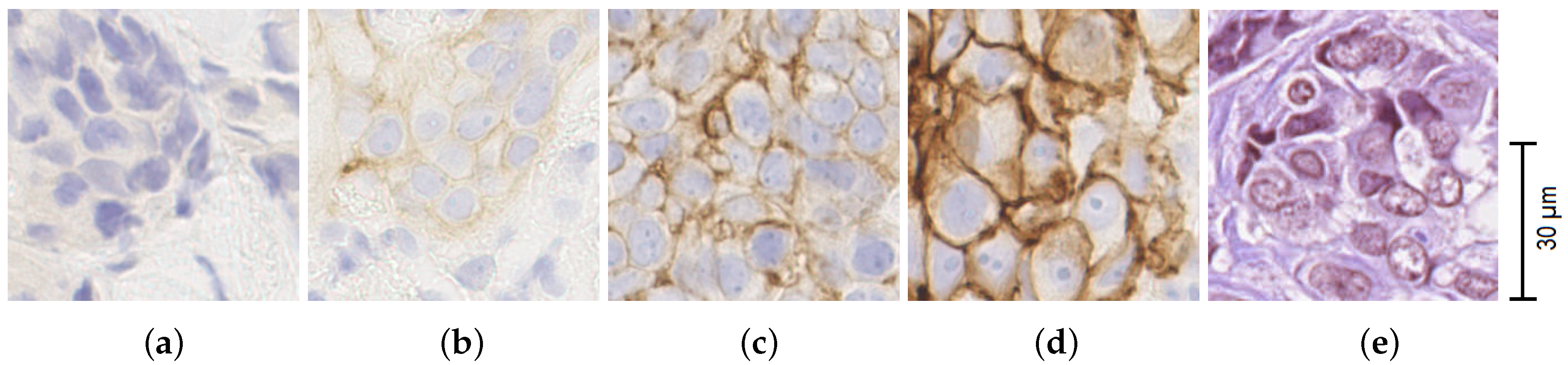
- IHC 0+: no staining or incomplete, barely perceptible membrane staining in 10% of tumour cells or less;
- –
- IHC 1+: incomplete, barely perceptible membrane staining in more than 10% of tumour cells;
- –
- IHC 2+: weak to moderate complete membrane staining in more than 10% of tumour cells;
- –
- IHC 3+: circumferential, complete, intense membrane staining in more than 10% of tumour cells.
2. Related Work
3. Methodology
3.1. Data Preprocessing
3.1.1. IHC Stained Slides
3.1.2. H&E Stained Slides
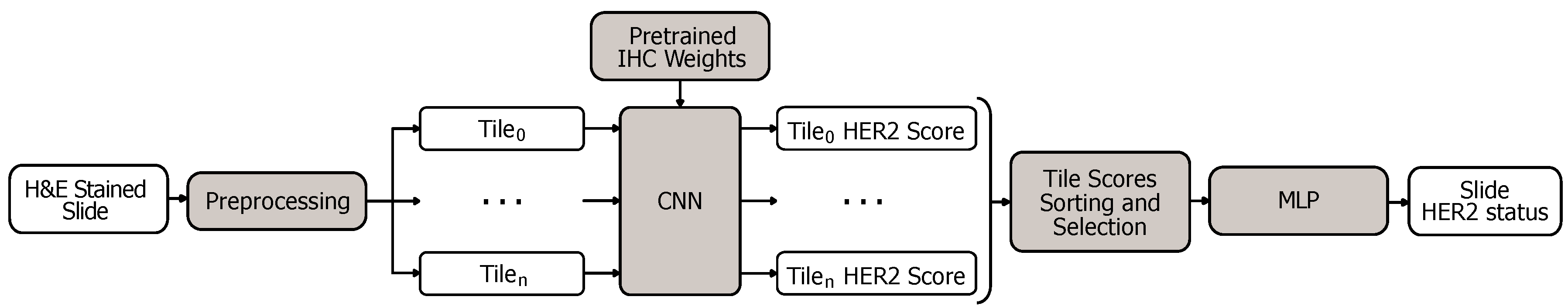
3.2. CNN for IHC Tile Scoring
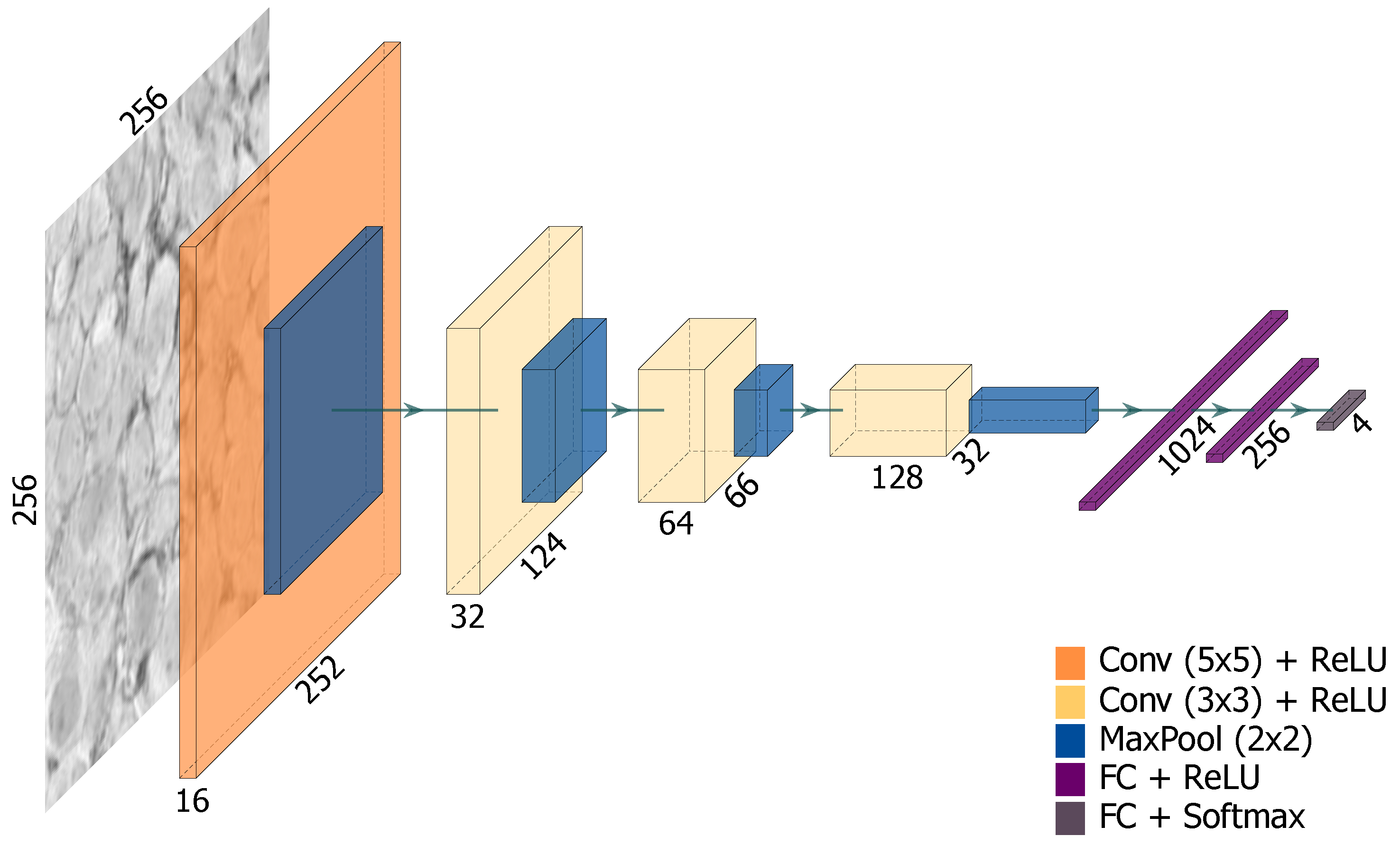
3.3. CNN for H&E Stained Slide Classification
4. Experimental Settings
4.1. Data
4.2. Training Details
5. Results and Discussion
5.1. Individual IHC Tile Scoring Results
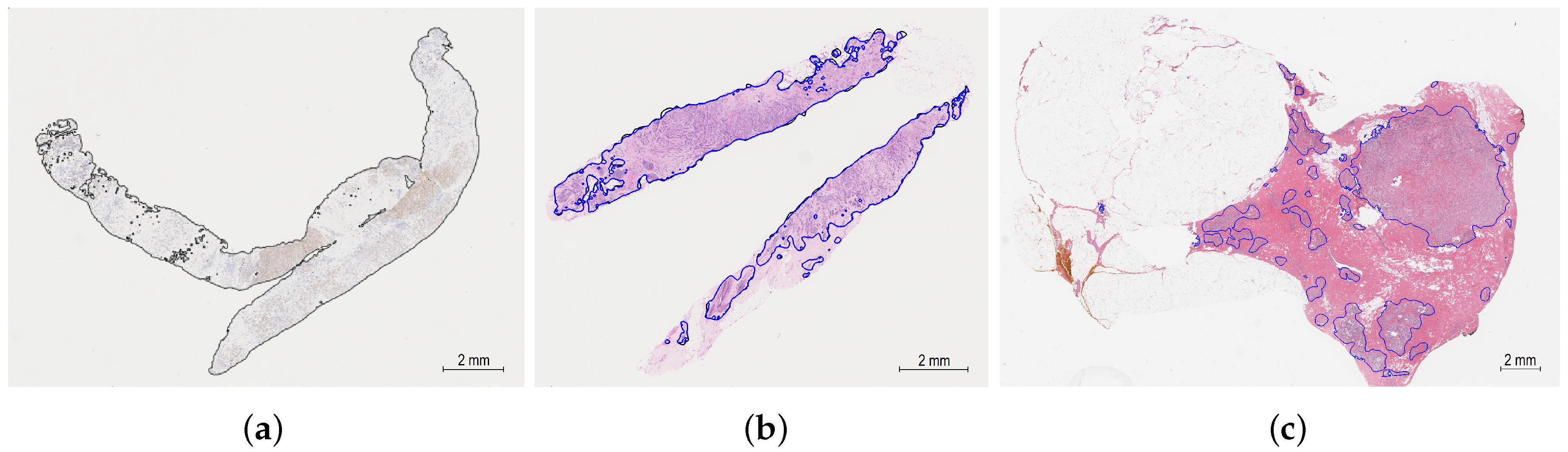
5.2. Invasive Tumor Tissue Segmentation
5.3. Slide Scoring
5.4. Ablation Study
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| Adam | Adaptive Moment Estimation |
| ASCO/CAP | American Society of Clinical Oncology/College of American Pathologists |
| BCa | Breast cancer |
| BRCA | TCGA-TCIA-BRCA |
| CNN | Convolutional Neural Network |
| H&E | Haematoxylin and Eosin |
| HER2 | Human Epidermal growth factor Receptor 2 |
| HER2SC | HER2 Scoring Contest dataset |
| IHC | Immunohistochemistry |
| ISH | In situ Hybridization |
| MIL | Multiple Instance Learning |
| MLP | Multilayer Perceptron |
| ROI | Regions of Interest |
| WSI | Whole Slide Images |
References
- American Cancer Society. Breast Cancer Facts & Figures 2017–2018. Available online: https://www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-statistics/breast-cancer-facts-and-figures/breast-cancer-facts-and-figures-2017-2018.pdf (accessed on 21 June 2020).
- Gandomkar, Z.; Brennan, P.; Mello-Thoms, C. Computer-based image analysis in breast pathology. J. Pathol. Inform. 2016, 7. [Google Scholar] [CrossRef] [PubMed]
- Veta, M.; Pluim, J.P.W.; van Diest, P.J.; Viergever, M.A. Breast Cancer Histopathology Image Analysis: A Review. IEEE Trans. Biomed. Eng. 2014, 61, 1400–1411. [Google Scholar] [CrossRef] [PubMed]
- American Society of Clinical Oncology (ASCO). Breast Cancer Guide. 2005–2020. Available online: https://www.cancer.net/cancer-types/breast-cancer/introduction (accessed on 21 June 2020).
- Rakha, E.A.; Pinder, S.E.; Bartlett, J.M.S.; Ibrahim, M.; Starczynski, J.; Carder, P.J.; Provenzano, E.; Hanby, A.; Hales, S.; Lee, A.H.S.; et al. Updated UK Recommendations for HER2 assessment in breast cancer. J. Clin. Pathol. 2015, 68, 93–99. [Google Scholar] [CrossRef] [PubMed]
- Goddard, K.; Weinmann, S.; Richert-Boe, K.; Chen, C.; Bulkley, J.; Wax, C. HER2 Evaluation and Its Impact on Breast Cancer Treatment Decisions. Public Health Genom. 2011, 15, 1–10. [Google Scholar] [CrossRef]
- Wolff, A.C.; Hammond, M.E.H.; Allison, K.H.; Harvey, B.E.; Mangu, P.B.; Bartlett, J.M.; Bilous, M.; Ellis, I.O.; Fitzgibbons, P.; Hanna, W.; et al. Human Epidermal Growth Factor Receptor 2 Testing in Breast Cancer: American Society of Clinical Oncology/College of American Pathologists Clinical Practice Guideline Focused Update. J. Clin. Oncol. 2018, 36, 2105–2122. [Google Scholar] [CrossRef]
- Hanna, W.M.; Barnes, P.J.; Chang, M.; Gilks, C.B.; Magliocco, A.M.; Rees, H.; Quenneville, L.; Robertson, S.J.; Sengupta, S.K.; Nofech-Mozes, S. Human epidermal growth factor receptor 2 testing in primary breast cancer in the era of standardized testing: A Canadian prospective study. J. Clin. Oncol. 2014, 32, 3967–3973. [Google Scholar] [CrossRef] [PubMed]
- HEROHE Challenge. Available online: https://ecdp2020.grand-challenge.org/ (accessed on 21 June 2020).
- Qaiser, T.; Mukherjee, A.; Reddy PB, C.; Munugoti, S.D.; Tallam, V.; Pitkäaho, T.; Lehtimäki, T.; Naughton, T.; Berseth, M.; Pedraza, A.; et al. HER2 challenge contest: A detailed assessment of automated HER2 scoring algorithms in whole slide images of breast cancer tissues. Histopathology 2018, 72, 227–238. [Google Scholar] [CrossRef]
- Clark, K.; Vendt, B.; Smith, K.; Freymann, J.; Kirby, J.; Koppel, P.; Moore, S.; Phillips, S.; Maffitt, D.; Pringle, M.; et al. The Cancer Imaging Archive (TCIA): Maintaining and Operating a Public Information Repository. J. Digit. Imaging 2013, 26, 1045–1057. [Google Scholar] [CrossRef]
- Lingle, W.; Erickson, B.J.; Zuley, M.L.; Jarosz, R.; Bonaccio, E.; Filippini, J.; Net, J.M.; Levi, L.; Morris, E.A.; Figler, G.G.; et al. Radiology Data from The Cancer Genome Atlas Breast Invasive Carcinoma [TCGA-BRCA] collection. Cancer Imaging Arch. 2016. [Google Scholar] [CrossRef]
- GitHub Repository with Code. Available online: https://github.com/spoliveira/HERclassHE.git (accessed on 21 June 2020).
- Madabhushi, A.; Lee, G. Image analysis and machine learning in digital pathology: Challenges and opportunities. Med. Image Anal. 2016, 33, 170–175. [Google Scholar] [CrossRef]
- Robertson, S.; Azizpour, H.; Smith, K.; Hartman, J. Digital image analysis in breast pathology—From image processing techniques to artificial intelligence. Transl. Res. 2017, 194, 19–35. [Google Scholar] [CrossRef] [PubMed]
- Tellez, D.; Balkenhol, M.; Otte-Höller, I.; van de Loo, R.; Vogels, R.; Bult, P.; Wauters, C.; Vreuls, W.; Mol, S.; Karssemeijer, N.; et al. Whole-Slide Mitosis Detection in H E Breast Histology Using PHH3 as a Reference to Train Distilled Stain-Invariant Convolutional Networks. IEEE Trans. Med Imaging 2018, 37, 2126–2136. [Google Scholar] [CrossRef]
- Cai, D.; Sun, X.; Zhou, N.; Han, X.; Yao, J. Efficient Mitosis Detection in Breast Cancer Histology Images by RCNN. In Proceedings of the 2019 IEEE 16th International Symposium on Biomedical Imaging (ISBI 2019), Venice, Italy, 8–11 April 2019; pp. 919–922. [Google Scholar] [CrossRef]
- Cruz-Roa, A.; Gilmore, H.; Basavanhally, A.; Feldman, M.; Ganesan, S.; Shih, N.; Tomaszewski, J.; Madabhushi, A.; González, F. High-throughput adaptive sampling for whole-slide histopathology image analysis (HASHI) via convolutional neural networks: Application to invasive breast cancer detection. PLoS ONE 2018, 13, e0196828. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Radulovic, M.; Kanjer, K.; Plataniotis, K.N. Discriminative Pattern Mining for Breast Cancer Histopathology Image Classification via Fully Convolutional Autoencoder. arXiv 2019, arXiv:1902.08670. [Google Scholar] [CrossRef]
- Romero, F.P.; Tang, A.; Kadoury, S. Multi-Level Batch Normalization In Deep Networks For Invasive Ductal Carcinoma Cell Discrimination In Histopathology Images. arXiv 2019, arXiv:1901.03684. [Google Scholar]
- Wan, T.; Cao, J.; Chen, J.; Qin, Z. Automated grading of breast cancer histopathology using cascaded ensemble with combination of multi-level image features. Neurocomputing 2017, 229, 34–44. [Google Scholar] [CrossRef]
- Cao, H.; Bernard, S.; Heutte, L.; Sabourin, R. Improve the Performance of Transfer Learning Without Fine-Tuning Using Dissimilarity-Based Multi-view Learning for Breast Cancer Histology Images. In Image Analysis and Recognition; Campilho, A., Karray, F., Ter Haar Romeny, B., Eds.; Springer International Publishing: Berlin/Heidelberg, Germany, 2018; pp. 779–787. [Google Scholar] [CrossRef]
- Vo, D.M.; Nguyen, N.Q.; Lee, S.W. Classification of breast cancer histology images using incremental boosting convolution networks. Inf. Sci. 2019, 482, 123–138. [Google Scholar] [CrossRef]
- Oscanoa, J.; Doimi, F.; Dyer, R.; Araujo, J.; Pinto, J.; Castaneda, B. Automated segmentation and classification of cell nuclei in immunohistochemical breast cancer images with estrogen receptor marker. In Proceedings of the 2016 38th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Orlando, FL, USA, 16–20 August 2016; pp. 2399–2402. [Google Scholar] [CrossRef]
- Saha, M.; Chakraborty, C.; Arun, I.; Ahmed, R.; Chatterjee, S. An Advanced Deep Learning Approach for Ki-67 Stained Hotspot Detection and Proliferation Rate Scoring for Prognostic Evaluation of Breast Cancer. Sci. Rep. 2017, 7. [Google Scholar] [CrossRef] [PubMed]
- Jamaluddin, M.F.; Fauzi, M.F.A.; Abas, F.S.; Lee, J.T.H.; Khor, S.Y.; Teoh, K.H.; Looi, L.M. Cell Classification in ER-Stained Whole Slide Breast Cancer Images Using Convolutional Neural Network. In Proceedings of the 2018 40th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Honolulu, HI, USA, 18–21 July 2018; pp. 632–635. [Google Scholar] [CrossRef]
- Rodner, E.; Simon, M.; Denzler, J. Deep bilinear features for Her2 scoring in digital pathology. Curr. Dir. Biomed. Eng. 2017, 3, 811–814. [Google Scholar] [CrossRef]
- Mukundan, R. A Robust Algorithm for Automated HER2 Scoring in Breast Cancer Histology Slides Using Characteristic Curves. In Medical Image Understanding and Analysis (MIUA); Springer: Cham, Switzerland, 2017; Volume 723, pp. 386–397. [Google Scholar] [CrossRef]
- Dou, Q.; Ouyang, C.; Chen, C.; Chen, H.; Heng, P.A. Unsupervised Cross-Modality Domain Adaptation of Convnets for Biomedical Image Segmentations with Adversarial Loss. In Proceedings of the 27th International Joint Conference on Artificial Intelligence (IJCAI); AAAI Press: Menlo Park, CA, USA, 2018; pp. 691–697. [Google Scholar] [CrossRef]
- Aderghal, K.; Khvostikov, A.; Krylov, A.; Benois-Pineau, J.; Afdel, K.; Catheline, G. Classification of Alzheimer Disease on Imaging Modalities with Deep CNNs Using Cross-Modal Transfer Learning. In Proceedings of the 2018 IEEE 31st International Symposium on Computer-Based Medical Systems (CBMS), Karlstad, Sweden, 18–21 June 2018; pp. 345–350. [Google Scholar] [CrossRef]
- Hadad, O.; Bakalo, R.; Ben-Ari, R.; Hashoul, S.; Amit, G. Classification of breast lesions using cross-modal deep learning. In Proceedings of the 2017 IEEE 14th International Symposium on Biomedical Imaging (ISBI 2017), Melbourne, Australia, 18–21 April 2017; pp. 109–112. [Google Scholar] [CrossRef]
- Bulten, W.; Bándi, P.; Hoven, J.; van de Loo, R.; Lotz, J.; Weiss, N.; van der Laak, J.; van Ginneken, B.; Hulsbergen-van de Kaa, C.; Litjens, G. Epithelium segmentation using deep learning in H&E-stained prostate specimens with immunohistochemistry as reference standard. Sci. Rep. 2019, 9. [Google Scholar] [CrossRef]
- Cruz-Roa, A.; Gilmore, H.L.; Basavanhally, A.; Feldman, M.D.; Ganesan, S.; Shih, N.C.; Tomaszewski, J.P.; Gonzalez, F.A.; Madabhushi, A. Accurate and reproducible invasive breast cancer detection in whole-slide images: A Deep Learning approach for quantifying tumor extent. Sci. Rep. 2017, 7. [Google Scholar] [CrossRef] [PubMed]
- Honari, S.; Molchanov, P.; Tyree, S.; Vincent, P.; Pal, C.; Kautz, J. Improving landmark localization with semi-supervised learning. In Proceedings of the 2018 IEEE/CVF Conference on Computer Vision and Pattern Recognition, Salt Lake City, UT, USA, 19–21 June 2018; pp. 1546–1555. [Google Scholar] [CrossRef]
- Chapelle, O.; Wu, M. Gradient descent optimization of smoothed information retrieval metrics. Inf. Retr. 2010, 13, 216–235. [Google Scholar] [CrossRef]
- Sedeen Viewer Software. Available online: https://pathcore.com/sedeen/ (accessed on 21 June 2020).
- Kingma, D.P.; Ba, J. Adam: A method for stochastic optimization. arXiv 2014, arXiv:1412.6980. [Google Scholar]
| True Class | |||||
|---|---|---|---|---|---|
| Prediction | 0 | 1 | 2 | 3 | |
| 0 | 490 | 132 | 2 | 0 | |
| 1 | 176 | 384 | 64 | 0 | |
| 2 | 45 | 159 | 419 | 1 | |
| 3 | 0 | 0 | 1 | 623 | |
| Accuracy | F1-Score | Precision | Recall | |
|---|---|---|---|---|
| HER2SC | 83.3% | 86.7% | 89.6% | 87.5% |
| BRCA | 53.8% | 21.5% | 81.2% | 31.5% |
| Method | Accuracy | F1-Score | Precision | Recall |
|---|---|---|---|---|
| MLP Aggregation: | ||||
| proposed method | 83.3% | 86.7% | 89.6% | 87.5% |
| w/out pretrained CNN weights | 62.5% | 48.1% | 39.1% | 62.5% |
| Median Aggregation: | ||||
| w/pretrained CNN weights | 50.0% | 43.3% | 78.6% | 50.0% |
| w/out pretrained CNN weights | 62.5% | 48.1% | 39.1% | 62.5% |
| Mean Aggregation: | ||||
| w/pretrained CNN weights | 50.0% | 43.3% | 78.6% | 50.0% |
| w/out pretrained CNN weights | 62.5% | 48.1% | 39.1% | 62.5% |
| Method | Accuracy | F1-Score | Precision | Recall |
|---|---|---|---|---|
| MLP Aggregation: | ||||
| proposed method | 53.3% | 21.5% | 81.2% | 31.5% |
| w/out pretrained CNN weights | 50.0% | 60.3% | 51.8% | 72% |
| Median Aggregation: | ||||
| w/pretrained CNN weights | 50.0% | 12.3% | 7.8% | 28.0% |
| w/out pretrained CNN weights | 52.2% | 63.5% | 66.5% | 72.0% |
| Mean Aggregation: | ||||
| w/pretrained CNN weights | 50.0% | 12.3% | 7.8% | 28.0% |
| w/out pretrained CNN weights | 52.2% | 63.5% | 66.5% | 72.0% |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oliveira, S.P.; Ribeiro Pinto, J.; Gonçalves, T.; Canas-Marques, R.; Cardoso, M.-J.; Oliveira, H.P.; Cardoso, J.S. Weakly-Supervised Classification of HER2 Expression in Breast Cancer Haematoxylin and Eosin Stained Slides. Appl. Sci. 2020, 10, 4728. https://doi.org/10.3390/app10144728
Oliveira SP, Ribeiro Pinto J, Gonçalves T, Canas-Marques R, Cardoso M-J, Oliveira HP, Cardoso JS. Weakly-Supervised Classification of HER2 Expression in Breast Cancer Haematoxylin and Eosin Stained Slides. Applied Sciences. 2020; 10(14):4728. https://doi.org/10.3390/app10144728
Chicago/Turabian StyleOliveira, Sara P., João Ribeiro Pinto, Tiago Gonçalves, Rita Canas-Marques, Maria-João Cardoso, Hélder P. Oliveira, and Jaime S. Cardoso. 2020. "Weakly-Supervised Classification of HER2 Expression in Breast Cancer Haematoxylin and Eosin Stained Slides" Applied Sciences 10, no. 14: 4728. https://doi.org/10.3390/app10144728
APA StyleOliveira, S. P., Ribeiro Pinto, J., Gonçalves, T., Canas-Marques, R., Cardoso, M.-J., Oliveira, H. P., & Cardoso, J. S. (2020). Weakly-Supervised Classification of HER2 Expression in Breast Cancer Haematoxylin and Eosin Stained Slides. Applied Sciences, 10(14), 4728. https://doi.org/10.3390/app10144728








