Allogeneic Demineralized Dentin Matrix Graft for Guided Bone Regeneration in Dental Implants
Abstract
1. Introduction
2. Materials and Methods
2.1. Source of Allogeneic Demineralized Dentin Matrix (Allo-DDM) and the Fabrication of Demineralized Dentin Matrix (DDM)
2.2. Study Design
2.3. Transplantation of DDM and Implant Placement
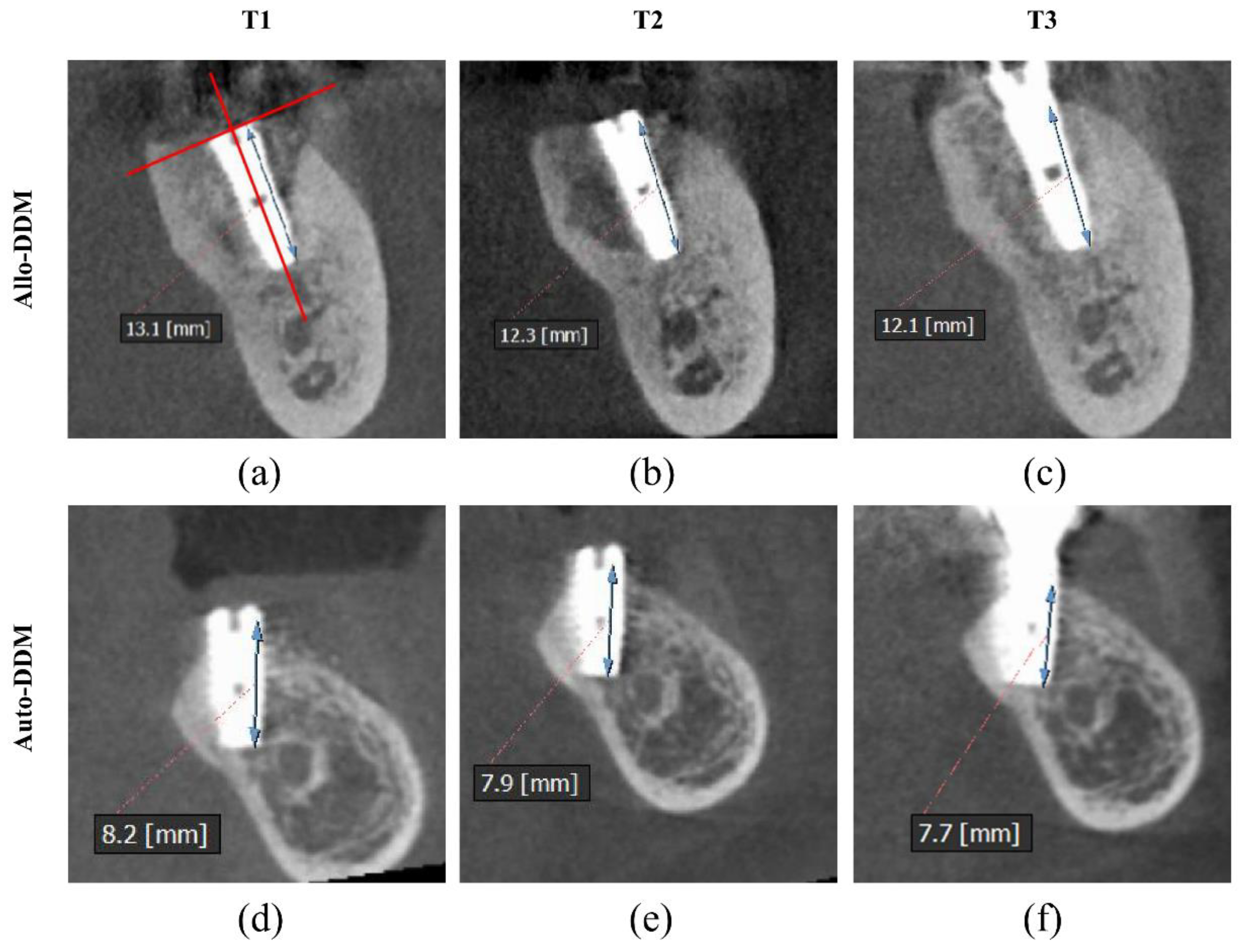
2.4. Measurement of Buccal Marginal Bone (BMB) Resorption Using Cone-Beam Computed Tomography (CBCT)
2.5. Statistical Analysis
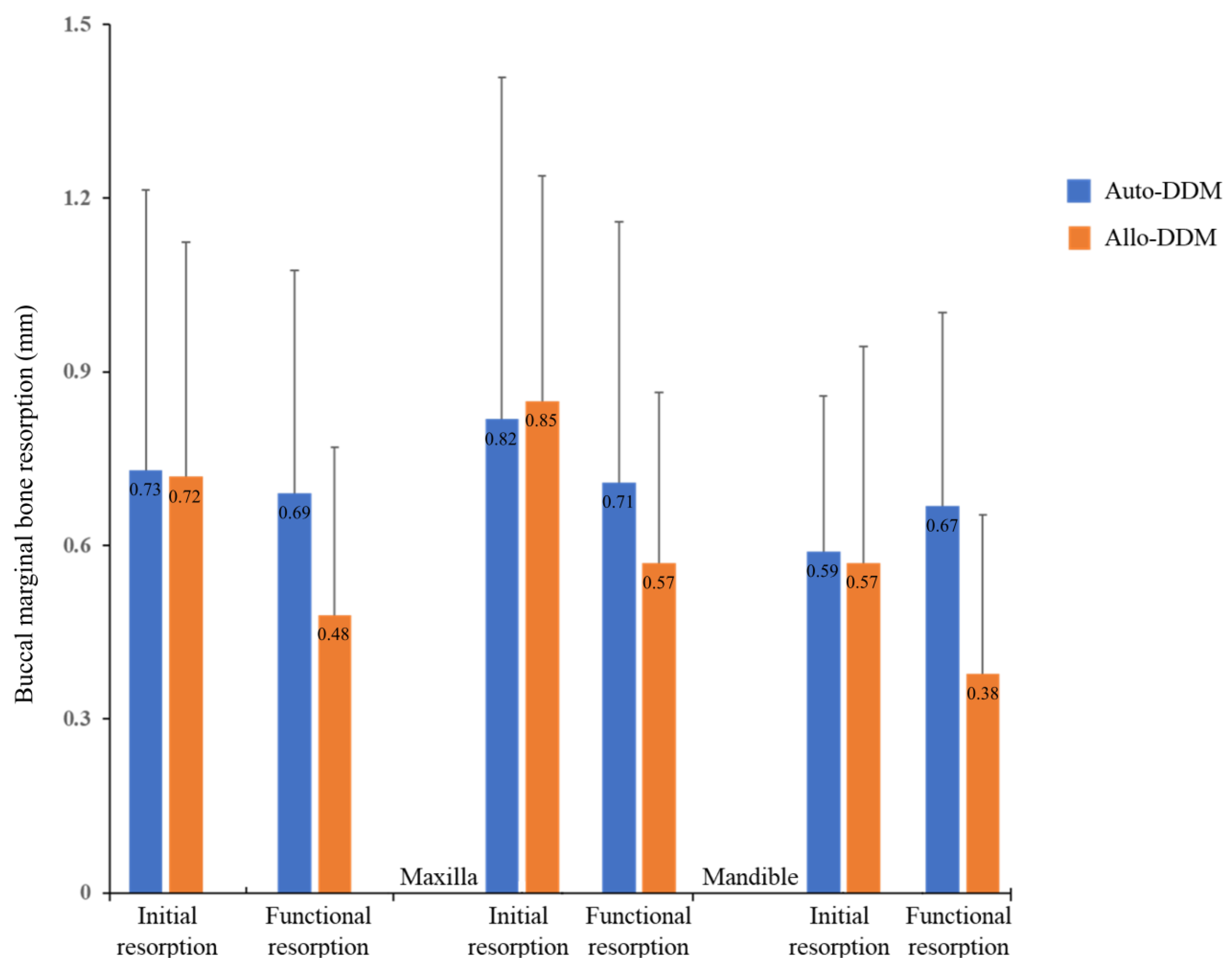
3. Results
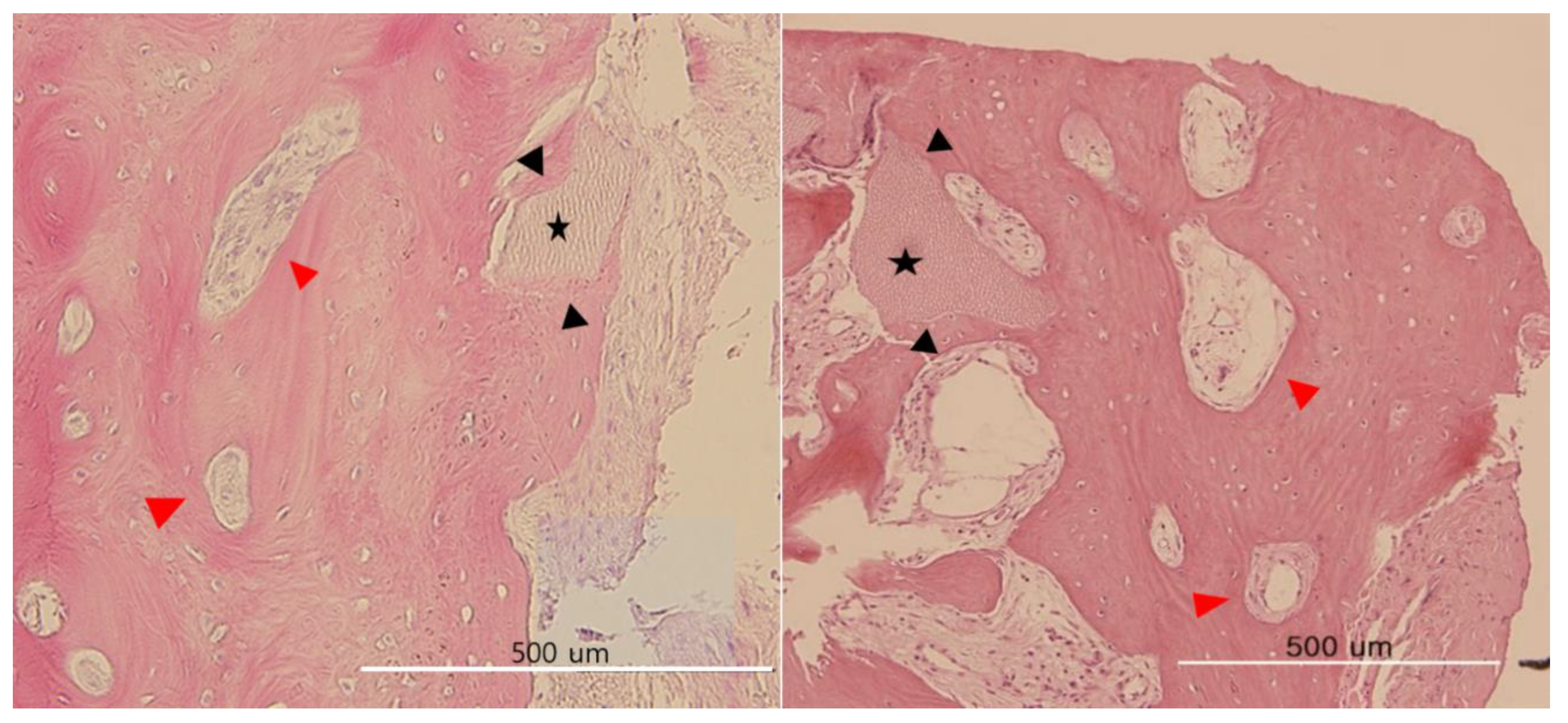
Histological Observations
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Kim, Y.K.; Kim, S.G.; Byeon, J.H.; Lee, H.J.; Um, I.U.; Lim, S.C.; Kim, S.Y. Development of a novel bone grafting material using autogenous teeth. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2010, 109, 496–503. [Google Scholar] [CrossRef]
- Pang, K.M.; Um, I.W.; Kim, Y.K.; Woo, J.M.; Kim, S.M.; Lee, J.H. Autogenous demineralized dentin matrix from extracted tooth for the augmentation of alveolar bone defect: A prospective randomized clinical trial in comparison with anorganic bovine bone. Clin. Oral Implant Res. 2017, 28, 809–815. [Google Scholar] [CrossRef]
- Ji-Young, L.; Young-Kyun, K.; Pil-Young, Y.; Ji-Su, O.; Su-Gwan, K. Guided bone regeneration using two types of non-resorbable barrier membranes. J. Korean Assoc. Oral Maxillofac. Surg. 2010, 36, 275–279. [Google Scholar]
- Masaru, M.; Okubo, N.; Shakya, M.; Kabir, M.A.; Yokozeki, K.; Zhu, B.; Ishikawa, M.; Kitamura, R.; Akazawa, T. Dentin Materials as Biological Scaffolds for Tissue Engineering. In Biomaterial-Supported Tissue Reconstruction or Regeneration; IntechOpen: London, UK, 2019; pp. 1–12. ISBN 978-1-83880-378-0. [Google Scholar]
- Murata, M.; Sato, D.; Hino, J.; Akazawa, T.; Tazaki, J.; Ito, K.; Arisue, M. Acid-insoluble human dentin as carrier material for recombinant human BMP-2. J. Biomed. Mater. Res. A 2012, 100, 571–577. [Google Scholar] [CrossRef] [PubMed]
- Marrazzo, P.; Paduano, F.; Palmieri, F.; Marrelli, M.; Tatullo, M. Highly Efficient In Vitro Reparative Behaviour of Dental Pulp Stem Cells Cultured with Standardised Platelet Lysate Supplementation. Stem Cells Int. 2016, 2016, 7230987. [Google Scholar] [CrossRef] [PubMed]
- Ballini, A.; Cantore, S.; Scacco, S.; Coletti, D.; Tatullo, M. Mesenchymal Stem Cells as Promoters, Enhancers, and Playmakers of the Translational Regenerative Medicine 2018. Stem Cells Int. 2018, 2018, 6927401. [Google Scholar] [CrossRef] [PubMed]
- Urist, M.R.; Dowell, T.A.; Hay, P.H.; Startes, B.S. Inductive Substrates for Bone Formation. Clin. Orthop. Relat. Res. 1968, 59, 59–96. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.K.; Lee, J.H.; Um, I.W.; Cho, W.J. Guided Bone Regeneration Using Demineralized Dentin Matrix: Long-Term Follow-Up. J. Oral. Maxillofac. Surg. 2016, 74, 515-e1. [Google Scholar] [CrossRef] [PubMed]
- Minetti, E.; Giacometti, E.; Gambardella, U.; Contessi, M.; Ballini, A.; Marenzi, G.; Celko, M.; Mastrangelo, F. Alveolar Socket Preservation with Different Autologous Graft Materials: Preliminary Results of a Multicenter Pilot Study in Human. Materials 2020, 13, 1153. [Google Scholar] [CrossRef]
- Li, P.; Zhu, H.; Huang, D. Autogenous DDM versus Bio-Oss granules in GBR for immediate implantation in periodontal postextraction sites: A prospective clinical study. Clin. Implant Dent. Relat. Res. 2018, 20, 923–928. [Google Scholar] [CrossRef]
- Minetti, E.; Palermo, A.; Contessi, M.; Gambardella, U.; Schmitz, J.; Giacometti, E.; Celko, M.; Trisi, P. Autologous tooth graft for maxillary sinus augmentation: A multicenter clinical study. Int. J. Growth Factors Stem Cells Dent. 2019, 2, 45–51. [Google Scholar] [CrossRef]
- Del Canto-Díaz, A.; de Elío-Oliveros, J.; Del Canto-Díaz, M.; Alobera-Gracia, M.A.; Del Canto-Pingarrón, M.; Martínez-González, J.M. Use of autologous tooth-derived graft material in the post-extraction dental socket. Pilot study. Med. Oral Patol. Oral Cir. Bucal 2019, 24, e53–e60. [Google Scholar] [CrossRef] [PubMed]
- Bang, G.; Urist, M.R. Bone induction in excavation chambers in matrix of decalcified dentin. Arch. Surg. 1967, 94, 781–789. [Google Scholar] [CrossRef]
- Bang, G.; Nordenram, Å.; Anneroth, G. Allogenic demineralized dentin implants in jaw defects of Java monkeys. Int. J. Oral Surg. 1972, 1, 126–136. [Google Scholar] [CrossRef]
- Bang, G. Induction of heterotopic bone formation by demineralized dentin in guinea pigs: Antigenicity of the dentin matrix. J. Oral Pathol. 1972, 1, 172–185. [Google Scholar] [CrossRef] [PubMed]
- Fugazzotto, P.A.; De Paoli, S.; Benfenati, S.P. The use of allogenic freeze-dried dentin in the repair of periodontal osseous defects in humans. Quintessence Int. 1986, 17, 461–477. [Google Scholar]
- Nordenram, A.; Bang, G.; Bernhoft, C.H. A clinical-radiographic study of allogenic demineralized dentin implants in cystic jaw cavities. Int. J. Oral Surg. 1975, 4, 61–64. [Google Scholar] [CrossRef]
- Kim, Y.K.; Kim, S.G.; Lim, S.C. Familial tooth bone graft for ridge and sinus augmentation: A report of two cases. J. Korean Assoc. Oral Maxillofac. Surg. 2014, 40, 37–42. [Google Scholar] [CrossRef]
- Kim, Y.K.; Bang, K.M.; Murata, M.; Mitsugi, M.; Um, I.W. Retrospective Clinical Study of Allogenic Demineralized Dentin Matrix for Alveolar Bone Repair. J. Hard Tissue Biol. 2017, 26, 95–102. [Google Scholar] [CrossRef]
- Joshi, C.P.; D’Lima, C.B.; Samat, U.C.; Karde, P.A.; Patil, A.G.; Dani, N.H. Comparative Alveolar Ridge Preservation Using Allogenous Tooth Graft versus Free-dried Bone Allograft: A Randomized, Controlled, Prospective, Clinical Pilot Study. Contemp. Clin. Dent. 2017, 8, 211–217. [Google Scholar] [CrossRef]
- Guidelines of Good Practice for Tooth Handling Institution. Korean Ministry of Health and Welfare. Available online: http://www.mohw.go.kr/upload/viewer/skin/doc.html?fn=1526013681690_20180511134121.pdf&rs=/upload/viewer/result/202006/ (accessed on 27 April 2018).
- Um, I.; Choi, S.; Kim, Y.; Pang, K.; Lee, J.; Lee, M.; Kim, B. Measurement of hepatitis B virus DNA in fresh versus processed dentin from chronically infected patients. J. Transl. Med. 2018, 16, 351. [Google Scholar] [CrossRef] [PubMed]
- Spellberg, B. The New Antibiotic Mantra-“Shorter Is Better”. JAMA Intern. Med. 2016, 176, 1254–1255. [Google Scholar] [CrossRef] [PubMed]
- Um, I.W.; Kim, Y.K.; Park, J.C.; Lee, J.H. Clinical application of autogenous demineralized dentin matrix loaded with recombinant human bone morphogenetic-2 for socket preservation: A case series. Clin. Implant. Dent. Relat. Res. 2019, 21, 4–10. [Google Scholar] [CrossRef] [PubMed]
- Jung, G.-U.; Jeon, T.-H.; Kang, M.-H.; Um, I.-W.; Song, I.-S.; Ryu, J.-J.; Jun, S.-H. Volumetric, Radiographic, and Histologic Analyses of Demineralized Dentin Matrix Combined with Recombinant Human Bone Morphogenetic Protein-2 for Ridge Preservation: A Prospective Randomized Controlled Trial in Comparison with Xenograft. Appl. Sci. 2018, 8, 1288. [Google Scholar] [CrossRef]
- Joshi, C.P.; Dani, N.H.; Khedkar, S.U. Alveolar ridge preservation using autogenous tooth graft versus beta-tricalcium phosphate alloplast: A randomized, controlled, prospective, clinical pilot study. J. Indian Soc. Periodontol. 2016, 20, 429–434. [Google Scholar] [CrossRef] [PubMed]
- Iasella, J.M.; Greenwell, H.; Miller, R.L.; Hill, M.; Drisko, C.; Bohra, A.A.; Scheetz, J.P. Ridge preservation with freeze-dried bone allograft and a collagen membrane compared to extraction alone for implant site development: A clinical and histologic study in humans. J. Periodontol. 2003, 74, 990–999. [Google Scholar] [CrossRef]
- Lekovic, V.; Kenney, E.B.; Weinlaender, M.; Han, T.; Klokkevold, P.; Nedic, M.; Orsini, M. A bone regenerative approach to alveolar ridge maintenance following tooth extraction. Report of 10 cases. J. Periodontol. 1997, 68, 563–570. [Google Scholar] [CrossRef]
- Simon, B.I.; Von Hagen, S.; Deasy, M.J.; Faldu, M.; Resnansky, D. Changes in alveolar bone height and width following ridge augmentation using bone graft and membranes. J. Periodontol. 2000, 71, 1774–1791. [Google Scholar] [CrossRef]
- Lekovic, V.; Camargo, P.M.; Klokkevold, P.R.; Weinlaender, M.; Kenney, E.B.; Dimitrijevic, B.; Nedic, M. Preservation of alveolar bone in extraction sockets using bioabsorbable membranes. J. Periodontol. 1998, 69, 1044–1049. [Google Scholar] [CrossRef]
- Papaspyridakos, P.; Chen, C.J.; Singh, M.; Weber, H.P.; Gallucci, G.O. Success criteria in implant dentistry: A systematic review. J. Dent. Res. 2012, 91, 242–248. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.Y.; Kim, Y.M.; Kim, J.Y.; Kim, M.R.; Kim, S.J. The retrospective study of marginal bone loss around dental implants according to different autogenous bone grafts. J. Korean Assoc. Oral Maxillofac. Surg. 2011, 37, 483–489. [Google Scholar] [CrossRef]
- Takuma, T.; Oishi, K.; Manabe, T.; Yoneda, S.; Nagata, T. Buccal bone resorption around posterior implants after surgery: A 1-year prospective study. Int. J. Oral Maxillofac. Implant. 2014, 29, 634–641. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Száva, D.-T.; Ormenișan, A.; Markovics, E.; Bögözi, B.; Mártha, K. Alveolar Bone Resorption Evaluation Around Single-piece Designed Bicortical Implants, Using Immediate Loading Protocol, Based on Orthopantomographs. J. Interdiscip. Med. 2017, 2, 328–331. [Google Scholar] [CrossRef]
- Trindade, R.; Albrektsson, T.; Tengvall, P.; Wennerberg, A. Foreign Body Reaction to Biomaterials: On Mechanisms for Buildup and Breakdown of Osseointegration. Clin. Implant Dent. Relat. Res. 2016, 18, 192–203. [Google Scholar] [CrossRef]
- Schwartz, O. Cell-mediated and humoral alloimmunreactions after subperiostal implantation of allogenic demineralized dentin in humans. Int. J. Oral Surg. 1983, 12, 95–105. [Google Scholar] [CrossRef]
- Carvalho, V.A.; Tosello Dde, O.; Salgado, M.A.; Gomes, M.F. Histomorphometric analysis of homogenous demineralized dentin matrix as osteopromotive material in rabbit mandibles. Int. J. Oral Maxillofac. Implant 2004, 19, 679–686. [Google Scholar]
- Gomes, M.F.; Banzi, E.C.; Destro, M.F.; Lavinicki, V.; Goulart, M. Homogenous demineralized dentin matrix for application in cranioplasty of rabbits with alloxan-induced diabetes: Histomorphometric analysis. Int. J. Oral Maxillofac. Implant 2007, 22, 939–947. [Google Scholar]
- Glowacki, J.; Kaban, L.B.; Murray, J.E.; Folkman, J.; Mulliken, J.B. Application of the biological principle of induced osteogenesis for craniofacial defects. Lancet 1981, 317, 959–962. [Google Scholar] [CrossRef]
- Tazaki, J.; Murata, M.; Yuasa, T.; Akazawa, T.; Ito, K.; Hino, J.; Nida, A.; Arisue, M.; Shibata, T. Autograft of human tooth and demineralized dentin matrices for bone augmentation. J. Ceram. Soc. Jpn. 2010, 118, 442–445. [Google Scholar] [CrossRef]
- Smith, J.G.; Smith, A.J.; Shelton, R.M.; Cooper, P.R. Antibacterial activity of dentine and pulp extracellular matrix extracts. Int. Endod. J. 2012, 45, 749–755. [Google Scholar] [CrossRef]
- Scarborough, N.L.; White, E.M.; Hughes, J.V.; Manrique, A.J.; Poser, J.W. Allograft safety: Viral inactivation with bone demineralization. Contemp. Orthop. 1995, 31, 257–261. [Google Scholar] [PubMed]
- Swenson, C.L.; Arnoczky, S.P. Demineralization for inactivation of infectious retrovirus in systemically infected cortical bone: In vitro and in vivo experimental studies. J. Bone Joint. Surg. Am. 2003, 85, 323–332. [Google Scholar] [CrossRef] [PubMed]

| Age (Years, SD) | p-Value | Buccal Bone Height (mm, SD) | ||||
|---|---|---|---|---|---|---|
| T1 | T2 | T3 | p-Value | |||
| Total (n = 96) | 57.13 (8.11) | 10.78 (1.84) | 10.15 (1.82) | 9.80 (1.64) | <0.001 * (Between each stage) | |
| Sex | ||||||
| Male (n = 59) | 56.13 (6.97) | 0.117 † | 11.31 (1.69) | 10.66 (1.81) | 10.22 (1.68) | T1: 0.397 † T2: 0.788 † T3: 0.576 † |
| Female (n = 37) | 58.73 (9.00) | 11.09 (1.81) | 10.45 (1.86) | 9.91 (1.80) | ||
| Location | ||||||
| Maxilla (n = 54) | 57.72 (6.97) | 0.410 † | 11.46 (1.69) | 10.68 (1.99) | 10.14 (1.87) | T1: 0.136 † T2: 0.531 † T3: 0.804 † |
| Mandible (n = 42) | 56.38 (8.93) | 10.92 (1.76) | 10.44 (1.60) | 10.15 (1.54) | ||
| Types of DDM | ||||||
| Auto-DDM (n = 44) | 56.45 (7.61) | 0.436 † | 11.76 (1.84) | 11.09 (1.70) | 10.45 (1.77) | T1: 0.004 † T2: 0.011 † T3: 0.065 † |
| Allo-DDM (n = 52) | 57.71 (8.11) | 10.78 (1.45) | 10.45 (1.82) | 9.80 (1.64) | ||
| Initial Resorption (T2-T1) | Functional Resorption (T3-T2) | p-Value | |
|---|---|---|---|
| Total | 0.72 (0.86) | 0.58 (0.70) | 0.272 * (Initial vs. Functional resorption) |
| Auto-DDM (n = 44) | 0.73 (0.97) | 0.69 (0.81) | Initial resorption: 0.973 † Functional resorption: 0.141 † |
| Allo-DDM (n = 52) | 0.72 (0.77) | 0.48 (0.58) | |
| Maxilla (n = 54) | 0.84 (0.98) | 0.64 (0.76) | Initial resorption: 0.145 † Functional resorption: 0.374 † |
| Mandible (n = 42) | 0.58 (0.66) | 0.51 (0.61) | |
| Maxilla | |||
| Auto-DDM (n = 26) | 0.82 (1.18) | 0.71 (0.90) | Initial resorption: 0.899 † Functional resorption: 0.502 † |
| Allo-DDM (n = 28) | 0.85 (0.78) | 0.57 (0.59) | |
| Mandible | |||
| Auto-DDM (n = 18) | 0.59 (0.54) | 0.67 (0.67) | Initial resorption: 0.889 † Functional resorption: 0.133 † |
| Allo-DDM (n = 24) | 0.57 (0.75) | 0.38 (0.55) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Um, I.-W.; Ku, J.-K.; Kim, Y.-M.; Yun, P.-Y.; Chang, N.-H.; Kim, Y.-K.; Choi, Y. Allogeneic Demineralized Dentin Matrix Graft for Guided Bone Regeneration in Dental Implants. Appl. Sci. 2020, 10, 4661. https://doi.org/10.3390/app10134661
Um I-W, Ku J-K, Kim Y-M, Yun P-Y, Chang N-H, Kim Y-K, Choi Y. Allogeneic Demineralized Dentin Matrix Graft for Guided Bone Regeneration in Dental Implants. Applied Sciences. 2020; 10(13):4661. https://doi.org/10.3390/app10134661
Chicago/Turabian StyleUm, In-Woong, Jeong-Kui Ku, Yu-Mi Kim, Pil-Young Yun, Na-Hee Chang, Young-Kyun Kim, and Yonghoon Choi. 2020. "Allogeneic Demineralized Dentin Matrix Graft for Guided Bone Regeneration in Dental Implants" Applied Sciences 10, no. 13: 4661. https://doi.org/10.3390/app10134661
APA StyleUm, I.-W., Ku, J.-K., Kim, Y.-M., Yun, P.-Y., Chang, N.-H., Kim, Y.-K., & Choi, Y. (2020). Allogeneic Demineralized Dentin Matrix Graft for Guided Bone Regeneration in Dental Implants. Applied Sciences, 10(13), 4661. https://doi.org/10.3390/app10134661








