Preparation, Sinterability, Electrical Transport and Thermal Expansion of Perovskite-Type La0.8Ca0.2CrO3 Composites
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Characterization
3. Results
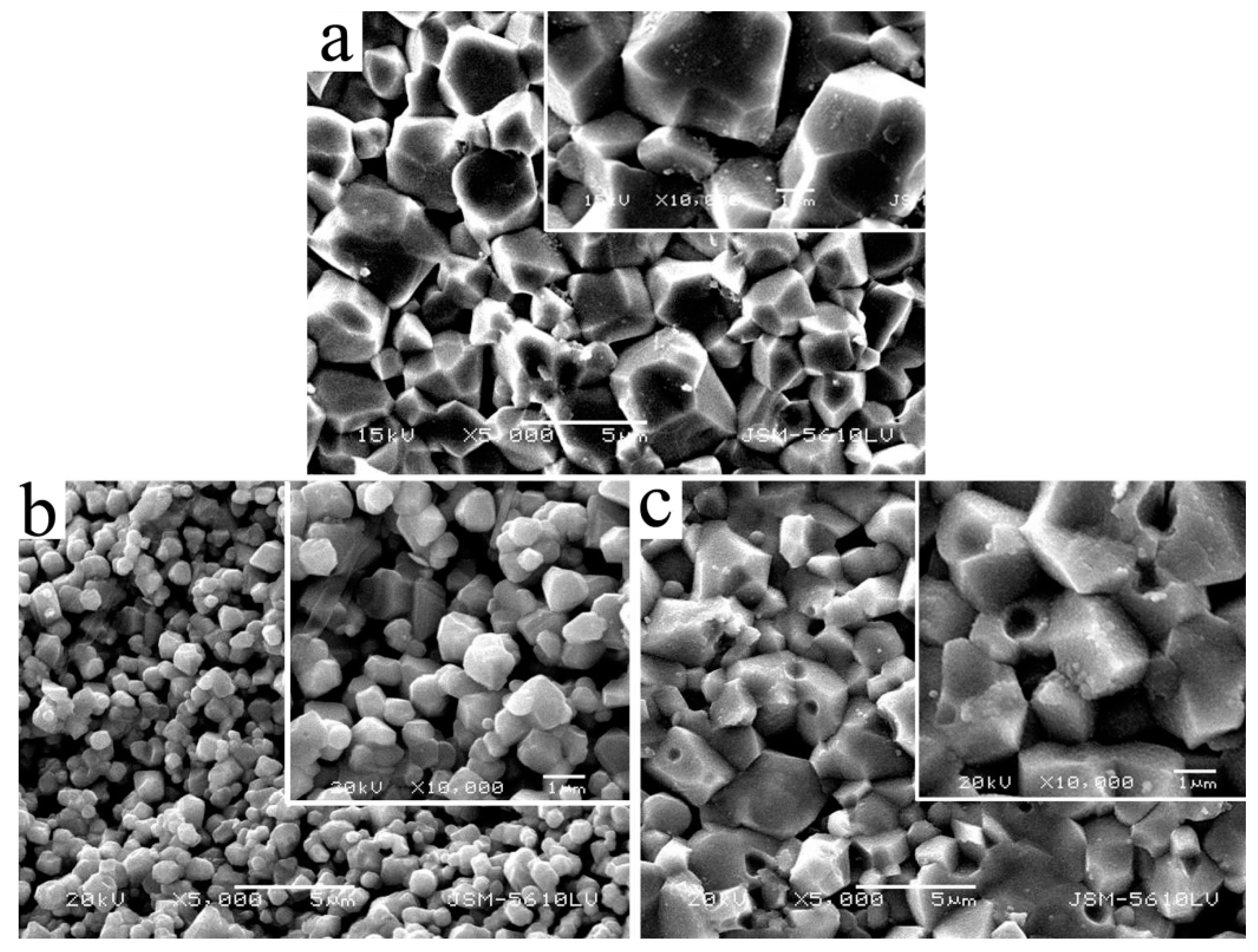
3.1. XRD and SEM of the La0.8Ca0.2CrO3 Powders
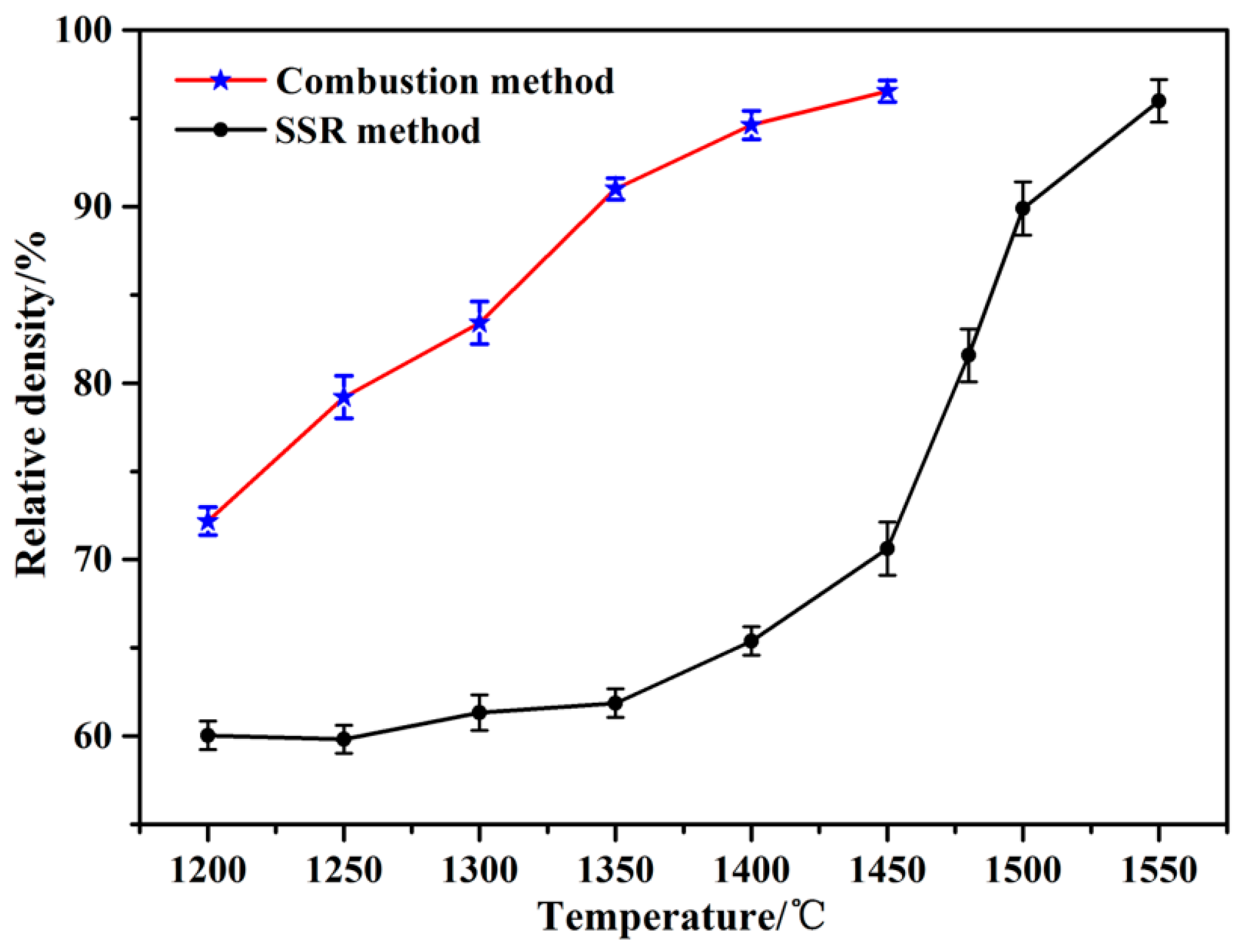
3.2. Sintering Characterization
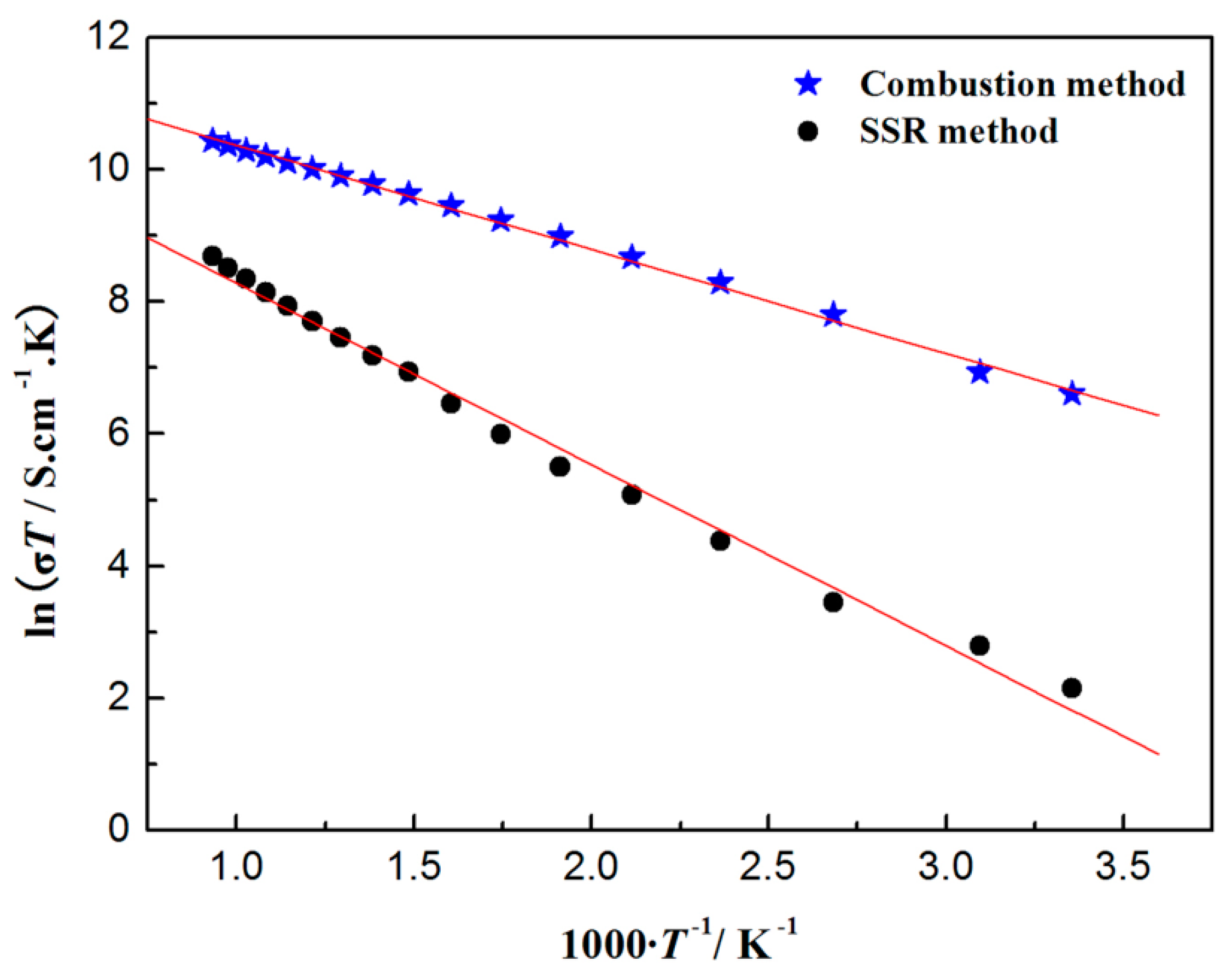
3.3. Electrical Transport
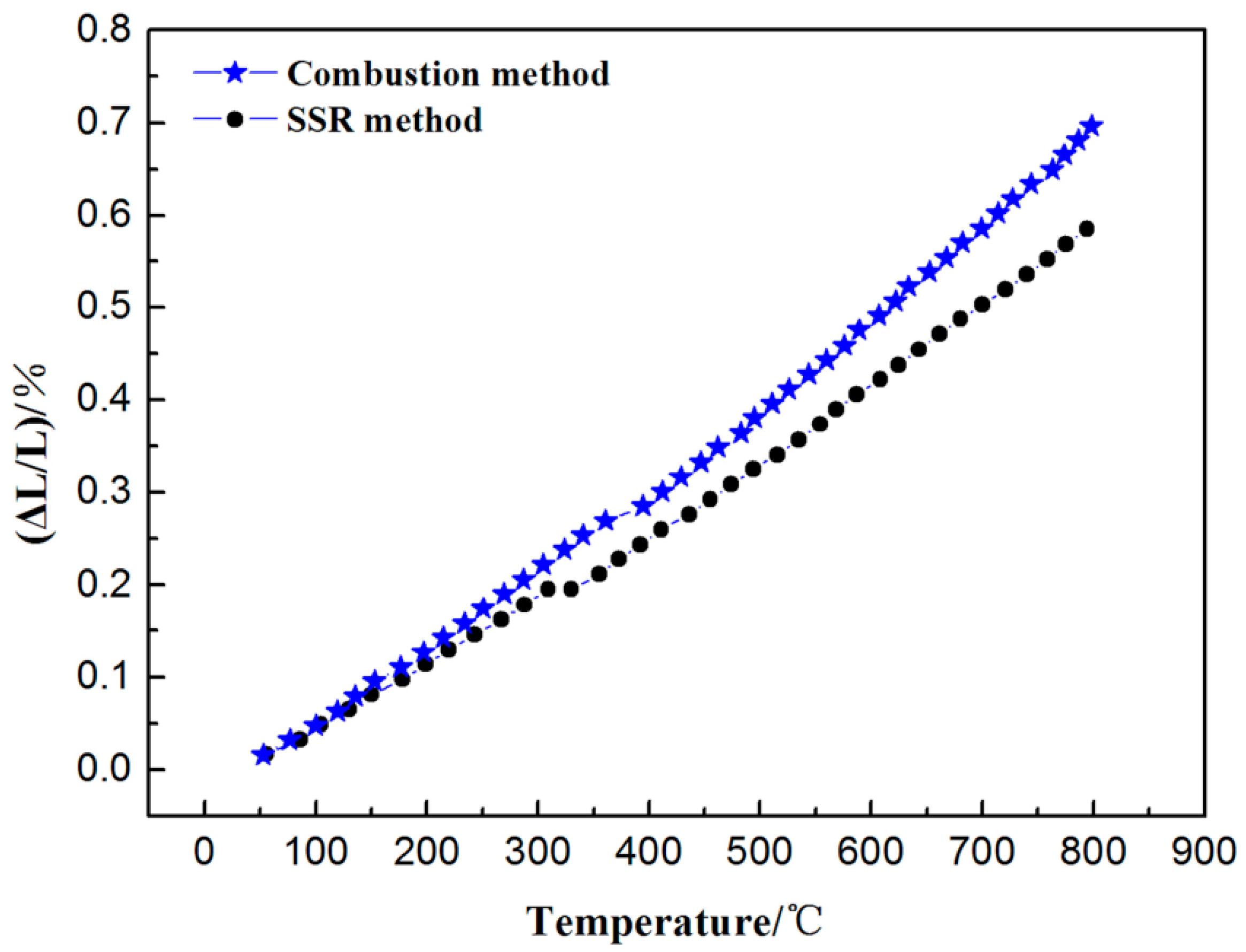
3.4. Thermal Expansion
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Feduska, W.; Isenberg, A.O. High-temperature solid oxide fuel cell-technical status. J. Power Sources 1983, 10, 89–102. [Google Scholar] [CrossRef]
- Minh, N. Ceramic Fuel Cells. J. Am. Ceram. Soc. 1993, 76, 563–588. [Google Scholar] [CrossRef]
- Zhu, W.Z.; Deevi, S.C. Development of interconnect materials for solid oxide fuel cells. Mater. Sci. Eng. A 2003, 348, 227–243. [Google Scholar] [CrossRef]
- Zhou, X.L.; Ma, J.; Deng, F.; Meng, G.Y.; Liu, X.Q. A high performance interconnecting ceramics for solid oxide fuel cells (SOFCs). Solid State Ionics 2007, 177, 3461–3466. [Google Scholar] [CrossRef]
- Singhal, S.C.; Kendall, K. (Eds.) High-Temperature Solid Oxide Fuel Cells: Fundamentals, Design and Applications; Elsevier: Amsterdam, The Netherlands, 2003. [Google Scholar] [CrossRef]
- Fergus, J. Lanthanum chromite-based materials for solid oxide fuel cell interconnects. Solid State Ionics 2004, 171, 1–15. [Google Scholar] [CrossRef]
- Groupp, L.; Adersen, H.U. Densification of La1-xSrxCrO3. J. Am. Ceram. Soc. 1976, 59, 449–450. [Google Scholar] [CrossRef]
- Yokokawa, H.; Sakai, N.; Kawada, T.; Dokiya, M. Thermodynamic analysis of reaction profiles between LaMO3 (M = Ni, Co, Mn) and ZrO2. J. Electrochem. Soc. 1991, 138, 2719–2726. [Google Scholar] [CrossRef]
- Mori, M.; Yamamoto, T.; Ichcikawa, T.; Takeda, Y. Dense sintered conditions and sintering mechanisms for alkaline earth metal (Mg, Ca and Sr)-doped LaCrO3 perovskites under reducing atmosphere. Solid State Ionics 2002, 148, 93–101. [Google Scholar] [CrossRef]
- Chick, L.; Liu, J.; Stevenson, J.; Armstrong, T.R.; McCready, D.E.; Maupin, G.D.; Coffey, G.W.; Coyle, C.A. Phase Transitions and Transient Liquid-Phase Sintering in Calcium-Substituted Lanthanum Chromite. J. Am. Ceram. Soc. 1997, 80, 2109–2120. [Google Scholar] [CrossRef]
- Kallarackel, T.; Gupta, S.; Singh, P. Thermodynamic Properties of LaCrO4, La2CrO6, and La2Cr3O12, and Subsolidus Phase Relations in the System Lanthanum-Chromium-Oxygen. J. Am. Ceram. Soc. 2013, 96, 3939–3948. [Google Scholar] [CrossRef]
- Stevenson, J.W.; Hallman, P.F.; Armstrong, T.R.; Chick, L.A. Sintering Behavior of Doped Lanthanum and Yttrium Manganite. J. Am. Ceram. Soc. 1995, 78, 507–512. [Google Scholar] [CrossRef]
- Yasuda, I.; Hikita, T. Formation of calcium chromate hydroxylapatite on the surface of a calcium-doped lanthanum chromite sintered body. J. Mater. Sci. 1994, 29, 2801–2805. [Google Scholar] [CrossRef]
- Masashi, M.; Yoshiko, H.; Nigel, M.S. Sintering behavior and mechanism of Sr-doped lanthanum chromites with a site excess composition in air. Solid State Ionics 1999, 123, 103–111. [Google Scholar]
- Guo, W.; Wang, Y.; Li, A.; Jiao, T.; Gao, F. Effect of sintering temperature on microstructure, electrical properties, and thermal expansion of perovskite-type La0.8Ca0.2CrO3 complex oxides synthesized by a combustion method. J. Electron. Mater. 2013, 42, 939–943. [Google Scholar] [CrossRef]
- Guo, W.; Xu, Q.; Huang, D.; Jiao, T.; Gao, F. Investigation on the calcining condition and sintering temperature of perovskite-type La0.7Ca0.3CrO3 complex oxides by a combustion method. Mater. Res. Bull. 2012, 47, 2067–2071. [Google Scholar] [CrossRef]
- Chick, L.; Pederson, L.; Maupin, G.; Maupin, J.; Bates, L.; Thomas, G. Glycine-Nitrate Combustion Synthesis of Oxide Ceramic Powder. Mater. Lett. 1990, 10, 6–12. [Google Scholar] [CrossRef]
- Armstrong, T.R.; Stevenson, J.W.; Hansinska, K.; McCready, D.E. Synthesis and Properties of Mixed Lanthanide Chromite Perovskites. J. Electrochem. Soc. 1998, 145, 4282–4289. [Google Scholar] [CrossRef]
- Xiong, H.; Zhang, G.J.; Zheng, J.Y. Synthesis, crystal structure and electric conductivity of La0.9Ca0.1Cr0.5B0.5O3 (B = Mn, Fe, Ni). Mater. Lett. 2001, 51, 61–67. [Google Scholar] [CrossRef]
- Mori, M.; Hiei, Y.; Yamamoto, T. Thermal Expansion Control Technique of Sr-Doped Lanthanum Chromite Perovskites by B-Site Doping for High-Temperature Solid Oxide Fuel Cell Separators. J. Am. Ceram. Soc. 2001, 84, 781–786. [Google Scholar] [CrossRef]
- Sakai, N.N.; Stolen, S. Heat capacity and thermodynamic properties of lanthanum(III) chromate(III): LaCrO3, at temperatures from 298.15 K. Evaluation of the thermal conductivity. J. Chem. Thermodyn. 1995, 27, 493–506. [Google Scholar] [CrossRef]
- Hayashi, H.; Watanabe, M.; Inaba, H. Measurement of Thermal Expansion Coefficient of LaCrO3. Thermochim. Acta 2000, 359, 77–85. [Google Scholar] [CrossRef]
- Gupta, S.; Mahapatra, M.; Singh, P. Phase transformation, thermal expansion and electrical conductivity of lanthanum chromite. Mater. Res. Bull. 2013, 48, 3262–3267. [Google Scholar] [CrossRef]
- Ge, L.; Zhang, M.; Wang, R.; Li, N.; Zhang, L.; Liu, S.; Jiao, T. Fabrication of CS/GA/RGO/Pd composite hydrogels for highly efficient catalytic reduction of organic pollutants. RSC Adv. 2020, 10, 15091–15097. [Google Scholar] [CrossRef]
- Wang, R.; Yan, X.; Ge, B.; Zhou, J.; Wang, M.; Zhang, L.; Jiao, T. Facile preparation of self-assembled black phosphorus-dye composite films for chemical gas sensors and surface-enhanced Raman scattering performances. ACS Sustainable Chem. Eng. 2020, 8, 4521–4536. [Google Scholar] [CrossRef]
- Feng, Y.; Yin, J.; Liu, S.; Wang, Y.; Li, B.; Jiao, T. Facile synthesis of Ag/Pd nanoparticles-loaded polyethyleneimine composite hydrogels with highly efficient catalytic reduction of 4-nitrophenol. ACS Omega 2020, 5, 3725–3733. [Google Scholar] [CrossRef]
- Zhang, L.; Yin, J.; Wei, K.; Li, B.; Jiao, T.; Chen, Y.; Zhou, J.; Peng, Q. Fabrication of hierarchical SrTiO3@MoS2 heterostructure nanofibers as efficient and low-cost electrocatalyst for hydrogen evolution reaction. Nanotechnology 2020, 31, 205604. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Liu, R.; Chen, H.; Li, H.; Guo, P. Synthesis of self-assembled nickel cobaltite microspheres and their electrocapacitive behavior in aqueous electrolytes. Colloid Surf. A 2020, 587, 124329. [Google Scholar] [CrossRef]
- Sun, J.; Yang, M.; Gong, Y.; Li, H.; Guo, P. Synthesis of Pd3Pb colloidal nanocrystal assembly and their electrocatalytic activity toward ethanol oxidation. Colloid Surf. A 2020, 586, 124224. [Google Scholar] [CrossRef]

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, L.; Jiang, M.; Yin, J.; Guo, W.; Jiao, T. Preparation, Sinterability, Electrical Transport and Thermal Expansion of Perovskite-Type La0.8Ca0.2CrO3 Composites. Appl. Sci. 2020, 10, 4634. https://doi.org/10.3390/app10134634
Liu L, Jiang M, Yin J, Guo W, Jiao T. Preparation, Sinterability, Electrical Transport and Thermal Expansion of Perovskite-Type La0.8Ca0.2CrO3 Composites. Applied Sciences. 2020; 10(13):4634. https://doi.org/10.3390/app10134634
Chicago/Turabian StyleLiu, Linlin, Mingmei Jiang, Juanjuan Yin, Wenfeng Guo, and Tifeng Jiao. 2020. "Preparation, Sinterability, Electrical Transport and Thermal Expansion of Perovskite-Type La0.8Ca0.2CrO3 Composites" Applied Sciences 10, no. 13: 4634. https://doi.org/10.3390/app10134634
APA StyleLiu, L., Jiang, M., Yin, J., Guo, W., & Jiao, T. (2020). Preparation, Sinterability, Electrical Transport and Thermal Expansion of Perovskite-Type La0.8Ca0.2CrO3 Composites. Applied Sciences, 10(13), 4634. https://doi.org/10.3390/app10134634





