A Textile-Based Microfluidic Platform for the Detection of Cytostatic Drug Concentration in Sweat Samples
Abstract
Featured Application
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of the Fabrics
2.2. Synthesis of Colloidal TiO2 Nanoparticles
2.3. Synthesis of PANI/TiO2 on CO and PET Fabrics
2.4. Preparation of the Sweat Samples
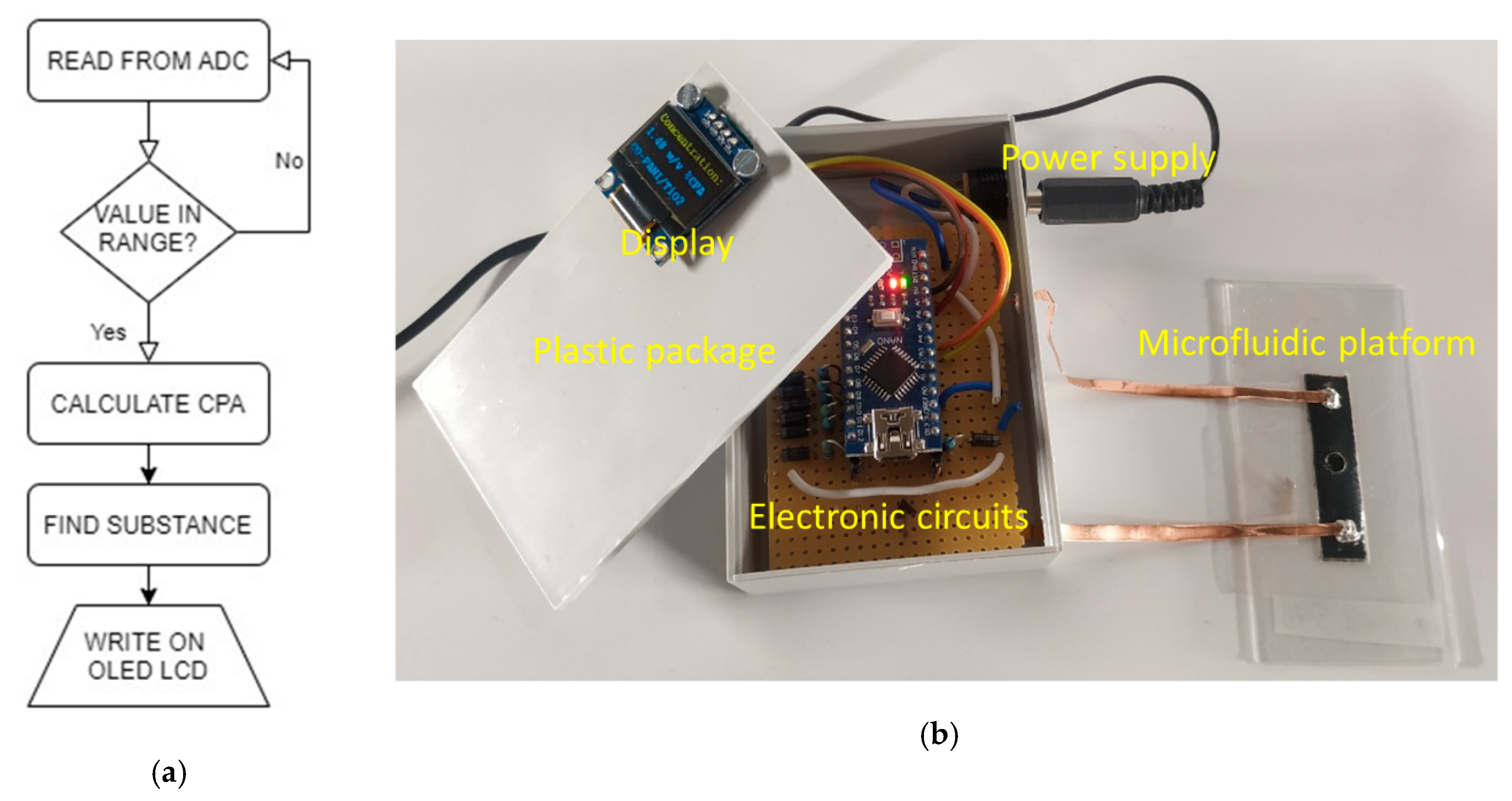
2.5. Design of the Microfluidic Platform
2.6. Characterization Methods
3. Results and Discussion
3.1. SEM and FTIR Analysis
3.2. Electrical Parameter Testing and Drug Detection
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Zhang, Y.; Cui, Y. Cotton-based wearable PEDOT:PSS electronic sensor for detecting acetone vapour. Flex. Print. Electron. 2017, 2, 042001. [Google Scholar] [CrossRef]
- Smith, R.E.; Totti, S.; Velliou, E.; Campagnolo, P.; Hingley-Wilson, S.M.; Ward, N.I.; Varcoe, J.R.; Crean, C. Development of a novel highly conductive and flexible cotton yarn for wearable pH sensor technology. Sens. Actuators B Chem. 2019, 287, 338–345. [Google Scholar] [CrossRef]
- Bhat, N.V.; Seshadri, D.T.; Radhakrishnan, S. Preparation, Characterization, and Performance of Conductive Fabrics: Cotton + PANI. Text. Res. J. 2004, 74, 155–166. [Google Scholar] [CrossRef]
- Tissera, N.D.; Wijesena, R.N.; Rathnayake, S.; Silva, R.M.; Nalin, K.M. Heterogeneous in situ polymerization of Polyaniline (PANI) nanofibers on cotton textiles: Improved electrical conductivity, electrical switching, and tuning properties. Carbohydr. Polym. 2018, 186, 35–44. [Google Scholar] [CrossRef]
- Onar, N.; Aksit, A.C.; Ebeoglugil, M.F.; Birlik, I.; Celik, E.; Ozdemir, I. Structural, Electrical, and Electromagnetic Properties of Cotton Fabrics Coated with Polyaniline and Polypyrrole. J. Appl. Polym. Sci. 2009, 114, 2003–2010. [Google Scholar] [CrossRef]
- Altinok, A.S.; Ucgul, I.; Oksuz, A.U. Production of polyester/polyaniline, cotton/polyaniline composite fabrics and examining electrical characteristics. Tekst. Appar./Tekst. Ve Konfeksiyon 2014, 24, 21–25. [Google Scholar]
- Engin, F.A.; Usta, I. Electromagnetic shielding effectiveness of polyester fabrics with polyaniline deposition. Text. Res. J. 2014, 84, 903–912. [Google Scholar] [CrossRef]
- Youssefi, M.; Motamedi, F. An electrically conductive hybrid polyaniline/silver-coated polyester fabric for smart applications. J. Ind. Text. 2019, 4, 1–16. [Google Scholar] [CrossRef]
- Hong, K.H.; Oh, K.W.; Kang, T.J. Polyaniline–nylon 6 composite fabric for ammonia gas sensor. J. Appl. Polym. Sci. 2004, 92, 37–42. [Google Scholar] [CrossRef]
- Hirase, R.; Shikata, T.; Shirai, M. Selective formation of polyaniline on wool by chemical polymerization, using potassium iodate. Synth. Met. 2004, 146, 73–77. [Google Scholar] [CrossRef]
- Nouri, M.; Haghighatkish, H.; Entezami, A.A.; Edrisi, E. Conductivity of textile fibres treated with aniline. Iran. Polym. J. 2000, 9, 49–58. [Google Scholar]
- Teli, M.; Dash, S.; Desai, P. Polyaniline Based Conductive Textiles. J. Inst. Eng. India Ser. E 2014, 95, 75–79. [Google Scholar] [CrossRef]
- Hoghoghifard, S.; Mokhtari, H.; Dehghani, S. Improving the conductivity of polyaniline coated polyester textile by optimizing the synthesis conditions. J. Ind. Text. 2016, 46, 611–623. [Google Scholar] [CrossRef]
- Choi, J.; Kang, D.; Han, S.; Kim, S.B.; Rogers, J.A. Thin, Soft, Skin-Mounted Microfluidic Networks with Capillary Bursting Valves for Chrono-Sampling of Sweat. Adv. Healthc. Mater. 2017, 6, 1601355. [Google Scholar] [CrossRef] [PubMed]
- Martín, A.; Kim, J.; Kurniawan, J.F.; Sempionatto, J.R.; Moreto, J.R.; Tang, G.; Campbell, A.S.; Shin, A.; Lee, M.Y.; Liu, X.; et al. Epidermal Microfluidic Electrochemical Detection System: Enhanced Sweat Sampling and Metabolite Detection. ACS Sens. 2017, 2, 1860–1868. [Google Scholar] [CrossRef] [PubMed]
- Alizadeh, A.; Burns, A.; Lenigk, R.; Gettings, R.; Ashe, J.; Porter, A.; McCaul, M.; Barrett, R.; Diamond, D.; White, P.; et al. A wearable patch for continuous monitoring of sweat electrolytes during exertion. Lab A Chip 2018, 18, 2632–2641. [Google Scholar] [CrossRef]
- Baysal, G.; Kök, F.N.; Trabzon, L.; Kızıl, H.; Göcek, İ.; Kayaoğlu, B.K. Microfluidic Nonwoven-Based Device as a Potential Biosensor for Sweat Analysis. Appl. Mech. Mater. 2014, 490–491, 274–279. [Google Scholar] [CrossRef]
- Koh, A.; Kang, D.; Xue, Y.; Lee, S.; Pielak, R.M.; Kim, J.; Hwang, T.; Min, S.; Banks, A.; Bastien, P.; et al. A soft, wearable microfluidic device for the capture, storage, and colorimetric sensing of sweat. Sci. Transl. Med. 2016, 8, 366ra165. [Google Scholar] [CrossRef]
- Garcia-Cordero, E.; Bellando, F.; Zhang, J.; Wildhaber, F.; Longo, J.; Guérin, H.; Ionescu, A.M. Three-Dimensional Integrated Ultra-Low-Volume Passive Microfluidics with Ion-Sensitive Field-Effect Transistors for Multiparameter Wearable Sweat Analyzers. ACS Nano 2018, 12, 12646–12656. [Google Scholar] [CrossRef]
- Reeder, J.T.; Choi, J.; Xue, Y.; Gutruf, P.; Hanson, J.; Liu, M.; Ray, T.; Bandodkar, A.J.; Avila, R.; Xia, W.; et al. Waterproof, electronics-enabled, epidermal microfluidic devices for sweat collection, biomarker analysis, and thermography in aquatic settings. Sci. Adv. 2019, 5, eaau6356. [Google Scholar] [CrossRef]
- Yuan, Z.; Hou, L.; Bariya, M.; Nyein, H.Y.Y.; Tai, L.C.; Ji, W.; Li, L.; Javey, A. A Multi-Modal Sweat Sensing Patch for Cross-Verification of Sweat Rate, Total Ionic Charge, and Na+ Concentration. Lab A Chip 2019, 19, 3179–3189. [Google Scholar] [CrossRef] [PubMed]
- Tai, L.C.; Gao, W.; Chao, M.; Bariya, M.; Ngo, Q.P.; Shahpar, Z.; Nyein, H.Y.Y.; Park, H.; Sun, J.; Jung, Y.; et al. Methylxanthine Drug Monitoring with Wearable Sweat Sensors. Adv. Mater. 2018, 30, 1707442. [Google Scholar] [CrossRef] [PubMed]
- Xiao, G.; He, J.; Chen, X.; Qiao, Y.; Wang, F.; Xia, Q.; Yu, L.; Lu, Z. A wearable, cotton thread/paper-based microfluidic device coupled with smartphone for sweat glucose sensing. Cellulose 2019, 26, 4553–4562. [Google Scholar] [CrossRef]
- Van Agthoven, M.; Sonneveld, P.; Verdonck, L.F.; Uyl-De Groot, C.A. Cost determinants in aggressive non-Hodgkin's lymphoma. Haematologica 2005, 90, 661–671. [Google Scholar]
- Lescoat, A.; Droitcourt, C.; Stock, N.; le Gall, F.; Dupuy, A. Vemurafenib-Induced Eccrine Squamous Syringometaplasia. Dermatology 2013, 226, 362–364. [Google Scholar] [CrossRef]
- Gupta, M.; Huang, V.; Linette, G.; Cornelius, L. Unusual complication of vemurafenib treatment of metastatic melanoma: Exacerbation of acantholytic dyskeratosis complicated by Kaposi varicelliform eruption. Arch. Dermatol. 2012, 148, 966–968. [Google Scholar] [CrossRef]
- Radoičić, M. Nanocomposites Based on Polyaniline and Titan (IV) Oxide: Synthesis, Characterization and Application in Photocatalysis. Ph.D. Thesis, University of Belgrade, Beograd, Serbia, 2013. [Google Scholar]
- Budavari, S.; O’Neil, M.J.; Smith, A.; Heckelman, P.E. (Eds.) The Merck Index, 11th ed.; Merck: Rahway, NJ, USA, 1989. [Google Scholar]
- Radoičić, M.; Ćirić-Marjanović, G.; Šaponjić, Z.; Mitrić, M.; Konstantinović, Z.; Stoiljković, M.; Nedeljković, J. Structural and magnetic properties of nanocomposites based on nanostructured polyaniline and titania nanotubes. J. Mater. Sci. 2013, 48, 5776–5787. [Google Scholar] [CrossRef]
- Radoičić, M.; Milošević, M.; Miličević, D.; Suljovrujić, E.; Ćirić-Marjanović, G.; Radetić, M.; Šaponjić, Z. Influence of TiO2 nanoparticles on formation mechanism of PANI/TiO2 nanocomposite coating on PET fabric and its structural and electrical properties. Surf. Coat. Technol. 2015, 278, 38–47. [Google Scholar] [CrossRef]
- Milovanovic, S.; Stamenic, M.; Markovic, D.; Radetic, M.; Zizovic, I. Solubility of thymol in supercritical carbon dioxide and its impregnation on cotton gauze. J. Supercrit. Fluid. 2013, 84, 173–181. [Google Scholar] [CrossRef]
- Tomšič, B.; Simončič, B.; Orel, B.; Vilčnik, A.; Spreizer, H. Biodegradability of cellulose fabric modified by imidazolidinone. Carbohyd. Polym. 2007, 69, 478–488. [Google Scholar] [CrossRef]
- Hofstetter, K.; Hinterstoisser, B.; Salmén, L. Moisture uptake in native cellulose—the roles of different hydrogen bonds: A dynamic FT-IR study using Deuterium exchange. Cellulose 2006, 13, 131–145. [Google Scholar] [CrossRef]
- Kondo, T. The assignment of IR absorption bands due to the freehydroxyl groups in cellulose. Cellulose 1997, 4, 281–292. [Google Scholar] [CrossRef]
- Łojewska, J.; Miskowiec, P.; Łojewski, T.; Proniewicz, L.M. Cellulose oxidative and hydrolytic degradation: In situ FTIR approach. Polym. Degrad. Stabil. 2005, 88, 512–520. [Google Scholar] [CrossRef]
- Radoičić, M.; Šaponjić, Z.; Nedeljković, J.; Ćirić-Marjanović, G.; Stejskal, J. Self-assembled polyaniline nanotubes and nanoribbons/titanium dioxide nanocomposites. Synth. Met. 2010, 160, 1325–1334. [Google Scholar] [CrossRef]
- Trchova, M.; Konyushenko, E.N.; Stejskal, J.; Šedenkova, I.; Holler, P.; Ćirić-Marjanović, G. Evolution of polyaniline nanotubes: The oxidation of aniline in water. J. Phys. Chem. B 2006, 110, 9461–9468. [Google Scholar] [CrossRef]
- Stejskal, J.; Sapurina, I.; Trchova, M.; Konyushenko, E.N.; Holler, P. The genesis of polyaniline nanotubes. Polymer 2006, 47, 8253–8262. [Google Scholar] [CrossRef]
- Janošević, A.; Ćirić-Marjanović, G.; Marjanović, B.; Holler, P.; Trchova, M.; Stejskal, J. Synthesis and characterization of conducting polyaniline 5 sulfosalicylate nanotubes. Nanotechnology 2008, 19, 135606. [Google Scholar] [CrossRef] [PubMed]
- Kang, E.T.; Neoh, K.G.; Tan, K.L. Polyaniline: A polymer with many interesting intrinsic redox states. Prog. Polym. Sci. 1998, 23, 277–324. [Google Scholar] [CrossRef]
- Socrates, G. Infrared and Raman Characteristic Group Frequencies: Tables and Charts; Wiley: New York, NY, USA, 2001; pp. 65–84, 107–109, 157–165, 249–261. [Google Scholar]
- Liu, G.; Alomari, M.; Sahin, B.; Snelgrove, S.E.; Edwards, J.; Mellinger, A.; Kaya, T. Real-time sweat analysis via alternating current conductivity of artificial and human sweat. Appl. Phys. Lett. 2015, 106, 133702. [Google Scholar] [CrossRef]
- Kar, P.; Choudhury, A. Electrical and dielectric properties of polyaniline doped with carboxyl-functionalized multiwalled carbon nanotube. Adv. Polym. Technol. 2013, 32, E760–E770. [Google Scholar] [CrossRef]
- Naeimirad, M.; Abuzade, R.A.; Babaahmadi, V.; Dabirian, F. Microfluidic through fibrous structures: Recent developments and future trends. Mater. Des. Process. Commun. 2019, 1, e78. [Google Scholar] [CrossRef]
- Allwood, M.; Stanley, A.; Wright, P. (Eds.) The Cytotoxics Handbook; Radcliffe Publishing: London, UK, 2002; p. 294. [Google Scholar]
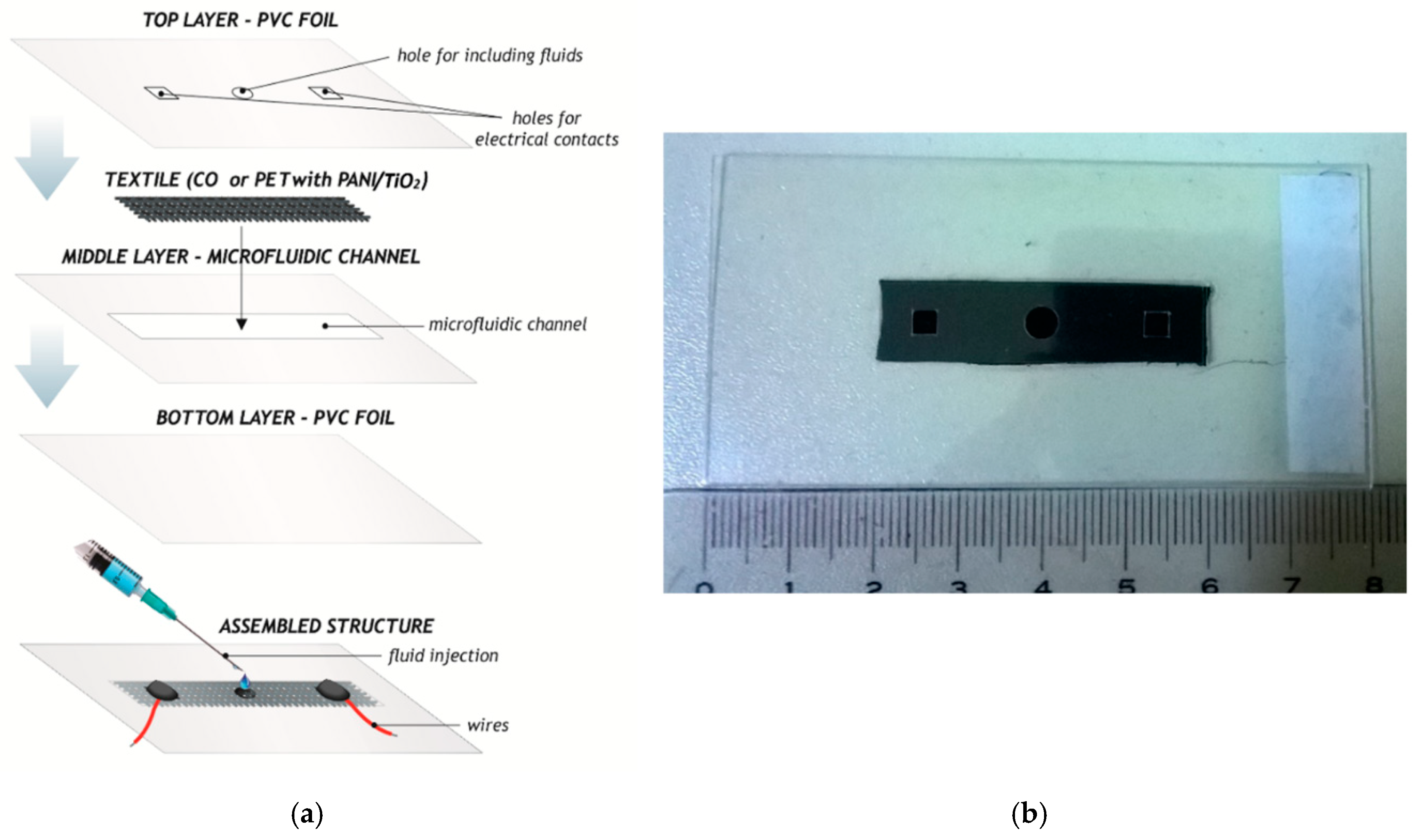
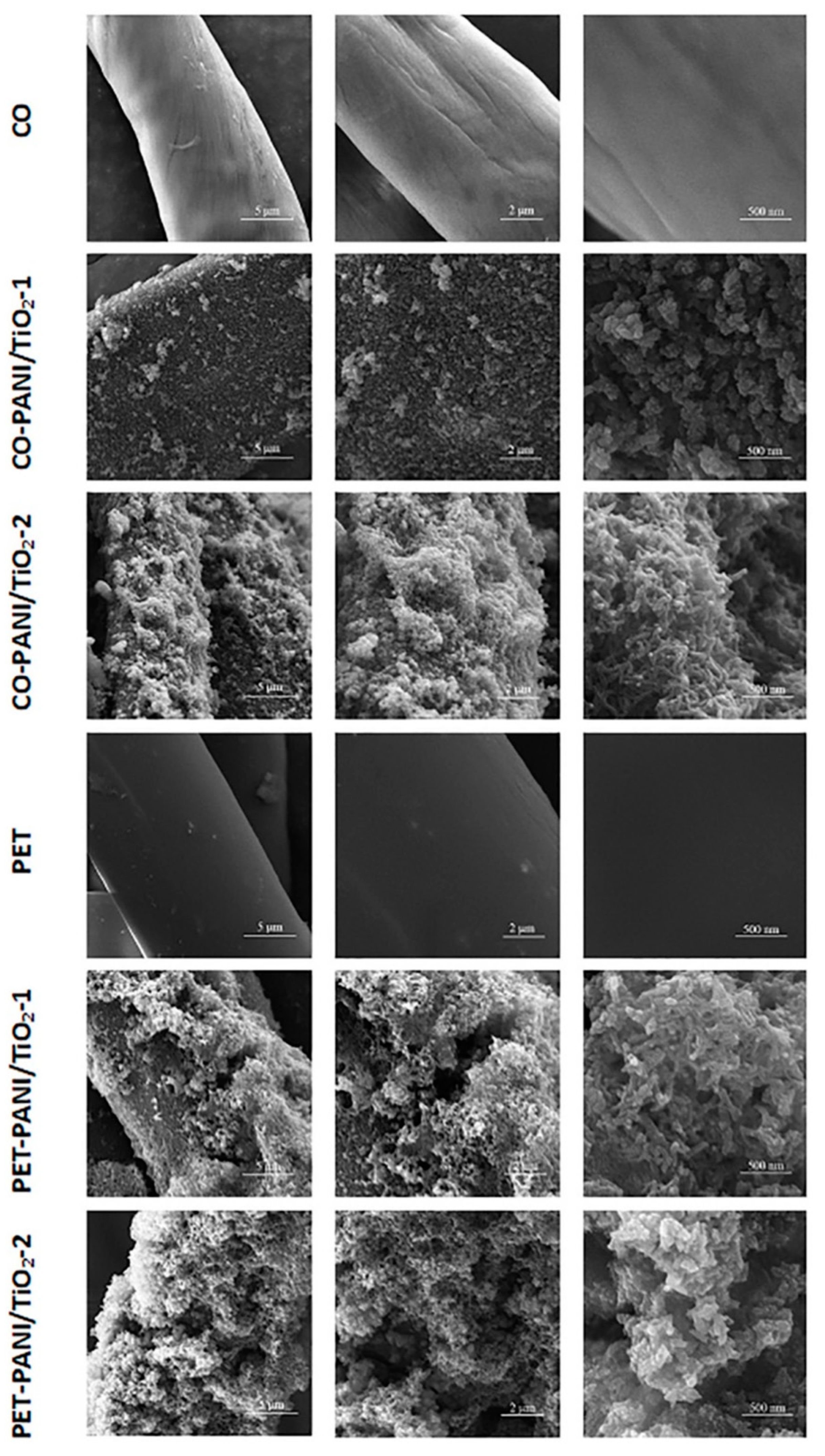

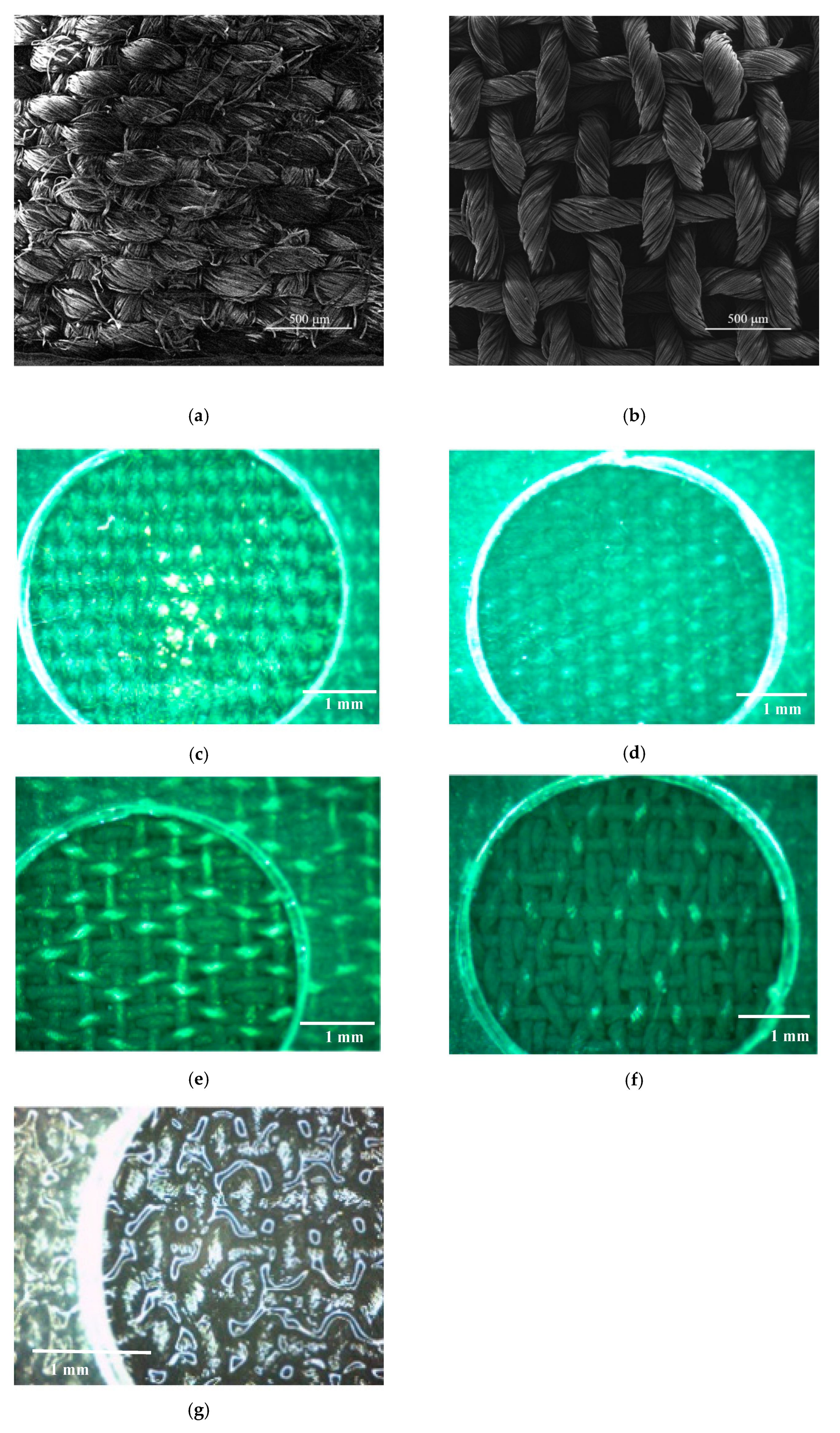
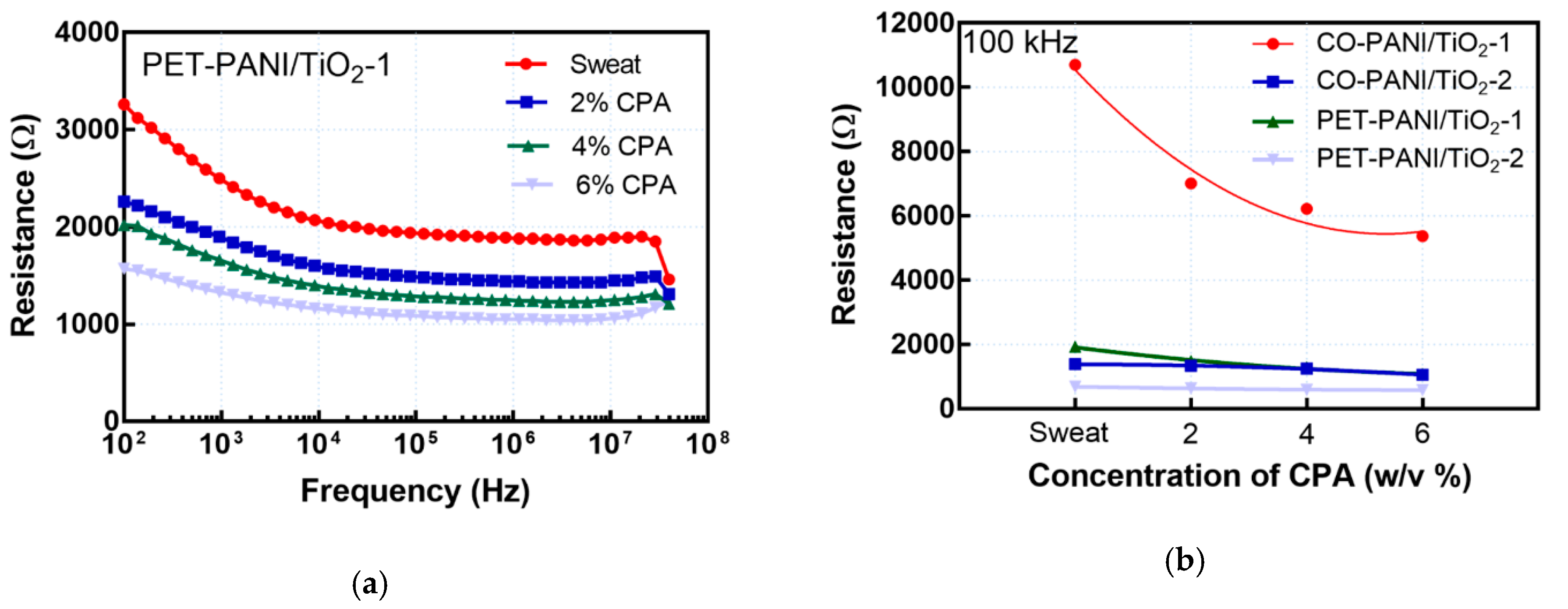
| Microfluidic Platform Type | Sensitivity |S| (Ω/w/v% of CPA) | R2 |
|---|---|---|
| CO-PANI/TiO2-1 | 888.33 | 0.8515 |
| CO-PANI/TiO2-2 | 55.00 | 0.9199 |
| PET-PANI/TiO2-1 | 141.66 | 0.9539 |
| PET-PANI/TiO2-2 | 17.00 | 0.9383 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stojanović, G.M.; Radetić, M.M.; Šaponjić, Z.V.; Radoičić, M.B.; Radovanović, M.R.; Popović, Ž.V.; Vukmirović, S.N. A Textile-Based Microfluidic Platform for the Detection of Cytostatic Drug Concentration in Sweat Samples. Appl. Sci. 2020, 10, 4392. https://doi.org/10.3390/app10124392
Stojanović GM, Radetić MM, Šaponjić ZV, Radoičić MB, Radovanović MR, Popović ŽV, Vukmirović SN. A Textile-Based Microfluidic Platform for the Detection of Cytostatic Drug Concentration in Sweat Samples. Applied Sciences. 2020; 10(12):4392. https://doi.org/10.3390/app10124392
Chicago/Turabian StyleStojanović, Goran M., Maja M. Radetić, Zoran V. Šaponjić, Marija B. Radoičić, Milan R. Radovanović, Željko V. Popović, and Saša N. Vukmirović. 2020. "A Textile-Based Microfluidic Platform for the Detection of Cytostatic Drug Concentration in Sweat Samples" Applied Sciences 10, no. 12: 4392. https://doi.org/10.3390/app10124392
APA StyleStojanović, G. M., Radetić, M. M., Šaponjić, Z. V., Radoičić, M. B., Radovanović, M. R., Popović, Ž. V., & Vukmirović, S. N. (2020). A Textile-Based Microfluidic Platform for the Detection of Cytostatic Drug Concentration in Sweat Samples. Applied Sciences, 10(12), 4392. https://doi.org/10.3390/app10124392








