The Possibility of Using the Probiotic Starter Culture Lacticaseibacillus rhamnosus LOCK900 in Dry Fermented Pork Loins and Sausages Produced Under Industrial Conditions
Abstract
Featured Application
Abstract
1. Introduction
2. Materials and Methods
2.1. Dry Fermented Meat Products’ Manufacturing Process
- Control LOINS, Control SAUSAGES—control samples of the study of dry fermented meat products without a probiotic starter culture LOCK900 addition;
- Probiotic LOINS, Probiotic SAUSAGES—samples of the study of dry fermented meat products with a probiotic starter culture LOCK900 addition.
2.1.1. Manufacturing of Dry Fermented Pork Loins
2.1.2. Manufacturing of Dry Fermented Sausages
2.2. Preparation of the Probiotic Starter Culture
2.3. Microbiological Analyses
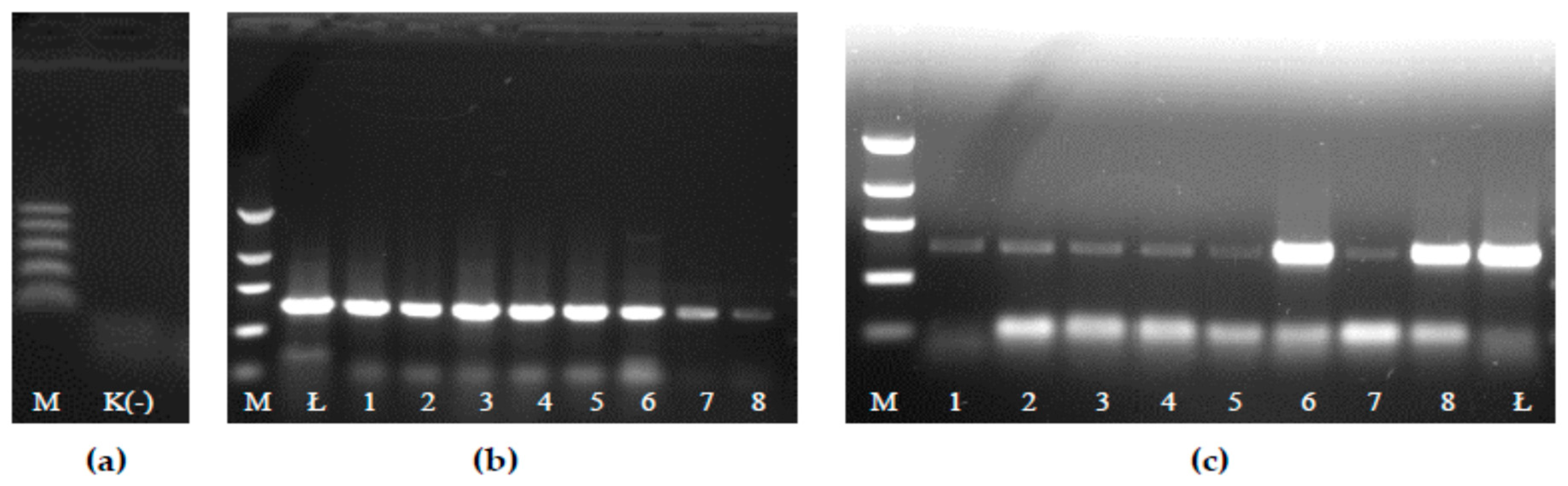
2.4. Confirmation of the Presence of the Probiotic Strain LOCK900 in Fermented Meat Products
2.5. Physical and Chemical Analyses
2.5.1. The pH Determination
2.5.2. Oxidation-Reduction Potential (ORP) Measurements
2.5.3. Lipid Oxidation
2.6. Statistical Analyses
3. Results
3.1. LAB and the Probiotic Strain LOCK900 Presence in Fermented Meat Products
3.2. Microbiological Quality and Safety
3.3. Physicochemical Quality and Safety
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- FAO/WHO. Guidelines for the Evaluations of Probiotics in Food; Report of a Joint FAO/WHO Working Group. Available online: http://www.fao.org/tempref/docrep/fao/009/a0512e/a0512e00.pdf (accessed on 5 April 2020).
- Rivera-Espinoza, Y.; Gallardo-Navarro, Y. Non-dairy probiotic products. Food Microbiol. 2010, 27, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Hill, C.; Guarner, F.; Reid, G.; Reid, G.; Gibson, G.R.; Merenstein, D.J.; Pot, B.; Morelli, L.; Canani, R.B.; Flint, H.J.; et al. Expert consensus document: The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 506–514. [Google Scholar] [CrossRef] [PubMed]
- Kołożyn-Krajewska, D.; Dolatowski, Z.J. Probiotic meat products and human nutrition. Process. Biochem. 2012, 47, 1761–1772. [Google Scholar] [CrossRef]
- Cocolin, L.; Rantsiou, K. Meat fermentation. In Handbook of Animal-Based Fermented Food and Beverage Technology, 2nd ed.; Hui, Y.H., Ed.; CRC Press: Boca Raton, FL, USA, 2012; pp. 533–535. [Google Scholar]
- Pidcock, K.; Heard, G.M.; Henriksson, A. Application of non-traditional meat starter cultures in production of Hulgarian salami. Int. J. Food Microbiol. 2002, 76, 75–81. [Google Scholar] [CrossRef]
- Papamanoli, E.; Tzanetakis, N.; Litopoulou-Tzanetaki, E.; Kotzekidou, P. Characterization of lactic acid bacteria isolated from a Greek dry-fermented sausage in respect of their technological and probiotic properties. Meat Sci. 2003, 65, 859–867. [Google Scholar] [CrossRef]
- Työppönen, S.; Petaja, E.; Mattila- Sandholm, T. Bioprotectives and probiotics for dry sausages. Int. J. Food Microbiol. 2003, 83, 233–244. [Google Scholar] [CrossRef]
- Klingberg, T.D.; Axelsson, L.; Naterstad, K.; Elsser, D.; Bude, B.B. Identification of potential starter cultures for Scandinavian-type fermented sausages. Int. J. Food Microbiol. 2005, 105, 419–431. [Google Scholar] [CrossRef]
- Benito, M.J.; Martin, A.; Aranda, E.; Perez-Nevado, F.; Ruiz-Moyano, S.; Cordoba, M.G. Characterization and selection of autochthonous lactic acid bacteria isolated from traditional Iberian dry-fermented salchichon and chorizo sausages. J. Food Sci. 2007, 72, M193–M201. [Google Scholar] [CrossRef]
- Rebucci, R.; Sangalli, L.; Fava, M.; Bersani, C.; Cantoni, C.; Baldi, A. Evaluation of functional aspects in Lactobacillus strains isolated from dry fermented sausages. J. Food Qual. 2007, 30, 187–201. [Google Scholar] [CrossRef]
- Prado, N.; Sampayo, M.; González, P.; Lombó, F.; Díaz, J. Physicochemical, sensory and microbiological characterization of Asturian Chorizo, a traditional fermented sausage manufactured in Northern Spain. Meat Sci. 2019, 156, 118–124. [Google Scholar] [CrossRef]
- Tabanelli, G.; Coloretti, F.; Chiavari, C.; Grazia, L.; Lanciotti, R.; Gardini, F. Effects of starter cultures and fermentation climate on the properties of two types of typical Italian dry fermented sausages produced under industrial conditions. Food Control. 2012, 26, 416–426. [Google Scholar] [CrossRef]
- Wójciak, K.M.; Dolatowski, Z.J.; Kołożyn-Krajewska, D.; Trząskowska, M. The effect of the Lactobacillus casei ŁOCK 0900 probiotic strain on the quality of dry-fermented sausage during chilling storage. J. Food Qual. 2012, 35, 353–365. [Google Scholar] [CrossRef]
- Kęska, P.; Stadnik, J. Taste-active peptides and amino acids of pork meat as components of dry-cured meat products: An in-silico study. J. Sens. Study 2017. [Google Scholar] [CrossRef]
- Pavli, F.G.; Argyri, A.A.; Chorianopoulos, N.G.; Nychas, G.J.E.; Tassou, C.C. Evaluation of Lactobacillus plantarum L125 strain with probiotic potential on physicochemical, microbiological and sensorial characteristics of dry-fermented sausages. LWT—Food Sci. Technol. 2020, 118, 108810. [Google Scholar] [CrossRef]
- Ayyash, M.; Liu, S.Q.; Al Mheiri, A.; Aldhaheri, M.; Raeisi, B.; Al-Nabulsi, A.; Osaili, T.; Olaimat, A. In vitro investigation of health-promoting benefits of fermented camel sausage by novel probiotic Lactobacillus plantarum: A comparative study with beef sausages. LWT—Food Sci. Technol. 2019, 99, 346–354. [Google Scholar] [CrossRef]
- Aymerich, T.; Martín, B.; Garriga, M.; Hugas, M. Microbial Quality and Direct PCR Identification of Lactic Acid Bacteria and Nonpathogenic Staphylococci from Artisanal Low-Acid Sausages. Appl. Environ. Microbiol. 2003, 69, 4583–4594. [Google Scholar] [CrossRef]
- Neffe-Skocińska, K.; Okoń, A.; Kołożyn-Krajewska, D.; Dolatowski, Z. Amino acid profile and sensory characteristics of dry fermented pork loins produced with a mixture of probiotic starter cultures. J. Sci. Food Agric. 2017, 97, 2953–2960. [Google Scholar] [CrossRef]
- Libera, J.; Latoch, A.; Wójciak, K.M. Utilization of Grape Seed Extract as a Natural Antioxidant in the Technology of Meat Products Inoculated with a Probiotic Strain of LAB. Foods 2020, 9, 103. [Google Scholar] [CrossRef]
- Neffe-Skocińska, K.; Wójciak, K.M.; Zielińska, D. Probiotic microorganisms in dry fermented meat products. In Probiotics and Prebiotics in Human Nutrition and Health, 1st ed.; Rao, V., Rao, L.G., Eds.; InTech: Rijeka, Croatia, 2016; pp. 279–300. [Google Scholar]
- Kęska, P.; Stadnik, J.; Wójciak, K.M.; Neffe-Skocińska, K. Physico-chemical and proteolytic changes during cold storage of dry-cured pork loins with probiotic strains of LAB. Int. J. Food Sci. Technol. 2020, 55, 1069–1079. [Google Scholar] [CrossRef]
- Aleksandrzak-Piekarczyk, T.; Koryszewska-Bagińska, A.; Bardowski, J. Genome Sequence of the Probiotic Strain Lactobacillus rhamnosus (Formerly Lactobacillus casei) LOCK900. Genome. Announc. 2013, 1, e00640-13. [Google Scholar] [CrossRef]
- ISO. Microbiology of the Food Chain—Horizontal Method for the Detection and Enumeration of Enterobacteriaceae—Part 2: Colony-Count Technique; ISO 21528-2:2017; International Organization for Standardization: Geneva, Switzerland, 2017. [Google Scholar]
- ISO. Microbiology of the Food Chain—Horizontal Method for the Detection, Enumeration and Serotyping of Salmonella—Part 1: Detection of Salmonella spp.; ISO 6579-1:2017; International Organization for Standardization: Geneva, Switzerland, 2017. [Google Scholar]
- ISO. Microbiology of the Food Chain—Horizontal Method for the Detection and Enumeration of Listeria monocytogenes and of Listeria spp.—Part 1: Detection Method; ISO 11290-1:2017; International Organization for Standardization: Geneva, Switzerland, 2017. [Google Scholar]
- Ward, L.J.H.; Timmins, M.J. Differentiation of Lactobacillus casei, Lactobacillus paracasei and Lactobacillus rhamnosus by polymerase chain reaction. Lett. Appl. Microbiol. 1999, 29, 90–92. [Google Scholar] [CrossRef] [PubMed]
- Pikul, J.; Leszczyński, D.E.; Kummerow, F.A. Evaluation of three modified TBA methods for measuring lipidoxidation in chicken meat. Food Chem. 1989, 37, 1309–1315. [Google Scholar] [CrossRef]
- Singh, V.P.; Pathak, V.; Verma, A.K. Fermented meat products: Organoleptic qualities and biogenic amines—A review. Am. J. Food Technol. 2012, 7, 278–288. [Google Scholar] [CrossRef][Green Version]
- Samappito, W.; Leenanon, B.; Levin, R.E. Microbiological Characteristics of ‘‘Mhom’’, a Thai Traditional Meat Sausage. Open Food Sci. J. 2011, 5, 31–36. [Google Scholar] [CrossRef]
- Kröckel, L. Chapter 5—The role of lactic acid bacteria in safety and flavour development of meat and meat products. In Lactic Acid Bacteria—R & D for Food, Health and Livestock Purposes, 1st ed.; Kongo, M., Ed.; InTech—Open Access Publisher: Rijeka, Croatia, 2013; Available online: http://dx.doi.org/10.5772/51117 (accessed on 5 April 2020). [CrossRef][Green Version]
- Zielińska, D.; Ołdak, A.; Rzepkowska, A.; Zieliński, K. Chapter 6—Enumeration and Identification of Probiotic Bacteria in Food Matrices. In Handbook of Food Bioengineering, Advances in Biotechnology for Food Industry, 1st ed.; Holban, A.M., Grumezescu, A.M., Eds.; Academic Press—Elsevier: Bucharest, Romania, 2018; pp. 167–196. [Google Scholar]
- Muniz da Silva, A.C.; Pena, P.O.; Pflanzer Júnior, S.B.; Silva do Nascimento, M. Effect of different dry aging temperatures on Listeria in nocua as surrogate for Listeria monocytogenes. Meat Sci. 2019, 157, 107884. [Google Scholar] [CrossRef]
- Olanya, O.M.; Hoshide, A.K.; Ijabadeniyi, O.A.; Ukukua, D.O.; Mukhopadhyayd, S.; Niemira, B.A.; Ayenie, O. Cost estimation of listeriosis (Listeria monocytogenes) occurrence in South Africa in 2017 and its food safety implications. Food Control. 2019, 102, 231–239. [Google Scholar] [CrossRef]
- Busani, L.; Scavia, G.; Luzzi, I.; Caprioli, A. Laboratory surveillance for prevention and control of foodborne zoonoses. Ann. Ist. Super. Sanita 2006, 42, 401–404. [Google Scholar]
- Gwida, M.; Hotzel, H.; Geue, L.; Tomaso, H. Occurrence of Enterobacteriaceae in Raw Meat and in Human Samples from Egyptian Retail Sellers. Int. Sch. Res. Not. 2014. [Google Scholar] [CrossRef]
- Paterson, D.L. Resistance in Gram-negative bacteria: Enterobacteriaceae. Am. J. Med. 2006, 119, S20–S28. [Google Scholar] [CrossRef]
- Wójciak, K.M.; Dolatowski, Z.J. Effect of acid whey on nitrosylmyoglobin concentration in uncured fermented sausage. LWT—Food Sci. Technol. 2015, 64, 713–719. [Google Scholar] [CrossRef]
- Trząskowska, M.; Kołożyn-Krajewska, D.; Wójciak, K. Microbiological quality of raw-fermented sausages with Lactobacillus casei LOCK0900 probiotic strain. Food Control. 2014, 35, 184–191. [Google Scholar] [CrossRef]
- Wójciak, K.M.; Karwowska, M.; Dolatowski, Z.J. Use of acid whey and mustard seed to replace nitrites during cooked sausage production. Meat Sci. 2014, 96, 750–756. [Google Scholar] [CrossRef] [PubMed]
- Ammor, M.S.; Mayo, B. Selection criteria for lactic acid bacteria to be used as functional starter cultures in dry fermented sausages. An update. Meat Sci. 2007, 76, 138–146. [Google Scholar] [CrossRef] [PubMed]
- Coll, C.F.; Giannuzzi, N.E.; Zaritzky, N.E. Mathematical modeling of microbial growth in ground beef from Argentina. Effect of lactic acid addition, temperature and packaging film. Meat Sci. 2008, 79, 509–520. [Google Scholar]
- Muthusamy, K.; Soundharrajan, I.; Srisesharam, S.; Kim, D.; Kuppusamy, P.; Lee, D.L.; Choi, K.C. Probiotic Characteristics and Antifungal Activity of Lactobacillus plantarum and Its Impact on Fermentation of Italian Ryegrass at Low Moisture. Appl. Sci. 2020, 10, 417. [Google Scholar] [CrossRef]
- Lorenzo, J.M.; Gomez, M.; Fonseca, S. Effect of commercial starter cultures on physicochemical characteristics, microbial counts and free fatty acid composition of dry-cured foal sausage. Food Control. 2014, 46, 382–389. [Google Scholar] [CrossRef]
- Lorenzo, J.M.; Sarriés, M.V.; Tateo, A.; Polidori, P.; Franco, D.; Lanza, M. Carcass characteristics, meat quality and nutritional value of horsemeat: A review. Meat Sci. 2014, 96, 1478–1488. [Google Scholar] [CrossRef]
- Macedo, R.E.F.; Pflanzer, O.S.; Gomes, C.L. Probiotic Meat Products. In Probiotic in Animals, 1st ed.; Rigobelo, I.C., Ed.; InTech—Open Access Publisher: Rijeka, Croatia, 2012; Available online: http://www.intechopen.com/books/probiotic-in-animals (accessed on 5 April 2020). [CrossRef][Green Version]
- Zhao, L.H.; Jin, Y.; Ma, C.W.; Song, H.; Li, H.; Wang, Z.; Xiao, S. Physico-chemical characteristics and free fatty acid composition of dry fermented mutton sausages as affected by the use of various combinations of starter cultures and spices. Meat Sci. 2011, 88, 761–766. [Google Scholar] [CrossRef]
- Okoń, A.; Stadnik, J.; Dolatowski, Z.J. Effect of probiotic bacteria on antiradical activity of peptides isolated from dry-cured loins. CyTA—J. Food 2017, 15, 382–390. [Google Scholar] [CrossRef]
- Ruiz--Moyano, S.; Martín, A.; Benito, M.J.; Hernández, A.; Casquete, R.; Córdoba, M.G. Application of Lactobacillus fermentum HL57 and Pediococcus acidilactici SP979 as potential probiotics in the manufacture of traditional Iberian dry-fermented sausages. Food Microbiol. 2011, 28, 839–847. [Google Scholar] [CrossRef]
- Essid, I.; Hassouna, M. Effect of inoculation of selected Staphylococcus xylosus and Lactobacillus plantarum strains on biochemical, microbiological and textural characteristics of a Tunisian dry fermented sausage. Food Control. 2013, 32, 707–714. [Google Scholar] [CrossRef]
- Chen, Q.; Kong, B.; Sun, Q.; Dong, F.; Liu, Q. Antioxidant potential of a unique LAB culture isolated from Harbin dry sausage: In vitro and in a sausage model. Meat Sci. 2015, 110, 180–188. [Google Scholar] [CrossRef] [PubMed]
- Sun, F.; Kong, B.; Chen, Q.; Han, Q.; Diao, X. N-nitrosoamine inhibition and quality preservation of Harbin dry sausages by inoculated with Lactobacillus pentosus, Lactobacillus curvatus and Lactobacillus sake. Food Control. 2017, 73, 1514–1521. [Google Scholar] [CrossRef]
| Kind of Sample | Glucose (g) | Sodium Ascorbate (g) | Spice Mix (g) | Probiotic Starter Culture LOCK900 (mL) |
|---|---|---|---|---|
| Control LOINS | 5 | 0.5 | - | - |
| Probiotic LOINS | 5 | 0.5 | - | 2 |
| Control SAUSAGES | 5 | 0.5 | 13 | - |
| Probiotic SAUSAGES | 5 | 0.5 | 13 | 2 |
| Samples (n = 12) | Time 0 | Time 1 | Time 2 |
|---|---|---|---|
| Number of LAB (log cfu g −1) | |||
| Control LOINS | 6.11aA ± 0.53 | 6.99aA ± 0.67 | 7.04aB ± 0.60 |
| Probiotic LOINS | 7.12 bA ± 0.87 | 7.54 bA ± 0.63 | 7.17 aA ± 0.43 |
| Control SAUSAGES | 6.89 aA ± 0.86 | 7.10 aB ± 0.95 | 7.31 aB ± 0.96 |
| Probiotic SAUSAGES | 8.08 bA ± 0.97 | 8.30 bA ± 0.87 | 8.21 bA ± 0.09 |
| Number of Isolates Identified as L. Rhamnosus (%) | |||
| Probiotic LOINS | 80 ± 6.67 | not analyzed | 92 ± 0.86 |
| Probiotic SAUSAGES | 84 ± 7.69 | not analyzed | 94 ± 5.09 |
| Samples (n = 12) | Time 0 | Time 1 | Time 2 |
|---|---|---|---|
| Enterobacteriaceae (log cfu mL −1) | |||
| Control LOINS | 4.25 aA ± 0.72 | 2.15 aB ± 0.20 | < 1.00aC |
| Control SAUSAGES | 5.67 bA ± 0.65 | 2.60 aB ± 0.67 | < 1.00aC |
| Probiotic LOINS | 4.41 aA ± 0.82 | < 1.00 bB | < 1.00 aB |
| Probiotic SAUSAGES | 4.60 aA ± 0.59 | < 1.00 bB | < 1.00 aB |
| Salmonella spp. | |||
| Control LOINS | absent in 25 g of product | ||
| Control SAUSAGES | |||
| Probiotic LOINS | |||
| Probiotic SAUSAGES | |||
| Listeria Monocytogenes | |||
| Control LOINS | absent in 25 g of product | ||
| Control SAUSAGES | |||
| Probiotic LOINS | |||
| Probiotic SAUSAGES | |||
| Samples (n = 12) | Time 0 | Time 1 | Time 2 |
|---|---|---|---|
| pH Value | |||
| Control LOINS | 5.69 dB ± 0.06 | 5.42 cA ± 0.14 | 5.47 dA ± 0.14 |
| Control SAUSAGES | 5.50 cB ± 0.07 | 5.39 cA ± 0.06 | 5.35 cA ± 0.05 |
| Probiotic LOINS | 4.96 bB ± 0.06 | 5.01 bC ± 0.02 | 4.91 bA ± 0.03 |
| Probiotic SAUSAGES | 4.90 aB ± 0.03 | 4.69 aA ± 0.06 | 4.66 aA ± 0.07 |
| ORP (mV) | |||
| Control LOINS | 305.70 aB ± 17.15 | 294.90 aB ± 9.39 | 276.00 aA ± 5.65 |
| Control SAUSAGES | 380.30 dB ± 4.15 | 348.20 bA ± 6.21 | 350.10 cA ± 3.99 |
| Probiotic LOINS | 316.30 cC ± 1.92 | 285.80 aB ± 3.44 | 275.70 aA ± 9.43 |
| Probiotic SAUSAGES | 300.60 bA ± 3.70 | 341.50 bC ± 9.29 | 327.70 bB ± 7.70 |
| TBARS (mg TDA kg−1) | |||
| Control LOINS | 0.42 aA ± 0.11 | 0.67 bB ± 0.16 | 0.47 aA ± 0,08 |
| Control SAUSAGES | 0.90 bA ± 0.12 | 1.13 cB ± 0.08 | 1.32 bC ± 0.07 |
| Probiotic LOINS | 1.34 cB ± 0.04 | 0.48 aA ± 0.05 | 0.46 aA ± 0,02 |
| Probiotic SAUSAGES | 1.51 cB ± 0.16 | 0.50 aA ± 0.10 | 0.49 aA ± 0.03 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Neffe-Skocińska, K.; Okoń, A.; Zielińska, D.; Szymański, P.; Sionek, B.; Kołożyn-Krajewska, D. The Possibility of Using the Probiotic Starter Culture Lacticaseibacillus rhamnosus LOCK900 in Dry Fermented Pork Loins and Sausages Produced Under Industrial Conditions. Appl. Sci. 2020, 10, 4311. https://doi.org/10.3390/app10124311
Neffe-Skocińska K, Okoń A, Zielińska D, Szymański P, Sionek B, Kołożyn-Krajewska D. The Possibility of Using the Probiotic Starter Culture Lacticaseibacillus rhamnosus LOCK900 in Dry Fermented Pork Loins and Sausages Produced Under Industrial Conditions. Applied Sciences. 2020; 10(12):4311. https://doi.org/10.3390/app10124311
Chicago/Turabian StyleNeffe-Skocińska, Katarzyna, Anna Okoń, Dorota Zielińska, Piotr Szymański, Barbara Sionek, and Danuta Kołożyn-Krajewska. 2020. "The Possibility of Using the Probiotic Starter Culture Lacticaseibacillus rhamnosus LOCK900 in Dry Fermented Pork Loins and Sausages Produced Under Industrial Conditions" Applied Sciences 10, no. 12: 4311. https://doi.org/10.3390/app10124311
APA StyleNeffe-Skocińska, K., Okoń, A., Zielińska, D., Szymański, P., Sionek, B., & Kołożyn-Krajewska, D. (2020). The Possibility of Using the Probiotic Starter Culture Lacticaseibacillus rhamnosus LOCK900 in Dry Fermented Pork Loins and Sausages Produced Under Industrial Conditions. Applied Sciences, 10(12), 4311. https://doi.org/10.3390/app10124311







