1. Introduction
Saponified fatty acids (soaps) are common anionic surfactants that are widely used as detergents and emulsifiers. Among fatty acid soaps, lauric acid soap is often used as a body cleanser, shampoo, dishwashing detergent, and laundry detergent, owing to its good foaming capacity and strong detergency. However, surfactants have been reported to affect the skin barrier function [
1,
2,
3].
The skin barrier consists of keratin proteins and intercellular lipids. Keratin, a cytoskeletal protein, is composed of a group of intermediate filaments composed of keratinocytes, which are differentiated and enucleated epidermal cells. Intercellular lipids have a lamellar structure in which the lipid molecules, mainly composed of ceramide, fatty acids, and cholesterol esters, are regularly arranged. Stratum corneum cells and intercellular lipids form a structure known as the brick and mortar model, establishing a skin barrier function [
4,
5,
6].
Previous studies on the effects of surfactants on the skin have reported a correlation with the alkyl chain length of surfactants [
7,
8,
9]. Kurosaki et al. evaluated the relationship between the carbon number of fatty acid surfactants and irritancy to skin (using skin roughness and degreasing power in the patch test as evaluation scales), and revealed that C12 alkyl anionic surfactants caused the strongest skin irritation [
10]. In addition, it has been reported that the skin barrier function is reduced due to the formation of a stratum corneum permeation path for a specific molecule, thereby increasing the skin permeability of the molecule. Using this phenomenon, the skin barrier function has been evaluated by measuring the invasiveness of substances or transepidermal water loss (TEWL) [
6,
11,
12,
13]. In vitro skin permeation assays, using the Franz diffusion cell system, are the most commonly used methods for assessing the skin absorption of drugs [
14]. Okasaka et al. developed a transmission index (TI) method to evaluate the effect of anionic surfactants on the skin barrier function, using the skin permeation rate of methylparaben as an index [
15].
Some previous studies have reported interesting findings on the effects of amphiphiles on the skin barrier function. Ionic liquids are amphiphilic substances similar to surfactants. Several interesting reviews of ionic liquids for enhancing drug skin permeation have been summarized [
16,
17]. Dobler et al. has reported the skin penetration effect by water-in-oil (W/O) or oil-in-water (O/W) emulsion, consisting of the hydrophobic ionic liquid or by the hydrophilic ionic liquid [
18]. Some ionic liquids are formed using fatty acids as raw materials and are known to significantly enhance the skin permeability of various drugs. Medrx Co. Ltd., has commercialized an ionic liquid composed of fatty acids and amines with high biocompatibility as a skin permeation enhancer [
19]. Kubota et al. investigated the mechanism by which fatty acid–aliphatic amine ionic liquids enhance the skin permeation of drugs [
20]. Their results have shown that the skin penetration-promoting effect of drugs differs depending on the composition of the ionic liquid. While the ionic liquid composed of octanoic acid and triisopropanolamine markedly enhanced the skin permeability of hydrophilic model drugs, the ionic liquid derived from isostearic acid, a branched-chain C18 fatty acid, has an inhibitory effect on the skin permeability of hydrophobic drugs [
20]. These results suggest that the structure of the alkyl chain of the amphiphile, including the surfactant, affects the skin barrier function. Hence, we investigated the effect of the alkyl structure of fatty acids on the skin barrier function using fatty acid soaps, which are common and simple amphiphiles. C18 fatty acids were used as the raw materials for the preparation of the fatty acid soaps. C18 fatty acids have many structural isomers with different branching and degree of unsaturation, and they are widely used as cosmetic or food ingredients or additives owing to their safety and high biocompatibility [
21,
22]. In this study, we used stearic acid as the straight chain fatty acid, isostearic acid as the branched-chain fatty acid, and oleic acid (18:1 (n-9)) and linoleic acid (18:2 (n-6)) as the unsaturated fatty acids. These fatty acids were neutralized with sodium hydroxide to prepare the saponified fatty acids. The effect of these structurally isomeric C18 fatty acid soaps on the skin barrier function was evaluated by the TI method described by Okasaka et al. [
15] Skin permeability assay was performed on the back skin of hairless mice using the Franz diffusion cell system. Methylparaben was used as an indicator. In parallel, the skin tissue injury was evaluated by microscopic observation.
2. Materials and Methods
2.1. Preparation of the Soaps
Fatty acid soaps were prepared by saponification reaction between the fatty acid and sodium hydroxide. The C18 fatty acids were prepared based on the classification of the alkyl structure. Stearic acid was prepared as a saturated straight-chain fatty acid. The optical isomeric mixture of isostearic acid was prepared as a saturated branched-chain fatty acid. Two types of C18 fatty acids were prepared as unsaturated fatty acids. Oleic acid was prepared as a monounsaturated fatty acid, while linoleic acid was prepared as a di-unsaturated fatty acid. For comparison with these sodium soaps, sodium laurate was prepared from lauric acid. All reagents were of first or special grade and purchased from Wako Pure Chemical Industries (Tokyo, Japan). A 1.0 mol/L sodium hydroxide aqueous solution was prepared by dissolving solid sodium hydroxide (Sigma-Aldrich, St. Louis, MO, USA) in deionized water. A 5 mL solution of 1 mol/L sodium hydroxide (5 mmol NaOH) was stirred on a hot plate stirrer (AS ONE Co., Osaka, Japan) maintained at 70 °C. Then, 5 mmol fatty acid was added to it and the mixture was stirred until it was completely homogenized. The resultant saponified fatty acid was adjusted to a 1% concentration by adding deionized water.
2.2. Skin Permeation Experiments and Evaluation of the Effect of Fatty Acid Soap on the Skin Barrier Function
The effect of different fatty acid soaps on the skin barrier function was assessed by the TI method, based on a previously described method [
15].
The skin permeability assay was performed using the Franz diffusion cell system (vertical reservoir, 8 mL; permeation area, 1 cm2) (PermGear, Hellertown, PA, USA). The reservoir chamber was filled with saline and warmed up to 37 °C. Laboskin® (Hoshino Laboratory Animals Inc., Ibaraki, Japan; certificated by the Japanese Society for Laboratory Animal Resources); the back skin from a 7-week-old male Hos:HR-1 hairless mouse, was divided into four parts and used as the transmission skin sample. There were no statistically significant differences in the head and tail sides as well as the right and left sides of the midline. A 1 mL 1% fatty acid soap solution or control sample (deionized water) was applied to the external surface of the skin for 1 h. After treatment, the test or control sample was removed and the skin was thoroughly washed five times each with 1 mL of deionized water followed by 2 mL of deionized water.
Methylparaben was purchased from Ueno Fine Chemical Industry Ltd. (Osaka, Japan). One hundred milligrams of methylparaben was accurately weighed using an electronic analytical scale AUX120 (Shimadzu, Kyoto, Japan) and dissolved in deionized water. To ensure complete the dissolution of methylparaben, the solution was stirred on a vortex mixer, followed by ultrasonication for 15 min. It was then diluted with 100 mL of deionized water to obtain a 0.100% (w/v) solution. After skin stimulation with soap, 1 mL of the 0.100% methylparaben solution was applied to the skin after washing twice. The donor cup was sealed with parafilm to prevent the evaporation of the methylparaben solution. The reservoir solution was stirred at approximately 500 rpm, using either 6 or 12 stations multi magnetic stirrer (AS ONE). The reservoir solution in the receptor cup was mixed 10 times with a micropipette. After 1 h, 200 µL of the sample was withdrawn and an equivalent volume of saline was replaced. The process was repeated after every hour for 6 h.
The concentration of methylparaben in the collected reservoir solution was measured by High Performance Liquid Chromatography (HPLC) method using an LC-20AB HPLC system (Shimadzu) with a Capcell Pak® 5 µm C18 MG-II column (internal diameter: 2.0 mm × 150 mm) (Shiseido, Tokyo, Japan). An accurately diluted sample of 0.002% methylparaben was used as the standard. The column oven temperature was set at 40 °C. The HPLC mobile phase, consisting of (A) 15 mM phosphoric acid (Nakarai tesque, Kyoto, Japan) and (B) methanol (for HPLC, Wako), was adjusted (A:B = 55:45), and the flow rate was maintained at 0.2 mL/min. Five microliters of the reservoir solution was injected using an autosampler SIL-20AC (Shimadzu). The peak area of the UV absorbance was measured at 256 nm and calibrated using the LCsolution 1.23 SP1 software (Shimadzu). The reference sample (0.001% methylparaben) was prepared by diluting the sample from the permeability test and was analyzed before and after the analysis of the test sample, to confirm the stability of methylparaben during the analysis. No interferences were observed from the blank skin extracts during the HPLC analysis of the collected reservoir solutions. Each experiment was repeated at least 4 times and statistical analysis was carried out.
The TI value was calculated as follows. The
Fluxss was determined from the quantity of permeate (
Q) with respect to time (
t), when the skin permeability indicated a linear correlation with time (Equation (1)). TI was determined as the relative value of the
Fluxss of control and the
Fluxss of the sample (Equation (2)). The
Fluxss value was calculated using the LINEST function on the time-concentration scatter plot in Microsoft Excel and statistical analysis using Welch’s
t-test and Pearson’s chi-square test were carried out:
2.3. Assessment of Skin Tissue Injury
The skin tissue injury caused by the fatty acid soap was assessed by microscopic observation, according to the method described in our previous study [
20]. The skin samples were treated with surfactant or control for 1 h before the assessment. After rough sectioning using surgical scissors, the skin samples were washed 3 times in 20 mL of Dulbecco’s recipe of phosphate buffered saline without Mg
2+ and Ca
2+ (PBS(−)) (10 mM, pH 7.4) for 5 min each. The tissue samples were fixed in 4% paraformaldehyde/0.1 M phosphate buffer (pH 7.4), stored overnight, and immersed in 20% sucrose/0.1 M phosphate buffer (pH 7.4) at 4 °C. The tissue blocks were embedded in an optical cutting temperature compound (Tissue-Tek, Sakura Finetek; Torrance, CA, USA) and snap-frozen in powdered dry ice. The frozen embedded tissue samples were cut to approximately 10 µm slices and air-dried. Then, the tissue slices were deposited on glass slides, moistened with water, and stained with Mayer’s hematoxylin (Wako Pure Chemical Industries) and eosin Y (Waldeck, Münster, Germany). After dehydration in graded anhydrous ethanol and Clear Plus (Falma Co.; Tokyo, Japan), a cover glass was used to seal the slices on the glass slides. Microscopic images were acquired using a BX51 bright field/fluorescence biological microscope (Olympus, Tokyo, Japan). The brightness and contrast of the images were automatically optimized using the ImageJ software.
4. Discussion
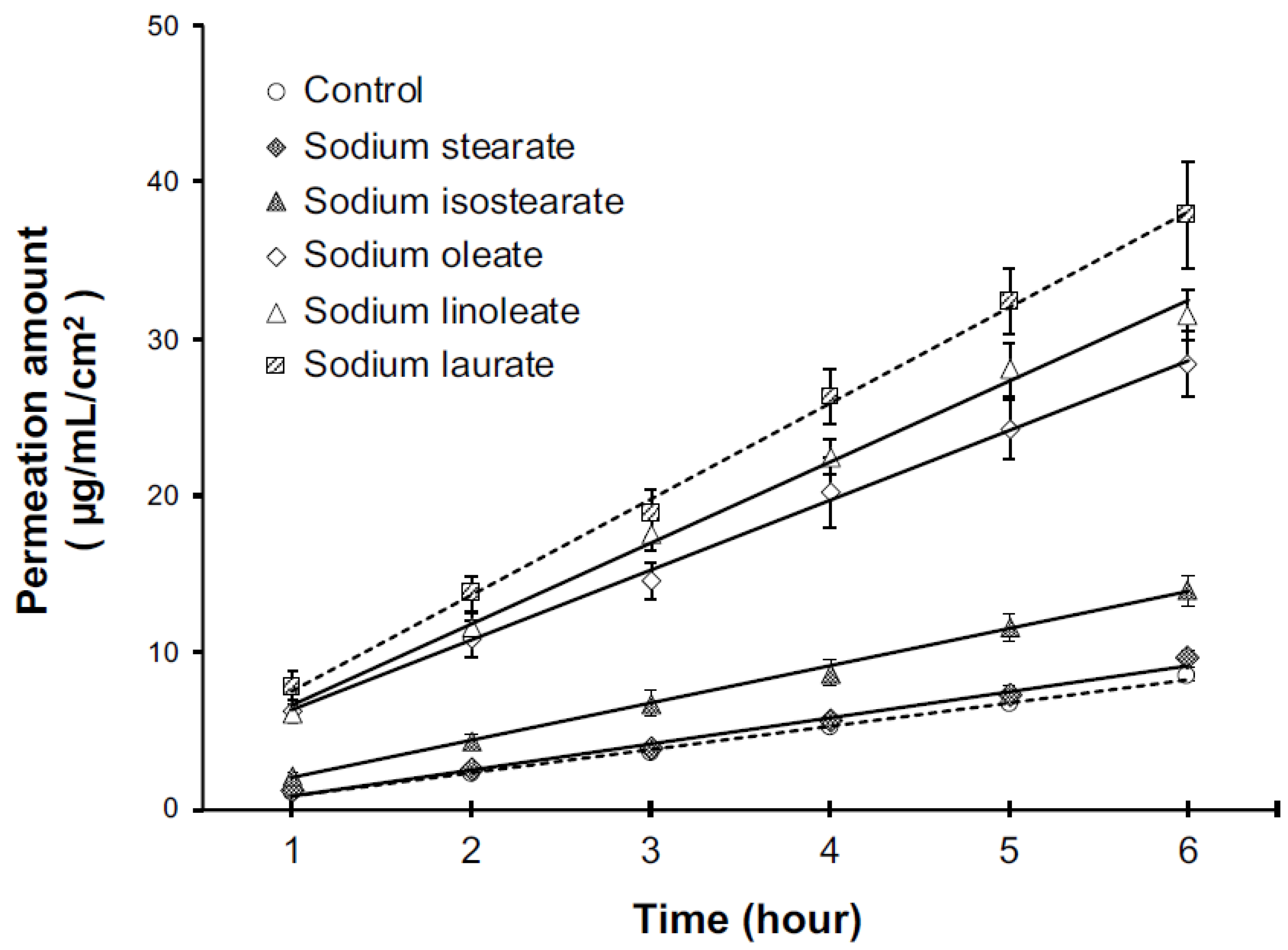
In this study, the effect of the structural differences in the various isomers belonging to the hydrophobic group of anionic surfactants on the skin barrier function was evaluated using the TI method. This method was developed for the purpose of the easy analysis of the effect of surfactants on the skin barrier function. It is an effective method for the comparative study of surfactants, provided the drug has a steady state in skin permeation (
Figure 1).
The TI value of the sodium laurate soap was approximately 4 (
Table 1), which was different from the value obtained in our previous study (2.53) [
15]. Of note, the sodium laurate soap assessed in the previous study was a cosmetic raw material-grade industrial product. On the other hand, the sodium laurate soap in this study was prepared using reagent-grade lauric acid and sodium hydroxide. Thus, it was assumed that the differences in purity and neutralization rates of the fatty acids led to the different TI values. From a qualitative point of view, previous studies have shown that sodium laurate soap has a significantly higher TI value than other anionic surfactants, indicating that the effect of sodium laurate soap on the skin barrier function is strong. Previous studies have reported that C12 fatty acid surfactants cause strong skin irritation [
10]. Based on the fact that the surfactants with strong skin irritation have high TI values, it was suggested that the TI value was related to skin irritation.
The TI values are expressed as the ratio of Fluxss of surfactant-treated skin to control-treated skin. The standard error (SE) was calculated from the unbiased variance of the combination of the Fluxss (n = 35) of control and Fluxss (n = 6–10) of the test sample.
The significant differences in the TI values between the C18 fatty acid soaps suggested that the effect of sodium fatty acid soaps on the skin barrier function was affected not only by the carbon number of alkyl structures, but also by their alkyl structures. When the fatty acids were arranged in increasing order of their respective TI values (saturated straight chain < saturated branched chain < monounsaturated ~< di-unsaturated), the sequence corresponded to the respective melting points of the fatty acids. The melting points of the saturated straight-chain stearic acid, saturated branched-chain isostearic acid, monounsaturated oleic acid, and di-unsaturated linoleic acid are 70, 16, ≤15, and −5 °C, respectively. There is a relationship between the melting point and solubility of a substance, with substances with higher melting points generally being less soluble [
23]. In soaps, generally, the Krafft point is proportionally affected by the melting point. The Krafft point increases as the number of carbon atoms in the alkyl chain increases. At the same time, if the hydrophilic groups are the same, the solubility in the water decreases as the carbon number increases. When an alkyl structure includes unsaturated groups, the Krafft point decreases and the water solubility increases. In addition, the solubility tends to increase as the hydrophilic group approaches the center of the alkyl chain [
24]. The difference in the solubility of the sodium soap was also confirmed by the change in the dissolution state during the soap preparation as well as the properties of the prepared soap. Based on these facts, it was assumed that the melting point, Krafft point, and the solubility of the raw fatty acid were the main factors affecting the skin barrier function. The relationship between the carbon number of surfactants and skin irritation is well known [
25,
26]. It can be said that their report is a mention to one aspect of the relationship between the melting point of fatty acids and the properties of surfactants on the skin.
The temperature conditions of the experiment are important because the solubility is affected by temperature. The TI value was calculated based on the skin permeation speed of methylparaben at 37 °C. The equilibrium at this temperature depends on the solubility of the fatty acid sodium soap. We predicted that the higher the solubility of the surfactant, the stronger the action on the keratinocyte intercellular lipid, and as a result, the skin permeability of the surfactant would be enhanced. In addition, because fatty acid soap is a weak acid and strong alkalin salt, the equilibrium state depends on the pH. Furthermore, the critical micelle concentration is affected by the temperature and the pH of the aqueous solution. Therefore, it is necessary to discuss the influence of the physicochemical properties of the soap molecules on the skin barrier function, based on the measurements of critical micelle concentration or the pH dependence of the solubility.
In this study, we also examined the effect of anionic surfactant on the skin barrier function from a perspective other than drug skin permeability. The microscopic observation of hematoxylin–eosin (HE) stained tissue sections provided information on cytotoxicity and the effects of the surfactant on the connective tissue, based on chromatin staining and the condition of the connection between the tissues and cells. The absence of chromatin in the epidermal layer of the sodium laurate soap-treated skin (
Figure 3g) indicated that the chromosome was damaged and the lipid bilayer of the cell membrane was probably damaged. This observation was similar to that seen upon the action of sodium lauryl sulfate (SDS), which exerts cytolytic effects due to its strong lipid solubility and protein denaturing activity [
7]. This phenomenon associated with sodium laurate soap, also known as ‘skin irritation’ [
10,
27,
28], results in the exfoliation of the stratum corneum, the lysis of epidermal cells, the disruption of the association between epidermal and dermal layers, and the cytotoxicity to cells in the dermal layers. The high TI value observed for the skin treated with sodium laurate soap could be attributed to the loss of the barrier function due to the physical damage of the skin tissue. However, although the unsaturated C18 fatty acid sodium soap had a TI value similar to that of the sodium laurate soap, no morphological damage to the skin tissue was observed (
Figure 3e,f). This suggested that the mechanism of the effect of unsaturated C18 fatty acid sodium soap on skin barrier function was different from that of sodium laurate soap.
Since microscopic examination does not provide information regarding the changes in intercellular lipids, the effect of unsaturated C18 fatty acid sodium soap on skin barrier function may be due to changes in intercellular lipids. At present, we do not have information to link the TI values with the effects of two important factors on the skin barrier function, namely stratum corneum protein and the keratinocyte intercellular lipid. The inferences from the inconsistency between the results of microscopic observations and the trends in TI values of each surfactant need to be separately experimentally verified. That is our task in the future.
According to the structural chemistry considerations, it is considered that the effect of surfactants on the skin barrier function represented by the TI value is affected by the melting point of the fatty acids in the soap molecule and the solubility of the soap affected by them. Some previous studies have discussed the effects of ion pair properties of anionic surfactants on the skin [
9,
29,
30,
31]. However, in this study, the effects of counter ion and the neutralization rate on the TI value were not examined. They are also important factors affecting soap solubility. Soap solubility and its effects on skin barrier function need to be further investigated. Generally, potassium soap has a higher solubility than sodium soap. The neutralization rate also affects the pH of the aqueous soap solution. This study only provides information on the effects of the fatty acid branching structure and the degree of unsaturation in fully neutralized sodium soap. Moreover, the information available from microscopy is very limited. For future studies, an investigation of the relationship between the ion pair properties of the surfactants and the skin barrier function is warranted. This can contribute to the development of the manufacturing method such as the solubility and the pH adjustment of the surfactant which is safe for the skin barrier function and the suggestion of the direction for uses.