Lipid Signalling in Human Immune Response and Bone Remodelling under Microgravity
Abstract
1. Microgravity in Human Health
1.1. Microgravity and Immune Response
1.2. Microgravity and Bone Remodelling
2. Bioactive Lipids in Inflammation and Bone Remodelling
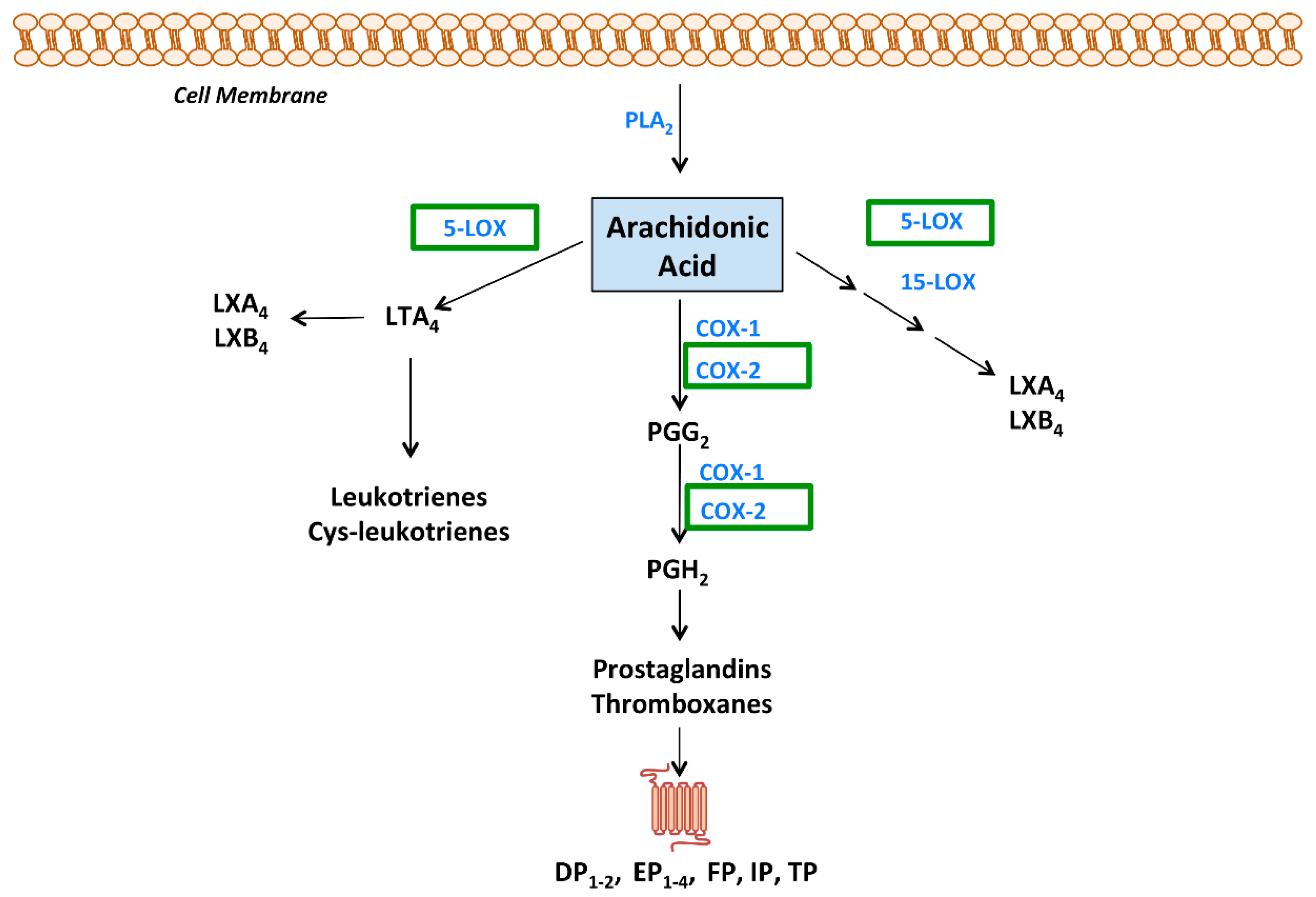
2.1. Eicosanoids: Chemistry, Signalling and Pathophysiology
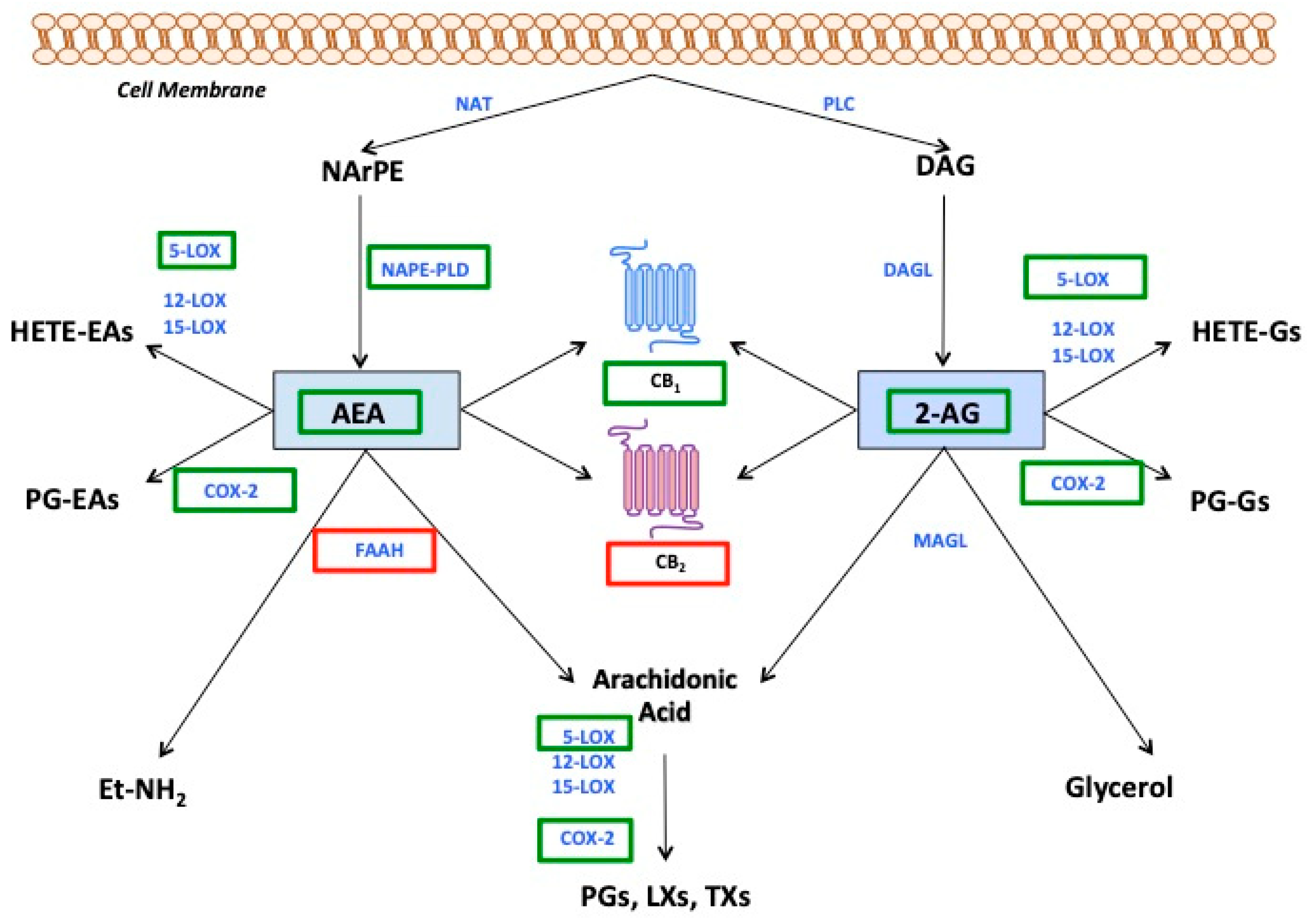
2.2. Endocannabinoids: Chemistry, Signalling and Pathophysiology
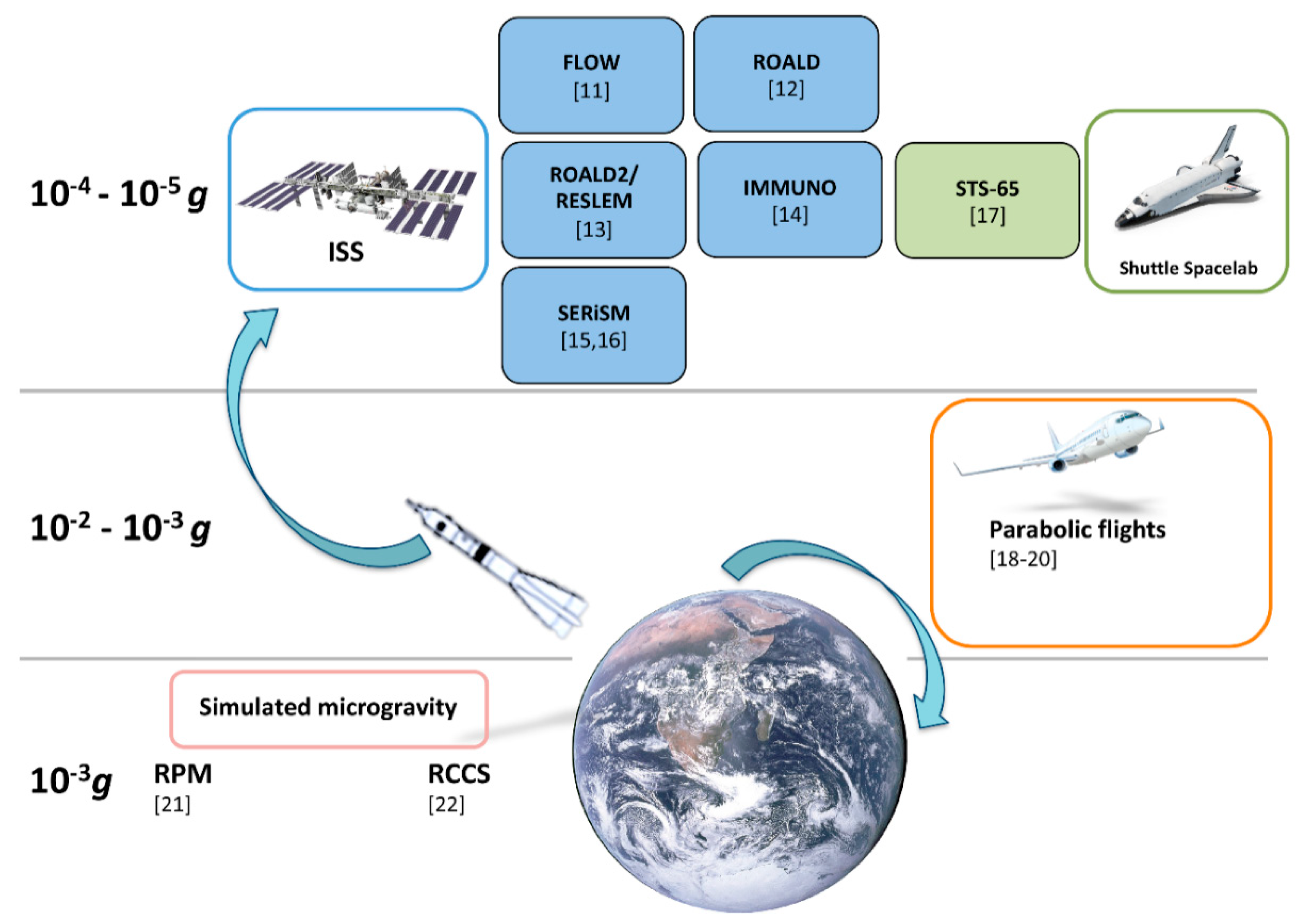
3. Lipid Signalling in Microgravity
3.1. Eicosanoids
3.2. Endocannabinoids
4. Conclusions and Future Perspectives
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hughes-Fulford, M. To infinity and beyond! Human spaceflight and life science. FASEB J. 2011, 25, 2858–2864. [Google Scholar] [CrossRef]
- Kimzey, S.L.; Johnson, P.C.; Ritzman, S.E.; Mengel, C.E. Hematology and immunology studies: The second manned Skylab mission. Aviat. Space Environ. Med. 1976, 47, 383–390. [Google Scholar]
- Cogoli, A.; Tschopp, A.; Fuchs-Bislin, P. Cell sensitivity to gravity. Science 1984, 225, 228–230. [Google Scholar] [CrossRef]
- Cogoli, A.; Bechler, B.; Cogoli-Greuter, M.; Criswell, S.B.; Joller, H.; Joller, P.; Hunzinger, E.; Muller, O. Mitogenic signal transduction in Tlymphocytes in microgravity. J. Leukoc. Biol. 1993, 53, 569–575. [Google Scholar] [CrossRef]
- Cogoli, A. Signal transduction in T lymphocytes in microgravity. Gravit. Space Biol. Bull. 1997, 10, 5–16. [Google Scholar]
- Otsuka, K.; Cornelissen, G.; Kubo, Y.; Hayashi, M.; Yamamoto, N.; Shibata, K.; Aiba, T.; Furukawa, S.; Ohshima, H.; Mukai, C. Intrinsic cardiovascular autonomic regulatory system of astronauts exposed long-term to microgravity in space: Observational study. npj Microgravity 2015, 1, 1–9. [Google Scholar] [CrossRef]
- White, R.J.; Averner, M. Humans in space. Nature 2001, 409, 1115–1118. [Google Scholar] [CrossRef]
- Setlow, R.B. The hazards of space travel. EMBO Rep. 2003, 4, 1013–1016. [Google Scholar] [CrossRef]
- Crucian, B.E.; Choukèr, A.; Simpson, R.J.; Mehta, S.; Marshall, G.; Smith, S.M.; Zwart, S.R.; Heer, M.; Ponomarev, S.; Whitmire, A.; et al. Immune System Dysregulation During Spaceflight: Potential Countermeasures for Deep Space Exploration Missions. Front. Immunol. 2018, 9. [Google Scholar] [CrossRef]
- Makedonas, G.; Mehta, S.; Choukèr, A.; Simpson, R.J.; Marshall, G.; Orange, J.S.; Aunon-Chancellor, S.; Smith, S.M.; Zwart, S.R.; Stowe, R.P.; et al. Specific Immunologic Countermeasure Protocol for Deep-Space Exploration Missions. Front. Immunol. 2019, 10. [Google Scholar] [CrossRef]
- Bacabac, R.G.; Van Loon, J.J.W.A.; de Blieck-Hogervorst, J.M.A.; Semeins, C.M.; Zandieh-Doulabi, B.; Helder, M.N.; Smit, T.H.; Klein-Nulend, J. Microgravity and bone cell mechanosensitivity: FLOW experiment during the DELTA mission. Microgravity Sci. Technol. 2007, 19, 133–137. [Google Scholar] [CrossRef]
- Battista, N.; Meloni, M.A.; Bari, M.; Mastrangelo, N.; Galleri, G.; Rapino, C.; Dainese, E.; Agrò, A.F.; Pippia, P.; Maccarrone, M. 5-Lipoxygenase-dependent apoptosis of human lymphocytes in the International Space Station: Data from the ROALD experiment. FASEB J. 2012, 26, 1791–1798. [Google Scholar] [CrossRef]
- Battista, N.; Di Tommaso, M.; Norfini, A.; Passerai, M.; Chiurchiù, V.; Maccarrone, M.; Bari, M. Altered Anandamide Metabolism in Microgravity: The “RESLEM” experiment. Front. Physiol. 2018, 9. [Google Scholar] [CrossRef]
- Buchheim, J.-I.; Matzel, S.; Rykova, M.; Vassilieva, G.; Ponomarev, S.; Nichiporuk, I.; Hörl, M.; Moser, D.; Biere, K.; Feuerecker, M.; et al. Stress Related Shift Toward Inflammaging in Cosmonauts After Long-Duration Space Flight. Front. Physiol. 2019, 10. [Google Scholar] [CrossRef]
- Gambacurta, A.; Merlini, G.; Ruggiero, C.; Diedenhofen, G.; Battista, N.; Bari, M.; Balsamo, M.; Piccirillo, S.; Valentini, G.; Mascetti, G.; et al. Human osteogenic differentiation in Space: Proteomic and epigenetic clues to better understand osteoporosis. Sci. Rep. 2019, 9, 8343. [Google Scholar] [CrossRef]
- Maccarrone, M.; Fava, M.; Battista, N.; Piccirillo, S.; Valentini, G.; Mascetti, G.; Gambacurta, A.; Bari, M. Opening the gate to the SERiSM project: From Earth to Space and back. Aerotec. Missili Spazio 2020, 99, 87–91. [Google Scholar] [CrossRef]
- Kumei, Y.; Shimokawa, H.; Katano, H.; Hara, E.; Akiyama, H.; Hirano, M.; Mukai, C.; Nagaoka, S.; Whitson, P.A.; Sams, C.F. Microgravity induces prostaglandin E2 and interleukin-6 production in normal rat osteoblasts: Role in bone demineralization. J. Biotechnol. 1996, 47, 313–324. [Google Scholar] [CrossRef]
- Maccarrone, M.; Finazzi-Agro, A. Microgravity increases the affinity of lipoxygenases for free fatty acids. FEBS Lett. 2001, 489, 283. [Google Scholar] [CrossRef]
- Choukèr, A.; Kaufmann, I.; Kreth, S.; Hauer, D.; Feuerecker, M.; Thieme, D.; Vogeser, M.; Thiel, M.; Schelling, G. Motion sickness, stress and the endocannabinoid system. PLoS ONE 2010, 5, e10752. [Google Scholar] [CrossRef]
- Strewe, C.; Feuerecker, M.; Nichiporuk, I.; Kaufmann, I.; Hauer, D.; Morukov, B.; Schelling, G.; Choukèr, A. Effects of parabolic flight and spaceflight on the endocannabinoid system in humans. Rev. Neurosci. 2012, 23, 673–680. [Google Scholar] [CrossRef]
- Maccarrone, M.; Bari, M.; Lorenzon, T.; Finazzi-Agrò, A. Altered gravity modulates prostaglandin H synthase in human K562 cells. J. Gravit. Physiol. 2000, 7, P61–P62. [Google Scholar]
- Gasperi, V.; Rapino, C.; Battista, N.; Bari, M.; Mastrangelo, N.; Angeletti, S.; Dainese, E.; Maccarrone, M. A functional interplay between 5-lipoxygenase and μ-calpain affects survival and cytokine profile of human Jurkat T lymphocyte exposed to simulated microgravity. Biomed. Res. Int. 2014, 2014, 782390. [Google Scholar] [CrossRef]
- Guéguinou, N.; Huin-Schohn, C.; Bascove, M.; Bueb, J.-L.; Tschirhart, E.; Legrand-Frossi, C.; Frippiat, J.-P. Could spaceflight-associated immune system weakening preclude the expansion of human presence beyond Earth’s orbit? J. Leukoc. Biol. 2009, 86, 1027–1038. [Google Scholar] [CrossRef]
- Frippiat, J.-P.; Crucian, B.E.; de Quervain, D.J.-F.; Grimm, D.; Montano, N.; Praun, S.; Roozendaal, B.; Schelling, G.; Thiel, M.; Ullrich, O.; et al. Towards human exploration of space: The THESEUS review series on immunology research priorities. npj Microgravity 2016, 2, 1–6. [Google Scholar] [CrossRef]
- Hauschild, S.; Tauber, S.; Lauber, B.; Thiel, C.S.; Layer, L.E.; Ullrich, O. T cell regulation in microgravity—The current knowledge from in vitro experiments conducted in space, parabolic flights and ground-based facilities. Acta Astronaut. 2014, 104, 365–377. [Google Scholar] [CrossRef]
- Crucian, B.E.; Cubbage, M.L.; Sams, C.F. Altered Cytokine Production by Specific Human Peripheral Blood Cell Subsets Immediately Following Space Flight. J. Interf. Cytokine Res. 2000, 20, 547–556. [Google Scholar] [CrossRef]
- Pippia, P.; Sciola, L.; Cogoli-greuter, M.; Meloni, M.A.; Spano, a.; Cogoli, A. Activation signals of T lymphocytes in microgravity. J. Biotechnol. 1996, 47, 215–222. [Google Scholar] [CrossRef]
- Hashemi, B.B.; Penkala, J.E.; Vens, C.; Huls, H.; Cubbage, M.; Sams, C.F. T cell activation responses are differentially regulated during clinorotation and in spaceflight. FASEB J. 1999, 13, 2071–2082. [Google Scholar] [CrossRef]
- Cooper, D.; Pellis, N.R. Suppressed PHA activation of T lymphocytes in simulated microgravity is restored by direct activation of protein kinase C. J. Leukoc. Biol. 1998, 63, 550–562. [Google Scholar] [CrossRef]
- Crucian, B.E.; Stowe, R.P.; Pierson, D.L.; Sams, C.F. Immune System Dysregulation Following Short- vs Long-Duration Spaceflight. Aviat. Space. Environ. Med. 2008, 79, 835–843. [Google Scholar] [CrossRef]
- Mukhopadhyay, S.; Saha, R.; Palanisamy, A.; Ghosh, M.; Biswas, A.; Roy, S.; Pal, A.; Sarkar, K.; Bagh, S. A systems biology pipeline identifies new immune and disease related molecular signatures and networks in human cells during microgravity exposure. Sci. Rep. 2016, 6, 25975. [Google Scholar] [CrossRef]
- Versari, S.; Longinotti, G.; Barenghi, L.; Maier, J.A.M.; Bradamante, S. The challenging environment on board the International Space Station affects endothelial cell function by triggering oxidative stress through thioredoxin interacting protein overexpression: The ESA-SPHINX experiment. FASEB J. 2013, 27, 4466–4475. [Google Scholar] [CrossRef]
- Girardi, C.; De Pittà, C.; Casara, S.; Calura, E.; Romualdi, C.; Celotti, L.; Mognato, M. Integration Analysis of MicroRNA and mRNA Expression Profiles in Human Peripheral Blood Lymphocytes Cultured in Modeled Microgravity. BioMed Res. Int. 2014, 2014, 1–16. [Google Scholar] [CrossRef]
- Ward, N.E.; Pellis, N.R.; Risin, S.A.; Risin, D. Gene expression alterations in activated human T-cells induced by modeled microgravity. J. Cell. Biochem. 2006, 99, 1187–1202. [Google Scholar] [CrossRef]
- Chang, T.T.; Walther, I.; Li, C.; Boonyaratanakornkit, J.; Galleri, G.; Meloni, M.A.; Cogoli, A.; Hughes-Fulford, M. The Rel/NF-κB pathway and transcription of immediate early genes in T cell activation are inhibited by microgravity. J. Leukoc. Biol. 2012, 92, 1133–1145. [Google Scholar] [CrossRef]
- McGinley, A.M.; Sutton, C.E.; Edwards, S.C.; Leane, C.M.; DeCourcey, J.; Teijeiro, A.; Hamilton, J.A.; Boon, L.; Djouder, N.; Mills, K.H.G. Interleukin-17A Serves a Priming Role in Autoimmunity by Recruiting IL-1β-Producing Myeloid Cells that Promote Pathogenic T Cells. Immunity 2020, 52, 342–356.e6. [Google Scholar] [CrossRef]
- Chang, T.T.; Spurlock, S.M.; Candelario, T.L.T.; Grenon, S.M.; Hughes-Fulford, M. Spaceflight impairs antigen-specific tolerance induction in vivo and increases inflammatory cytokines. FASEB J. 2015, 29, 4122–4132. [Google Scholar] [CrossRef]
- Shi, L.; Tian, H.; Wang, P.; Li, L.; Zhang, Z.; Zhang, J.; Zhao, Y. Spaceflight and simulated microgravity suppresses macrophage development via altered RAS/ERK/NFκB and metabolic pathways. Cell. Mol. Immunol. 2020, 1–14. [Google Scholar] [CrossRef]
- Guéguinou, N.; Jeandel, J.; Kaminski, S.; Baatout, S.; Ghislin, S.; Frippiat, J.-P. Modulation of Iberian Ribbed Newt Complement Component C3 by Stressors Similar to those Encountered during a Stay Onboard the International Space Station. Int. J. Mol. Sci. 2019, 20, 1579. [Google Scholar] [CrossRef]
- Wang, C.; Chen, H.; Luo, H.; Zhu, L.; Zhao, Y.; Tian, H.; Wang, R.; Shang, P.; Zhao, Y. Microgravity activates p38 MAPK-C/EBPβ pathway to regulate the expression of arginase and inflammatory cytokines in macrophages. Inflamm. Res. 2015, 64, 303–311. [Google Scholar] [CrossRef]
- Li, N.; Wang, C.; Sun, S.; Zhang, C.; Lü, D.; Chen, Q.; Long, M. Microgravity-Induced Alterations of Inflammation-Related Mechanotransduction in Endothelial Cells on Board SJ-10 Satellite. Front. Physiol. 2018, 9, 1025. [Google Scholar] [CrossRef] [PubMed]
- Xia, M.; Nguyen, T.P.; Bao, J.-X.; Meng, N.; Boini, K.M.; Li, P.-L. Microgravity-Induced Activation of Nlrp3 Inflammasomes in Mouse Vascular Endothelial Cells. FASEB J. 2016, 30, 1204.9. [Google Scholar] [CrossRef]
- De Groot, N.S.; Burgas, M.T. Is membrane homeostasis the missing link between inflammation and neurodegenerative diseases? Cell. Mol. Life Sci. 2015, 72, 4795–4805. [Google Scholar] [CrossRef] [PubMed]
- Miguel, L.; Owen, D.M.; Lim, C.; Liebig, C.; Evans, J.; Magee, A.I.; Jury, E.C. Primary Human CD4+ T Cells Have Diverse Levels of Membrane Lipid Order That Correlate with Their Function. J. Immunol. 2011. [Google Scholar] [CrossRef] [PubMed]
- Fessler, M.B.; Parks, J.S. Intracellular Lipid Flux and Membrane Microdomains as Organizing Principles in Inflammatory Cell Signaling. J. Immunol. 2011, 187, 1529–1535. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, D.; Banerjee, S.; Sen, A.; Banerjee, K.K.; Das, P.; Roy, S. Leishmania donovani Affects Antigen Presentation of Macrophage by Disrupting Lipid Rafts. J. Immunol. 2005, 175, 3214–3224. [Google Scholar] [CrossRef]
- Kohn, F.P.M.; Hauslage, J. The gravity dependence of pharmacodynamics: The integration of lidocaine into membranes in microgravity. npj Microgravity 2019, 5, 1–6. [Google Scholar] [CrossRef]
- Akiyama, T.; Horie, K.; Hinoi, E.; Hiraiwa, M.; Kato, A.; Maekawa, Y.; Takahashi, A.; Furukawa, S. How does spaceflight affect the acquired immune system? npj Microgravity 2020, 6, 1–7. [Google Scholar] [CrossRef]
- Chiurchiù, V.; Leuti, A.; Maccarrone, M. Bioactive Lipids and Chronic Inflammation: Managing the Fire Within. Front. Immunol. 2018, 9, 38. [Google Scholar] [CrossRef]
- Smith, S.M.; Abrams, S.A.; Davis-Street, J.E.; Heer, M.; O’Brien, K.O.; Wastney, M.E.; Zwart, S.R. Fifty years of human space travel: Implications for bone and calcium research. Annu. Rev. Nutr. 2014, 34, 377–400. [Google Scholar] [CrossRef]
- Zaidi, M.; Yuen, T.; Sun, L.; Rosen, C.J. Regulation of Skeletal Homeostasis. Endocr. Rev. 2018, 39, 701–718. [Google Scholar] [CrossRef] [PubMed]
- Rodionova, N.V.; Oganov, V.S.; Zolotova, N.V. Ultrastructural changes in osteocytes in microgravity conditions. Adv. Space Res. 2002, 30, 765–770. [Google Scholar] [CrossRef]
- Aguirre, J.I.; Plotkin, L.I.; Stewart, S.A.; Weinstein, R.S.; Parfitt, A.M.; Manolagas, S.C.; Bellido, T. Osteocyte apoptosis is induced by weightlessness in mice and precedes osteoclast recruitment and bone loss. J. Bone Miner. Res. 2006, 21, 605–615. [Google Scholar] [CrossRef] [PubMed]
- Cardoso, L.; Herman, B.C.; Verborgt, O.; Laudier, D.; Majeska, R.J.; Schaffler, M.B. Osteocyte apoptosis controls activation of intracortical resorption in response to bone fatigue. J. Bone Miner. Res. 2009, 24, 597–605. [Google Scholar] [CrossRef]
- Plotkin, L.I.; Mathov, I.; Aguirre, J.I.; Parfitt, A.M.; Manolagas, S.C.; Bellido, T. Mechanical stimulation prevents osteocyte apoptosis: Requirement of integrins, Src kinases, and ERKs. Am. J. Physiol. Cell Physiol. 2005, 28, C633–C643. [Google Scholar] [CrossRef]
- Zayzafoon, M.; Gathings, W.E.; McDonald, J.M. Modeled Microgravity Inhibits Osteogenic Differentiation of Human Mesenchymal Stem Cells and Increases Adipogenesis. Endocrinology 2004, 145, 2421–2432. [Google Scholar] [CrossRef]
- Caillot-Augusseau, A.; Lafage-Proust, M.-H.; Soler, C.; Pernod, J.; Dubois, F.; Alexandre, C. Bone formation and resorption biological markers in cosmonauts during and after a 180-day space flight (Euromir 95). Clin. Chem. 1998. [Google Scholar] [CrossRef]
- Nabavi, N.; Khandani, A.; Camirand, A.; Harrison, R.E. Effects of microgravity on osteoclast bone resorption and osteoblast cytoskeletal organization and adhesion. Bone 2011, 49, 965–974. [Google Scholar] [CrossRef]
- Hughes-Fulford, M.; Lewis, M.L. Effects of microgravity on osteoblast growth activation. Exp. Cell Res. 1996, 224, 103–109. [Google Scholar] [CrossRef]
- Tamma, R.; Colaianni, G.; Camerino, C.; Di Benedetto, A.; Greco, G.; Strippoli, M.; Vergari, R.; Grano, A.; Mancini, L.; Mori, G.; et al. Microgravity during spaceflight directly affects in vitro osteoclastogenesis and bone resorption. FASEB J. 2009, 23, 2549–2554. [Google Scholar] [CrossRef]
- Raisz, L.G. Prostaglandins and bone: Physiology and pathophysiology. Osteoarthr. Cartil. 1999, 7, 419–421. [Google Scholar] [CrossRef] [PubMed]
- Blackwell, K.A.; Raisz, L.G.; Pilbeam, C.C. Prostaglandins in bone: Bad cop, good cop? Trends Endocrinol. Metab. 2010, 21, 294–301. [Google Scholar] [CrossRef] [PubMed]
- Rossi, F.; Tortora, C.; Punzo, F.; Bellini, G.; Argenziano, M.; Di Paola, A.; Torella, M.; Perrotta, S. The Endocannabinoid/Endovanilloid System in Bone: From Osteoporosis to Osteosarcoma. Int. J. Mol. Sci. 2019, 20, 1919. [Google Scholar] [CrossRef] [PubMed]
- Bab, I.; Ofek, O.; Tam, J.; Rehnelt, J.; Zimmer, A. Endocannabinoids and the Regulation of Bone Metabolism. J. Neuroendocrinol. 2008, 20, 69–74. [Google Scholar] [CrossRef]
- Sims, S.M.; Panupinthu, N.; Lapierre, D.M.; Pereverzev, A.; Dixon, S.J. Lysophosphatidic acid: A potential mediator of osteoblast–osteoclast signaling in bone. Biochim. Biophys. Acta (BBA) Mol. Cell Biol. Lipids 2013, 1831, 109–116. [Google Scholar] [CrossRef]
- Hannun, Y.A.; Obeid, L.M. Principles of bioactive lipid signalling: Lessons from sphingolipids. Nat. Rev. Mol. Cell Biol. 2008, 9, 139–150. [Google Scholar] [CrossRef]
- Basil, M.C.; Levy, B.D. Specialized pro-resolving mediators: Endogenous regulators of infection and inflammation. Nat. Rev. Immunol. 2015, 16, 51–67. [Google Scholar] [CrossRef]
- Hishikawa, D.; Hashidate, T.; Shimizu, T.; Shindou, H. Diversity and function of membrane glycerophospholipids generated by the remodeling pathway in mammalian cells. J. Lipid Res. 2014, 55, 799–807. [Google Scholar] [CrossRef]
- Ulrich, C.M.; Bigler, J.; Potter, J.D. Non-steroidal anti-inflammatory drugs for cancer prevention: Promise, perils and pharmacogenetics. Nat. Rev. Cancer 2006, 6, 130–140. [Google Scholar] [CrossRef]
- Smith, W.L.; Urade, Y.; Jakobsson, P.-J. Enzymes of the Cyclooxygenase Pathways of Prostanoid Biosynthesis. Chem. Rev. 2011, 111, 5821–5865. [Google Scholar] [CrossRef]
- Mazaleuskaya, L.L.; Lawson, J.A.; Li, X.; Grant, G.; Mesaros, C.; Grosser, T.; Blair, I.A.; Ricciotti, E.; FitzGerald, G.A. A broad-spectrum lipidomics screen of antiinflammatory drug combinations in human blood. JCI Insight 2016, 1. [Google Scholar] [CrossRef] [PubMed]
- Schwab, J.M.; Serhan, C.N. Lipoxins and new lipid mediators in the resolution of inflammation. Curr. Opin. Pharmacol. 2006, 6, 414–420. [Google Scholar] [CrossRef] [PubMed]
- Aoki, T.; Narumiya, S. Prostaglandins and chronic inflammation. Trends Pharmacol. Sci. 2012, 33, 304–311. [Google Scholar] [CrossRef] [PubMed]
- Marzo, V.D.; Bifulco, M.; Petrocellis, L.D. The endocannabinoid system and its therapeutic exploitation. Nat. Rev. Drug Discov. 2004, 3, 771–784. [Google Scholar] [CrossRef]
- Cristino, L.; Bisogno, T.; Di Marzo, V. Cannabinoids and the expanded endocannabinoid system in neurological disorders. Nat. Rev. Neurol. 2020, 16, 9–29. [Google Scholar] [CrossRef]
- Friedman, D.; French, J.A.; Maccarrone, M. Safety, efficacy, and mechanisms of action of cannabinoids in neurological disorders. Lancet Neurol. 2019, 18, 504–512. [Google Scholar] [CrossRef]
- Maccarrone, M.; Bab, R.; Biro, T.; Cabral, G.A.; Dey, S.K.; Di Marzo, V.; Konje, J.C.; Kunos, G.; Mechoulam, R.; Pacher, P.; et al. Endocannabinoid signaling at the periphery: 50 years after THC. Trends Pharmacol. Sci. 2015, 36, 277–296. [Google Scholar] [CrossRef]
- Maccarrone, M. Metabolism of the Endocannabinoid Anandamide: Open Questions after 25 Years. Front. Mol. Neurosci. 2017, 10, 166. [Google Scholar] [CrossRef]
- Dinh, T.P.; Carpenter, D.; Leslie, F.M.; Freund, T.F.; Katona, I.; Sensi, S.L.; Kathuria, S.; Piomelli, D. Brain monoglyceride lipase participating in endocannabinoid inactivation. Proc. Natl. Acad. Sci. USA 2002, 99, 10819–10824. [Google Scholar] [CrossRef]
- Baggelaar, M.P.; den Dulk, H.; Florea, B.I.; Fazio, D.; Perruzza, D.; Bernabò, N.; Raspa, M.; Janssen, A.P.A.; Scavizzi, F.; Barboni, B.; et al. ABHD2 Inhibitor Identified by Activity-Based Protein Profiling Reduces Acrosome Reaction. ACS Chem. Biol. 2019, 14, 2943. [Google Scholar] [CrossRef]
- Cencioni, M.T.; Chiurchiù, V.; Catanzaro, G.; Borsellino, G.; Bernardi, G.; Battistini, L.; Maccarrone, M. Anandamide Suppresses Proliferation and Cytokine Release from Primary Human T-Lymphocytes Mainly via CB 2 Receptors. PLoS ONE 2010, 5, e8688. [Google Scholar] [CrossRef] [PubMed]
- Cencioni, M.T.; Bisicchia, E.; De Bardi, M.; Gasperini, C.; Borsellino, G.; Centonze, D.; Battistini, L.; Maccarrone, M. Distinct modulation of human myeloid and plasmacytoid dendritic cells by anandamide in multiple sclerosis. Ann. Neurol. 2013, 73, 626–636. [Google Scholar] [CrossRef]
- Gokoh, M.; Kishimoto, S.; Oka, S.; Sugiura, T. 2-Arachidonoylglycerol enhances the phagocytosis of opsonized zymosan by HL-60 cells differentiated into macrophage-like cells. Biol. Pharm. Bull. 2007, 30, 1199–1205. [Google Scholar] [CrossRef] [PubMed]
- Centonze, D.; Bari, M.; Rossi, S.; Prosperetti, C.; Furlan, R.; Fezza, F.; De Chiara, V.; Battistini, L.; Bernardi, G.; Bernardini, S.; et al. The endocannabinoid system is dysregulated in multiple sclerosis and in experimental autoimmune encephalomyelitis. Brain 2007, 130, 2543–2553. [Google Scholar] [CrossRef]
- Centonze, D.; Finazzi-Agrò, A.; Bernardi, G.; Maccarrone, M. The endocannabinoid system in targeting inflammatory neurodegenerative diseases. Trends Pharmacol. Sci. 2007, 28, 180–187. [Google Scholar] [CrossRef]
- Chiurchiù, V.; Leuti, A.; Maccarrone, M. Cannabinoid Signaling and Neuroinflammatory Diseases: A Melting pot for the Regulation of Brain Immune Responses. J. Neuroimmune Pharmacol. 2015, 10, 268–280. [Google Scholar] [CrossRef] [PubMed]
- Maccarrone, M.; Battista, N.; Meloni, M.; Bari, M.; Galleri, G.; Pippia, P.; Cogoli, A.; Finazzi-Agrò, A. Creating conditions similar to those that occur during exposure of cells to microgravity induces apoptosis in human lymphocytes by 5-lipoxygenase-mediated mitochondrial uncoupling and cytochrome c release. J. Leukoc. Biol. 2003, 73, 472–481. [Google Scholar] [CrossRef]
- Durand, M.; Gallant, M.A.; de Brum-Fernandes, A.J. Prostaglandin D2 receptors control osteoclastogenesis and the activity of human osteoclasts. J. Bone Miner. Res. 2008, 23, 1097–1105. [Google Scholar] [CrossRef]
- Tian, X.Y.; Zhang, Q.; Zhao, R.; Setterberg, R.B.; Zeng, Q.Q.; Ma, Y.F.; Jee, W.S.S. Continuous infusion of PGE2 is catabolic with a negative bone balance on both cancellous and cortical bone in rats. J. Musculoskelet. Neuronal Interact. 2007, 7, 372–381. [Google Scholar]
- Tian, X.Y.; Zhang, Q.; Zhao, R.; Setterberg, R.B.; Zeng, Q.Q.; Iturria, S.J.; Ma, Y.F.; Jee, W.S.S. Continuous PGE2 leads to net bone loss while intermittent PGE2 leads to net bone gain in lumbar vertebral bodies of adult female rats. Bone 2008, 42, 914–920. [Google Scholar] [CrossRef]
- Burger, E.H.; Klein-Nulend, J. Microgravity and Bone Cell Mechanosensitivity. Bone 1998, 22, 127S–130S. [Google Scholar] [CrossRef]
- Hughes-Fulford, M. Function of the cytoskeleton in gravisensing during spaceflight. Adv. Space Res. 2003, 32, 1585–1593. [Google Scholar] [CrossRef]
- Bari, M.; Battista, N.; Merlini, G.; Fava, M.; Ruggiero, C.; Piccirillo, S.; Valentini, G.; Mascetti, G.; Gambacurta, A.; Maccarrone, M. The SERiSM project: Preliminary data on human stem cell reprogramming in microgravity. Front. Physiol. 2018, 9. [Google Scholar] [CrossRef]
- Ofek, O.; Attar-Namdar, M.; Kram, V.; Dvir-Ginzberg, M.; Mechoulam, R.; Zimmer, A.; Frenkel, B.; Shohami, E.; Bab, I. CB2 cannabinoid receptor targets mitogenic Gi protein-cyclin D1 axis in osteoblasts. J. Bone Miner. Res. 2011, 26, 308–316. [Google Scholar] [CrossRef]
- Sophocleous, A.; Landao-Bassonga, E.; Van’t Hof, R.J.; Idris, A.I.; Ralston, S.H. The type 2 cannabinoid receptor regulates bone mass and ovariectomy-induced bone loss by affecting osteoblast differentiation and bone formation. Endocrinology 2011, 152, 2141–2149. [Google Scholar] [CrossRef]
- Idris, A.I.; Ralston, S.H. Role of cannabinoids in the regulation of bone remodeling. Front. Endocrinol. 2012, 3. [Google Scholar] [CrossRef]
- Idris, A.I.; Sophocleous, A.; Landao-Bassonga, E.; van’t Hof, R.J.; Ralston, S.H. Regulation of bone mass, osteoclast function, and ovariectomy-induced bone loss by the type 2 cannabinoid receptor. Endocrinology 2008, 149, 5619–5626. [Google Scholar] [CrossRef]
- Idris, A.I.; Sophocleous, A.; Landao-Bassonga, E.; Canals, M.; Milligan, G.; Baker, D.; van’t Hof, R.J.; Ralston, S.H. Cannabinoid receptor type 1 protects against age-related osteoporosis by regulating osteoblast and adipocyte differentiation in marrow stromal cells. Cell Metab. 2009, 10, 139–147. [Google Scholar] [CrossRef]
- Chiurchiù, V.; Leuti, A.; Dalli, J.; Jacobsson, A.; Battistini, L.; Maccarrone, M.; Serhan, C.N. Proresolving lipid mediators resolvin D1, resolvin D2, and maresin 1 are critical in modulating T cell responses. Sci. Transl. Med. 2016, 8, 353ra111. [Google Scholar] [CrossRef]
- Chiurchiù, V.; Leuti, A.; Saracini, S.; Fontana, D.; Finamore, P.; Giua, R.; Padovini, L.; Incalzi, R.A.; Maccarrone, M. Resolution of inflammation is altered in chronic heart failure and entails a dysfunctional responsiveness of T lymphocytes. FASEB J. 2019, 33, 909–916. [Google Scholar] [CrossRef]
| Sample | Target | Effect of Microgravity | Experimental Setup | Reference |
|---|---|---|---|---|
| PBMCs | AEA | Enhanced production | ISS | [13] |
| Human Blood | AEA | Enhanced production | ISS | [14] |
| Human Blood | AEA | Enhanced production | Parabolic flight | [19,20] |
| PBMCs | FAAH | Down-regulation (both at transcriptional and protein level) | ISS | [13] |
| PBMCs | NAPE-PLD | Up-regulation (both at transcriptional and protein level) | ISS | [13] |
| Osteoblasts | PGE2 | Enhanced production | Shuttle Spacelab | [17] |
| hBDSCs | CB1 | Enhanced production | ISS | [16,93] |
| hBDSCs | CB2 | Lower production | ISS | [16,93] |
| Purified enzyme | LOX-1 | Enhanced activity | Parabolic flight | [18] |
| K562 cells | COX-2 | Enhanced activity | RPM | [21] |
| Lymphocytes, U937 cells | 5-LOX | Enhanced activity | RPM | [12,87] |
| PBMCs | 5-LOX | Enhanced activity | ISS | [12] |
| Jurkat T cells | 5-LOX | Enhanced activity | RCCS | [22] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fava, M.; Leuti, A.; Maccarrone, M. Lipid Signalling in Human Immune Response and Bone Remodelling under Microgravity. Appl. Sci. 2020, 10, 4309. https://doi.org/10.3390/app10124309
Fava M, Leuti A, Maccarrone M. Lipid Signalling in Human Immune Response and Bone Remodelling under Microgravity. Applied Sciences. 2020; 10(12):4309. https://doi.org/10.3390/app10124309
Chicago/Turabian StyleFava, Marina, Alessandro Leuti, and Mauro Maccarrone. 2020. "Lipid Signalling in Human Immune Response and Bone Remodelling under Microgravity" Applied Sciences 10, no. 12: 4309. https://doi.org/10.3390/app10124309
APA StyleFava, M., Leuti, A., & Maccarrone, M. (2020). Lipid Signalling in Human Immune Response and Bone Remodelling under Microgravity. Applied Sciences, 10(12), 4309. https://doi.org/10.3390/app10124309







