Process Control Strategies in Chemical Looping Gasification—A Novel Process for the Production of Biofuels Allowing for Net Negative CO2 Emissions
Abstract
1. Introduction
2. Modelling Methods
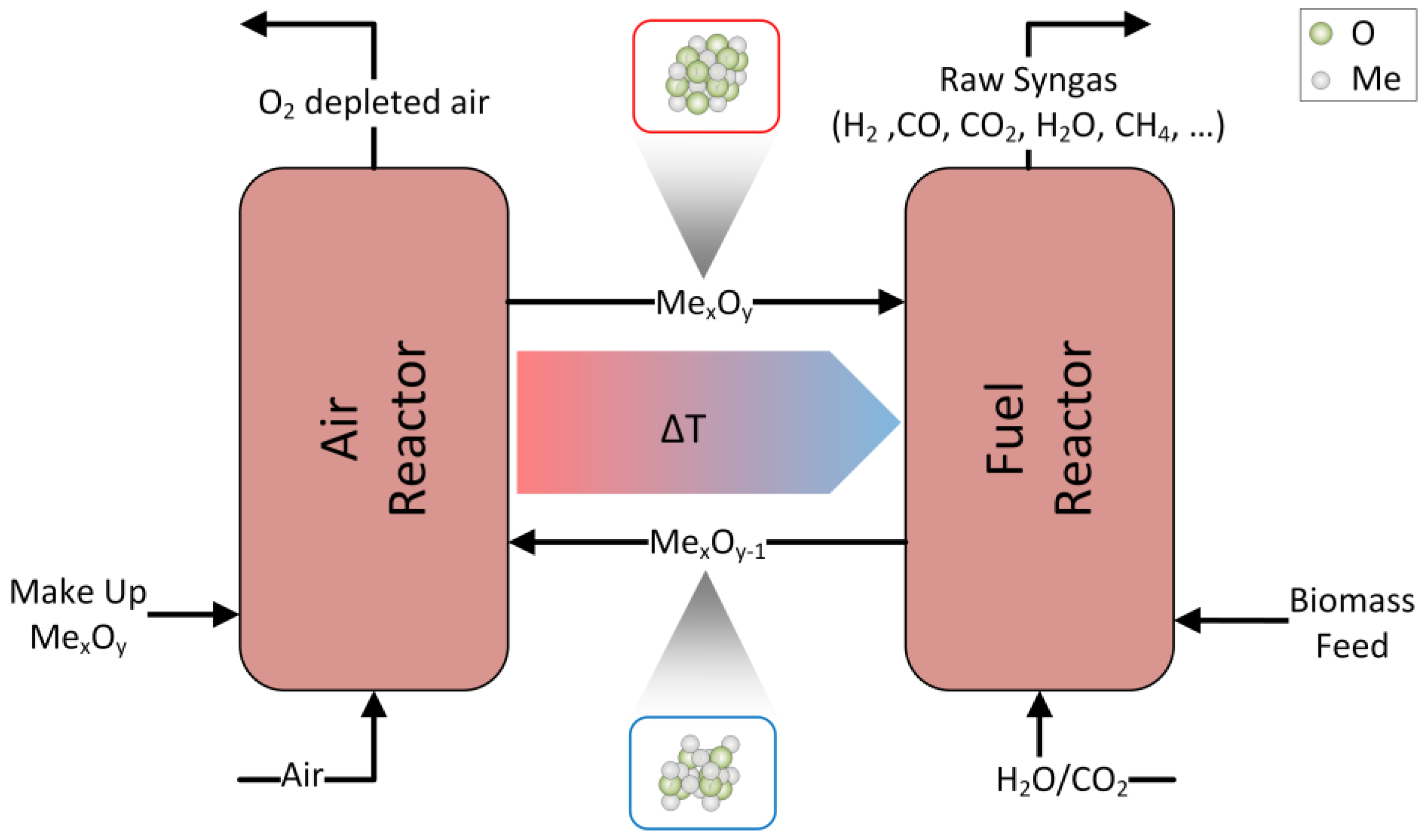
2.1. Description of the Process Model
- Prior to any calculation, an initial solid mass flow is given into the system (INIT), to model the circulating solid OC mass. Instead of estimating the actual solid loss, the approach of Ohlemüller et al. [25], setting the total OC loss (OCLOSS) to 1% of the circulating mass to achieve fast flowsheet conversion, was adopted. The same amount of fresh solids was constantly fed to the AR (MAKEUP), to achieve constant solid circulation.
- For both reactors, cyclones are employed to achieve solid-gas separation. The FR products are separated into a gas (FRGAS) and solid (SOLTOSEP) stream, via CYCL1 (separation efficiency 100%). Similarly, the AR products are separated into a gas (ARFLUE) and solid stream (OCO-TOFR) in CYLC2 (separation efficiency 100%).
- All streams entering the process are fed at ambient temperature ( = 25 °C), except for the stream STEAM, which is fed as saturated steam (120 °C).
- The steam and the air entering the FR/AR are preheated to a designated inlet temperature (, ). If not stated otherwise, the inlet temperature of both streams (STEAM, AIR) when entering the FR/AR is set to 400 °C.
- As Aspen Plus™ is not equipped to handle solid fuels, the biomass feedstock (FUEL) is fed to the decomposer (DECOMP), where it is decomposed into its pyrolysis products (DEVOLAD). The heat of pyrolysis (Q-DECOMP) is transferred to the fuel reactor. A detailed description of the decomposer block is given in Section 2.2.
- The pyrolysis products (DEVOLAD), the gasification agent (STEAMX), the OC recycled from the AR (OCO-TOFR), and the CO2 required for solid feeding and loop seal fluidization (SCREWFLU) are mixed (FRIN) before entering the fuel reactor.
- Subsequently, the educts entering the fuel reactor (FR) are converted into reaction products according to the chemical equilibrium at the given boundary conditions (TFR, PFR = 1 atm).
- The solids leaving CYCL1 are separated into the OC fed to the AR (OCR-TOAR) and a stream containing carbon and ash (SOL) in the solids separation (SOLSEP). This separation signifies the removal of bed material (i.e., OC, ash and unconverted feedstock) from the FR via sluicing during operation. Additionally, a fraction of the oxygen carrier material is removed from the system (OCLOSS), to model OC losses via sluicing and attrition.
- The OC makeup stream (MAKEUP) and the inlet air (AIRX) are mixed (ARIN) before being fed to the AR.
- Inside the air reactor (AR) the reduced OC and the unreacted char react with the oxygen contained in the air according to the chemical equilibrium at the given boundary conditions (TAR, PAR = 1 atm).
2.2. Decomposer
2.3. Boundary Conditions
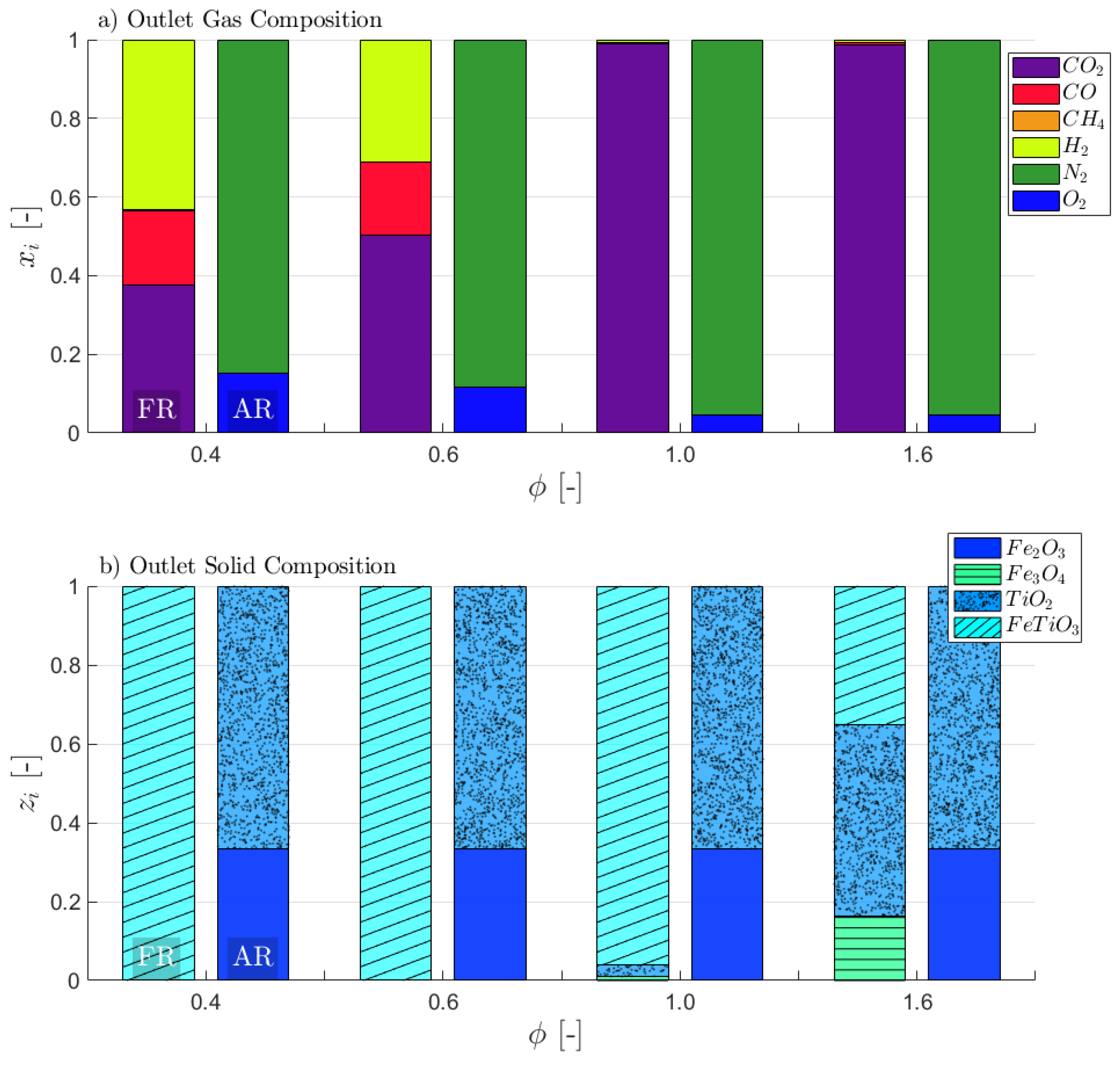
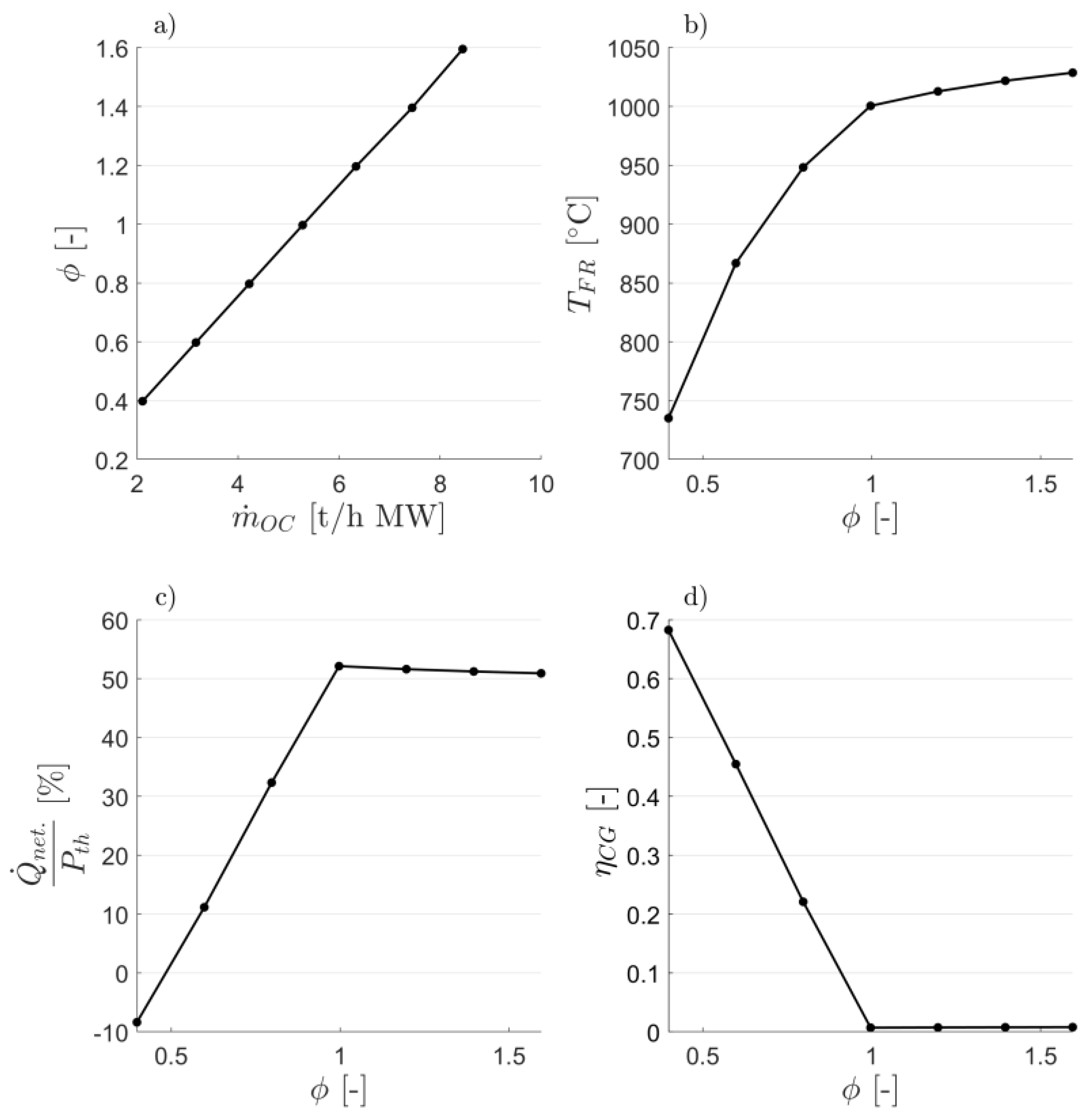
3. Results and Discussion
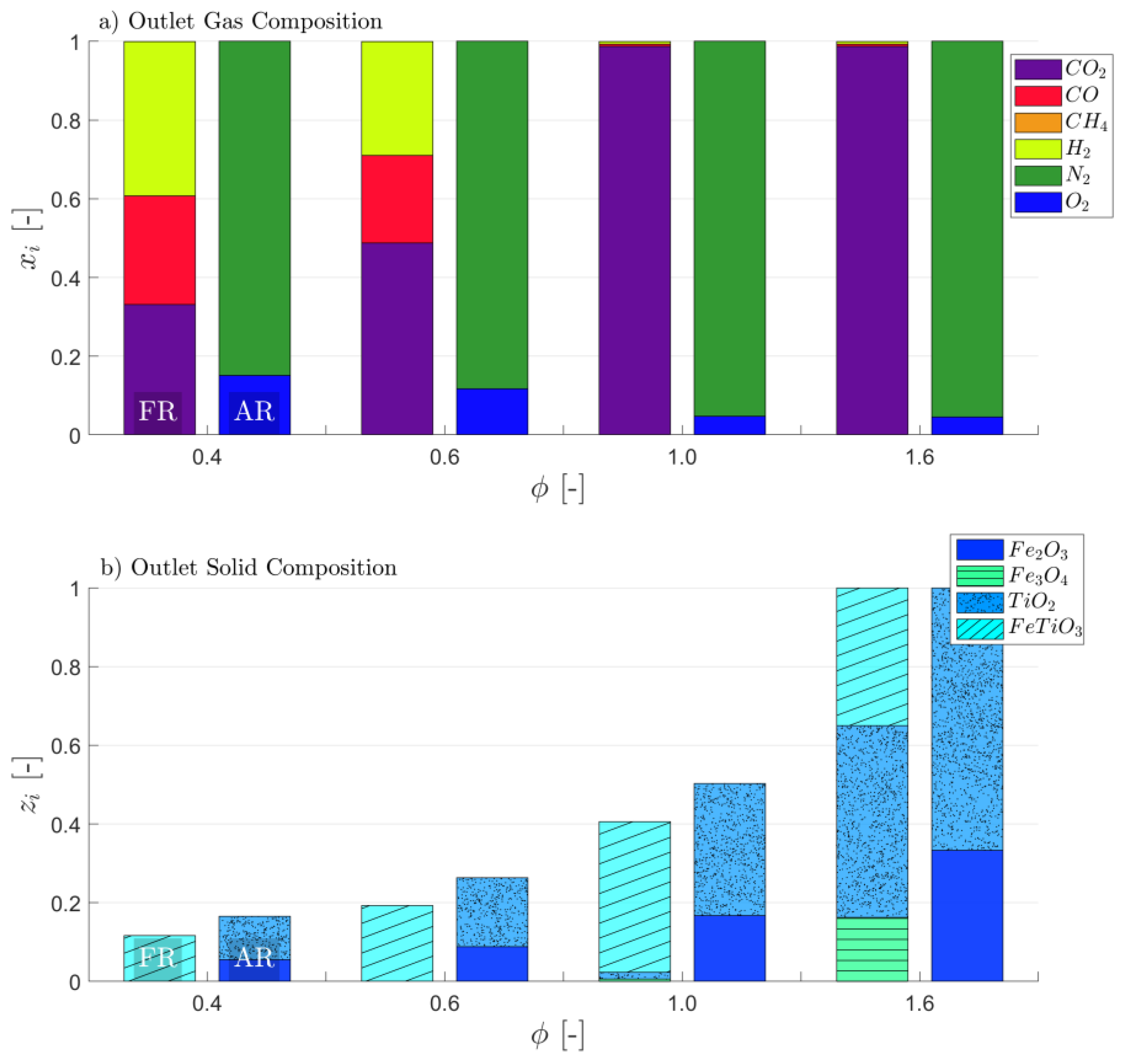
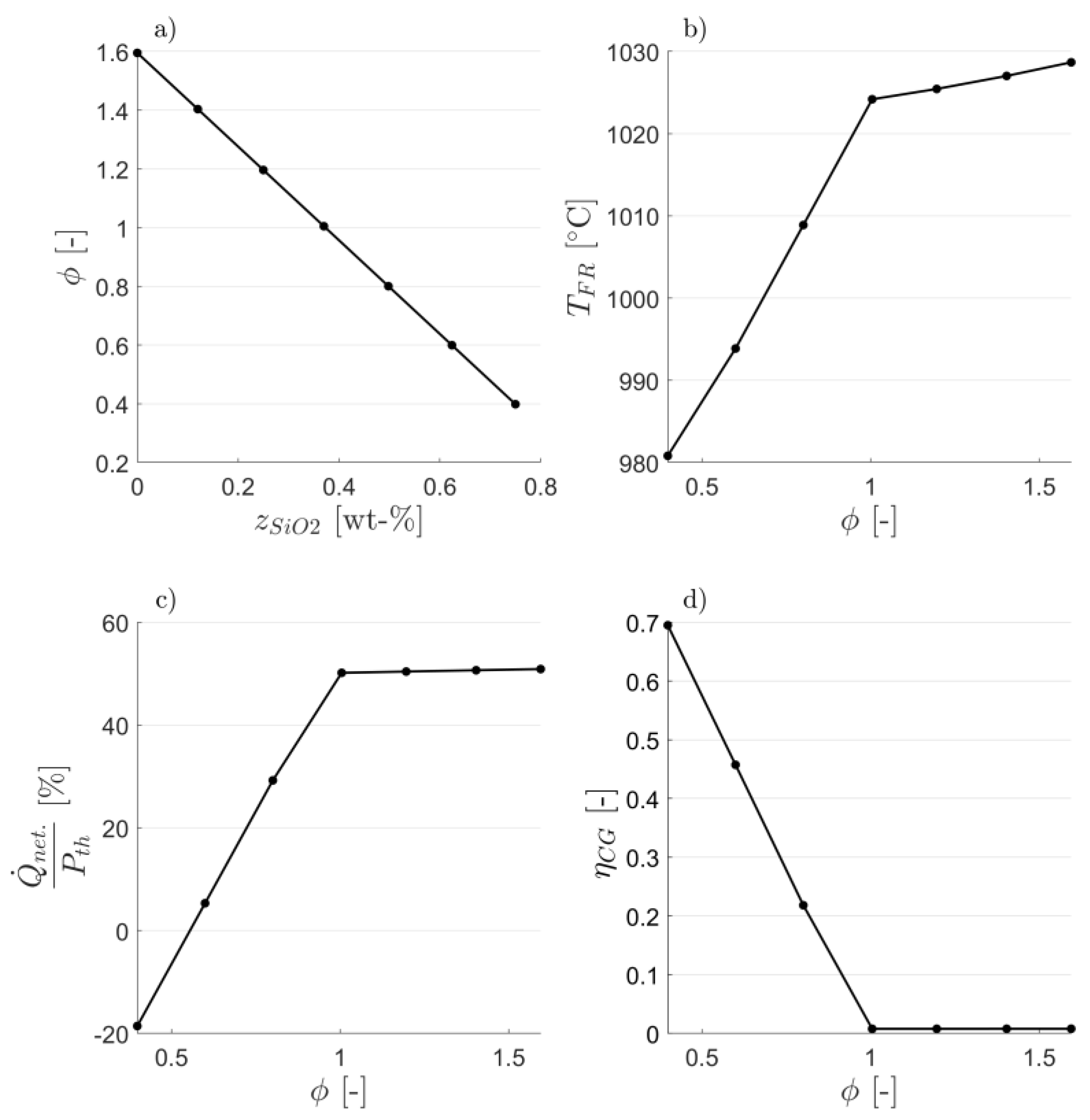
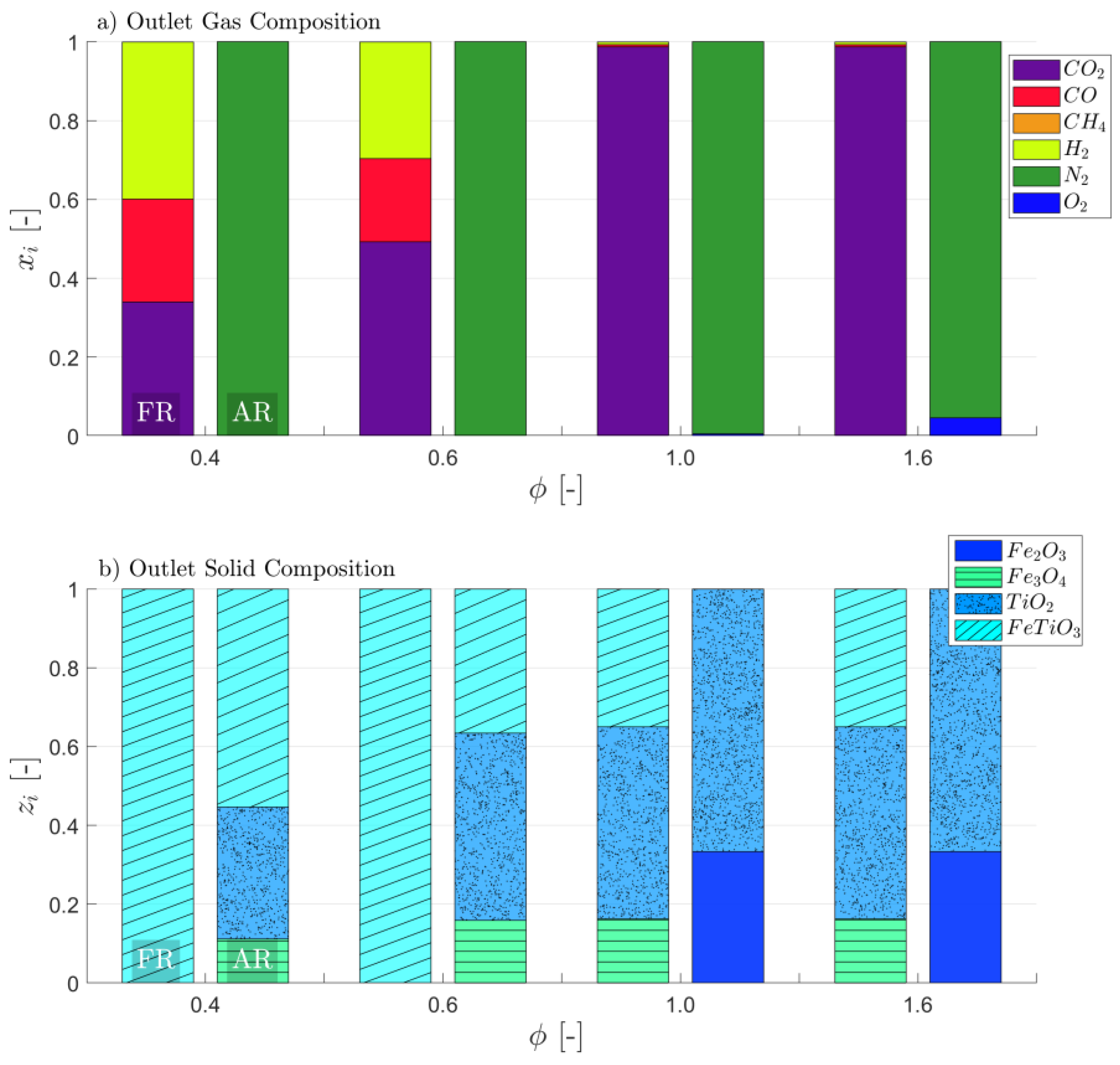
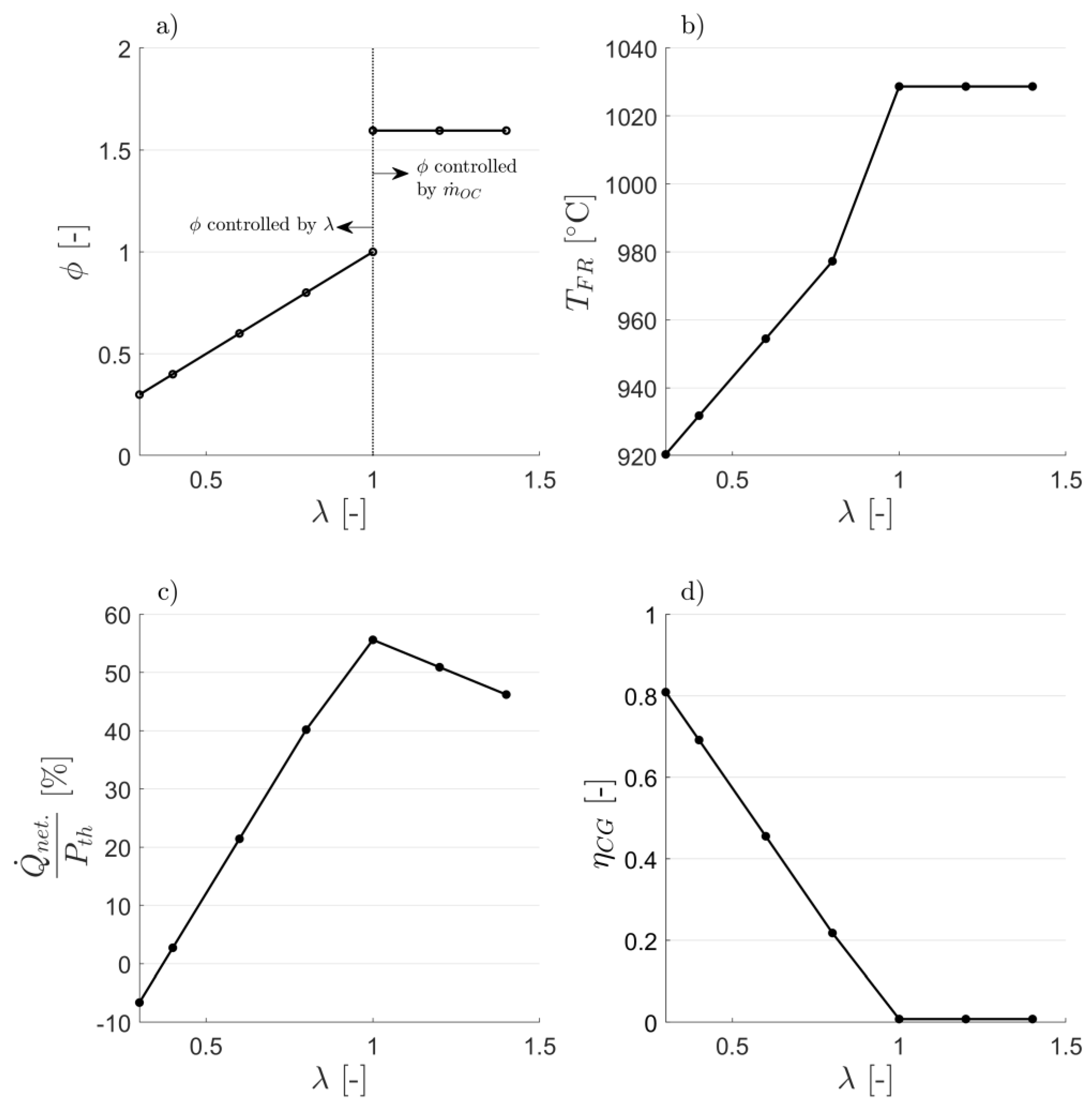
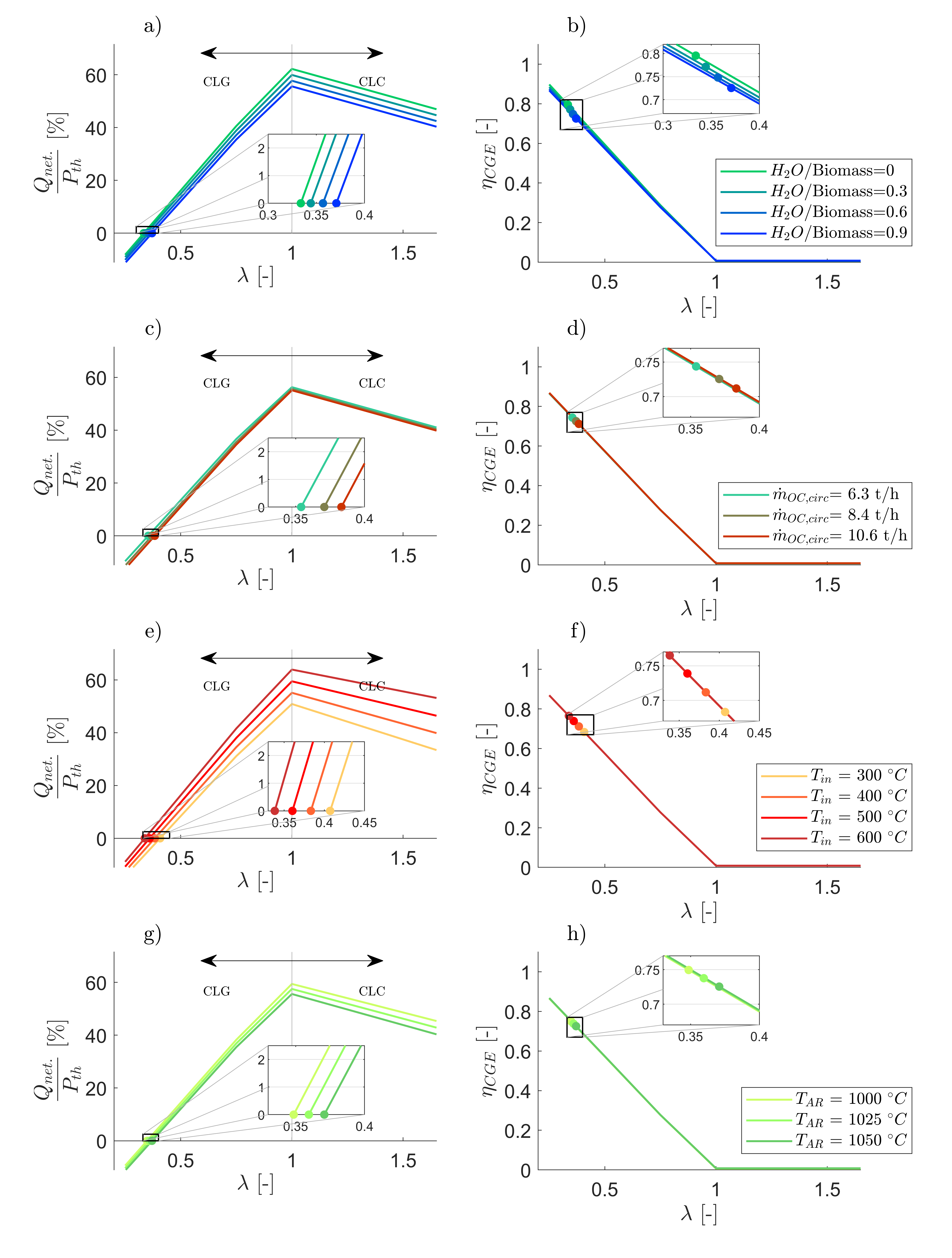
3.1. Attaining CLG Behavior
3.2. Reduction of OC Circulation
3.3. Dilution of OC with Inert Bed Material
3.4. Reduction of Air-to-Fuel Equivalence Ratio
3.5. Optimizing CLG Efficiency
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Nomenclature
| Symbol | Explanation | Unit |
| Enthalpy of stream i | kJ/kg | |
| Mass flow of component/element i | kg/h | |
| Molar mass of component/element i | g/mole | |
| Mole flow of component/element i | kmole/h | |
| Power | kW | |
| Pressure | bar | |
| Oxygen transport capacity of oxygen carrier | - | |
| Mass flow of air entering the AR | kg/h | |
| Temperature | °C | |
| Mass/mole fraction in gas phase | - | |
| Conversion of component i | - | |
| Mass yield of component/element i from substance j | - | |
| Mass/mole fraction in solid phase | - | |
| Carbon capture efficiency | - | |
| Cold gas efficiency | - | |
| Air-to-fuel equivalence ratio | - | |
| Oxygen carrier-to-fuel equivalence ratio | - |
| Subscript | Explanation |
| AR | Air reactor |
| devol. | Devolatilization. |
| FR | Fuel reactor |
| init | Initial |
| net | net |
| O | Oxygen |
| OC | Oxygen Carrier |
| ox | Oxidation |
| red | Reduction |
| s | Solid |
| stoich | Stoichiometric |
| th | Thermal |
| Abbreviation | Explanation |
| AR | Air Reactor |
| ASU | Air Separation Unit |
| CGE | Cold Gas Efficiency |
| CLC | Chemical Looping Combustion |
| CLG | Chemical Looping Gasification |
| FR | Fuel Reactor |
| GHGE | Greenhouse Gas Emissions |
| LHV | Lower Heating Value |
| OC | Oxygen Carrier |
| RED II | European Union Renewable Energy Directive |
| WGS | Water-Gas-Shift |
Appendix A. Boundary Conditions for CLG Process Model
| Parameter | Approach 1 * | Approach 2 * | Approach 3 * | Unit |
|---|---|---|---|---|
| 730–1030 | 980–1030 | 930–1030 | °C | |
| 1050 | 1050 | 1050 | °C | |
| / | 1.013 | 1.013 | 1.013 | bar |
| 200.4 | 200.4 | 200.4 | kg/h | |
| 180.4 | 180.4 | 180.4 | kg/h | |
| 40.1 | 40.1 | 40.1 | kg/h | |
| 1362.6 | 1362.6 | 454–1590 | kg/h | |
| 25 | 25 | 25 | °C | |
| / | 400 | 400 | 400 | °C |
| 2.11–8.45 | 8.45 | 8.45 | t/h | |
| 0 | 0–75 | 0 | wt-% |
Appendix B. Shifting from CLC to CLG Operation through Variations in the Air-to-Fuel Equivalence Ratio
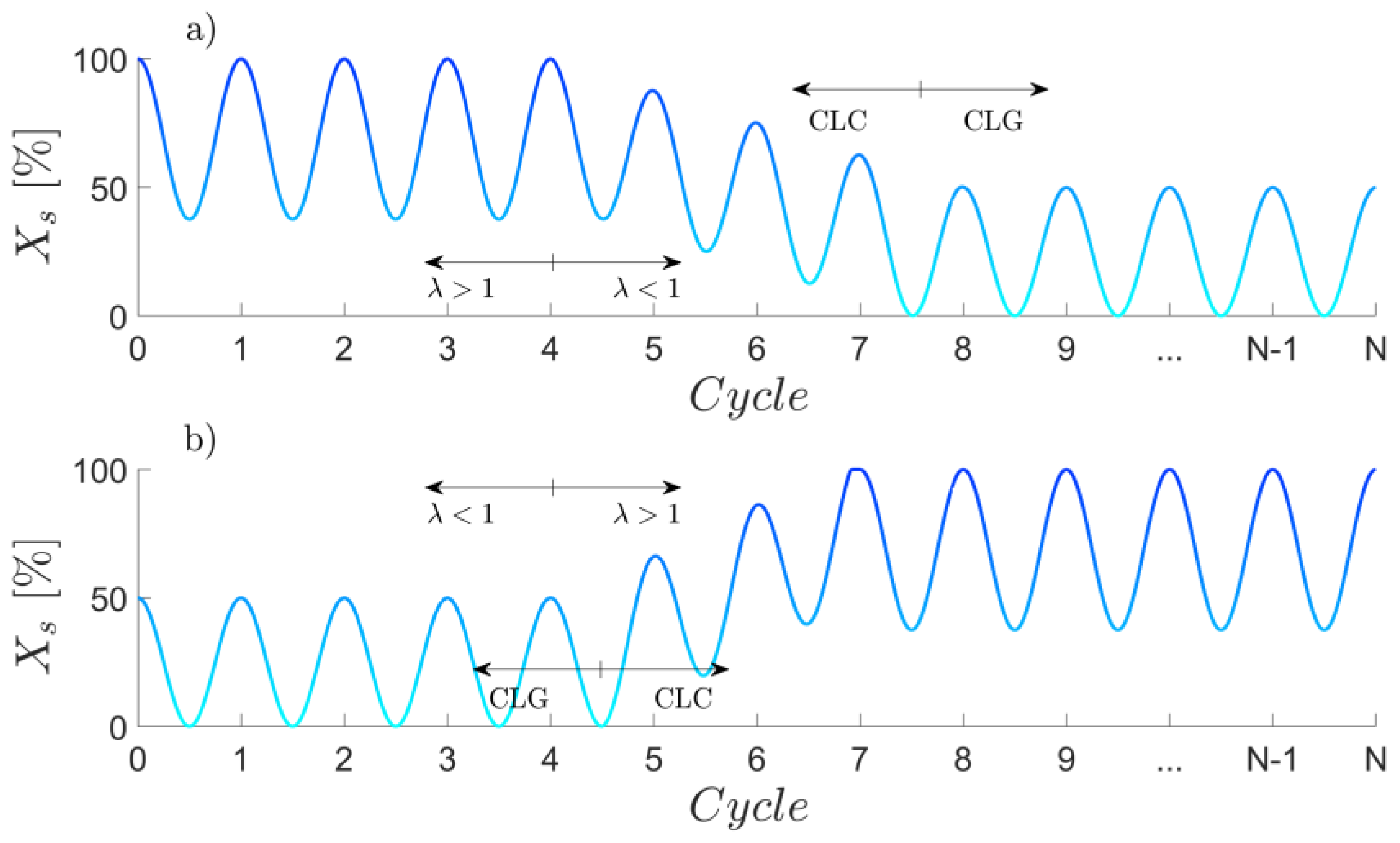
Appendix C. Char Conversion in an Sub-Stoichiometrically Operated AR

References
- International Energy Agency (IEA). Key World Energy Statistics 2018; International Energy Agency: Paris, France, 2018. [Google Scholar]
- Energy Information Administration. Statistics Data Browser—Electricity Generation from Renewables by Source. Available online: https://www.iea.org/statistics/ (accessed on 23 August 2019).
- European Commission. Transport Emissions—A European Strategy for Low-Emission Mobility. Available online: https://ec.europa.eu/clima/policies/transport_en (accessed on 23 August 2019).
- EUR-Lex. Directive (EU) 2018/2001 of the European Parliament and of the Council of 11 December 2018 on the Promotion of the Use of Energy from Renewable Sources (Text with EEA Relevance). Available online: https://eur-lex.europa.eu/legal-content/en/TXT/?uri=CELEX:32018L2001 (accessed on 12 June 2020).
- Carrasco, J.E.; Monti, A.; Tayeb, J.; Kiel, J.; Girio, F.; Matas, B.; Santos Jorge, R. Strategic Research and Innovation Agenda 2020; EERA: Brussels, Belgium, 2020; p. 82. [Google Scholar]
- De, S.; Agarwal, A.K.; Moholkar, V.S.; Thallada, B. Coal and Biomass Gasification: Recent Advances and Future Challenges; Springer: Berlin/Heidelberg, Germany, 2018. [Google Scholar]
- Higman, C.; Van der Burgt, M. Gasification, 2nd ed.; Gulf Professional Publishing: Houston, TX, USA; Elsevier Science: Amsterdam, The Netherlands, 2008. [Google Scholar]
- Barrio, M.; Gbel, B.; Rimes, H.; Henriksen, U.; Hustad, J.E.; Srensen, L.H. Steam gasification of wood char and the effect of hydrogen inhibition on the chemical kinetics. In Progress in Thermochemical Biomass Conversion; Bridgwater, A.V., Ed.; Blackwell Science Ltd.: Oxford, UK, 2001; pp. 32–46. [Google Scholar]
- Hansen, L.K.; Rathmann, O.; Olsen, A.; Poulsen, K. Steam Gasification of Wheat Straw, Barley Straw, Willow and Giganteus; Risø National Laboratory: Roskilde, Denmark, 1997. [Google Scholar]
- Klose, W.; Wolki, M. On the intrinsic reaction rate of biomass char gasification with carbon dioxide and steam. Fuel 2005, 84, 885–892. [Google Scholar] [CrossRef]
- Ollero, P.; Serrera, A.; Arjona, R.; Alcantarilla, S. The CO2 gasification kinetics of olive residue. Biomass Bioenergy 2003, 24, 151–161. [Google Scholar] [CrossRef]
- Barrio, M.; Hustad, J.E. CO2 Gasification of birch char and the effect of CO inhibition on the calculation of chemical kinetics. In Progress in Thermochemical Biomass Conversion; Bridgwater, A.V., Ed.; Blackwell Science Ltd.: Oxford, UK, 2001; pp. 47–60. [Google Scholar]
- Basu, P. Biomass Gasification and Pyrolysis; Elsevier: Amsterdam, The Netherlands, 2010. [Google Scholar]
- Ge, H.; Zhang, H.; Guo, W.; Song, T.; Shen, L. System simulation and experimental verification: Biomass-based integrated gasification combined cycle (BIGCC) coupling with chemical looping gasification (CLG) for power generation. Fuel 2019, 241, 118–128. [Google Scholar] [CrossRef]
- Huang, Z.; Zhang, Y.; Fu, J.; Yu, L.; Chen, M.; Liu, S.; He, F.; Chen, D.; Wei, G.; Zhao, K.; et al. Chemical looping gasification of biomass char using iron ore as an oxygen carrier. Int. J. Hydrogen Energy 2016, 41, 17871–17883. [Google Scholar] [CrossRef]
- Karl, J.; Pröll, T. Steam gasification of biomass in dual fluidized bed gasifiers: A review. Renew. Sust. Energ. Rev. 2018, 98, 64–78. [Google Scholar] [CrossRef]
- Xu, G.; Murakami, T.; Suda, T.; Matsuzawa, Y.; Tani, H. The superior technical choice for dual fluidized bed gasification. Ind. Eng. Chem. Res. 2006, 45, 2281–2286. [Google Scholar] [CrossRef]
- Aigner, I.; Pfeifer, C.; Hofbauer, H. Co-gasification of coal and wood in a dual fluidized bed gasifier. Fuel 2011, 90, 2404–2412. [Google Scholar] [CrossRef]
- Huang, Z.; He, F.; Feng, Y.; Zhao, K.; Zheng, A.; Chang, S.; Wei, G.; Zhao, Z.; Li, H. biomass char direct chemical looping gasification using NiO-modified iron ore as an oxygen carrier. Energy Fuels 2014, 28, 183–191. [Google Scholar] [CrossRef]
- Ge, H.; Guo, W.; Shen, L.; Song, T.; Xiao, J. Biomass gasification using chemical looping in a 25 kWth reactor with natural hematite as oxygen carrier. Chem. Eng. J. 2016, 286, 174–183. [Google Scholar] [CrossRef]
- Guo, Q.; Cheng, Y.; Liu, Y.; Jia, W.; Ryu, H.-J. Coal chemical looping gasification for syngas generation using an iron-based oxygen carrier. Ind. Eng. Chem. Res. 2014, 53, 78–86. [Google Scholar] [CrossRef]
- Huang, Z.; He, F.; Feng, Y.; Zhao, K.; Zheng, A.; Chang, S.; Li, H. Synthesis gas production through biomass direct chemical looping conversion with natural hematite as an oxygen carrier. Bioresour. Technol. 2013, 140, 138–145. [Google Scholar] [CrossRef] [PubMed]
- Huseyin, S.; Wei, G.; Li, H.; He, F.; Huang, Z. Chemical-looping gasification of biomass in a 10 kWth interconnected fluidized bed reactor using Fe2O3/Al2O3 oxygen carrier. J. Fuel Chem. Technol. 2014, 42, 922–931. [Google Scholar] [CrossRef]
- Adanez, J.; Abad, A.; Garcia-Labiano, F.; Gayan, P.; De Diego, L.F. Progress in chemical-looping combustion and reforming technologies. Prog. Energy Combust. Sci. 2012, 38, 215–282. [Google Scholar] [CrossRef]
- Ohlemüller, P.; Alobaid, F.; Abad, A.; Adanez, J.; Ströhle, J.; Epple, B. Development and validation of a 1D process model with autothermal operation of a 1 MWth chemical looping pilot plant. Int. J. Greenh. Gas Control 2018, 73, 29–41. [Google Scholar] [CrossRef]
- Markström, P.; Linderholm, C.; Lyngfelt, A. Chemical-looping combustion of solid fuels—Design and operation of a 100 kW unit with bituminous coal. Int. J. Greenh. Gas Control 2013, 15, 150–162. [Google Scholar] [CrossRef]
- Cuadrat, A.; Abad, A.; García-Labiano, F.; Gayán, P.; De Diego, L.F.; Adánez, J. Effect of operating conditions in Chemical-Looping Combustion of coal in a 500 Wth unit. Int. J. Greenh. Gas Control 2012, 6, 153–163. [Google Scholar] [CrossRef]
- Pérez-Vega, R.; Abad, A.; García-Labiano, F.; Gayán, P.; De Diego, L.F.; Adánez, J. Coal combustion in a 50 kWth chemical looping combustion unit: Seeking operating conditions to maximize CO2 capture and combustion efficiency. Int. J. Greenh. Gas Control 2016, 50, 80–92. [Google Scholar] [CrossRef]
- Ströhle, J.; Orth, M.; Epple, B. Chemical looping combustion of hard coal in a 1 MWth pilot plant using ilmenite as oxygen carrier. Appl. Energy 2015, 157, 288–294. [Google Scholar] [CrossRef]
- Cao, Y.; Casenas, B.; Pan, W.-P. Investigation of chemical looping combustion by solid fuels. 2. Redox reaction kinetics and product characterization with coal, biomass, and solid waste as solid fuels and CuO as an oxygen carrier. Energy Fuels 2006, 20, 1845–1854. [Google Scholar] [CrossRef]
- Leion, H.; Mattisson, T.; Lyngfelt, A. Solid fuels in chemical-looping combustion. Int. J. Greenh. Gas Control 2008, 2, 180–193. [Google Scholar] [CrossRef]
- Virginie, M.; Adánez, J.; Courson, C.; De Diego, L.F.; García-Labiano, F.; Niznansky, D.; Kiennemann, A.; Gayán, P.; Abad, A. Effect of Fe–olivine on the tar content during biomass gasification in a dual fluidized bed. Appl. Catal. B Environ. 2012, 121–122, 214–222. [Google Scholar] [CrossRef]
- Kuhn, J.N.; Zhao, Z.; Felix, L.G.; Slimane, R.B.; Choi, C.W.; Ozkan, U.S. Olivine catalysts for methane and tar-steam reforming. Appl. Catal. B Environ. 2008, 81, 14–26. [Google Scholar] [CrossRef]
- Mendiara, T.; Johansen, J.M.; Utrilla, R.; Geraldo, P.; Jensen, A.D.; Glarborg, P. Evaluation of different oxygen carriers for biomass tar reforming (I): Carbon deposition in experiments with toluene. Fuel 2011, 90, 1049–1060. [Google Scholar] [CrossRef]
- Larsson, A.; Israelsson, M.; Lind, F.; Seemann, M.; Thunman, H. Using ilmenite to reduce the tar yield in a dual fluidized bed gasification system. Energy Fuels 2014, 28, 2632–2644. [Google Scholar] [CrossRef]
- Milne, T.A.; Evans, R.J.; Abatzaglou, N. Biomass Gasifier “Tars”: Their Nature, Formation, and Conversion; National Renewable Energy Laboratory: Golden, CO, USA, 1998.
- Ge, H.; Guo, W.; Shen, L.; Song, T.; Xiao, J. Experimental investigation on biomass gasification using chemical looping in a batch reactor and a continuous dual reactor. Chem. Eng. J. 2016, 286, 689–700. [Google Scholar] [CrossRef]
- Leion, H.; Jerndal, E.; Steenari, B.-M.; Hermansson, S.; Israelsson, M.; Jansson, E.; Johnsson, M.; Thunberg, R.; Vadenbo, A.; Mattisson, T.; et al. Solid fuels in chemical-looping combustion using oxide scale and unprocessed iron ore as oxygen carriers. Fuel 2009, 88, 1945–1954. [Google Scholar] [CrossRef]
- Fan, L.-S. Chemical Looping Systems for Fossil Energy Conversions; Wiley-AIChE: Hoboken, NJ, USA, 2010. [Google Scholar]
- Mayer, K.; Penthor, S.; Pröll, T.; Hofbauer, H. The different demands of oxygen carriers on the reactor system of a CLC plant—Results of oxygen carrier testing in a 120 kWth pilot plant. Appl. Energy 2015, 157, 323–329. [Google Scholar] [CrossRef]
- Ohlemüller, P.G. Untersuchung von Chemical-Looping-Combustion im Megawatt-Maßstab; Cuvillier: Göttingen, Germany, 2019. [Google Scholar]
- De Diego, L.F.; Garcı´a-Labiano, F.; Gayán, P.; Celaya, J.; Palacios, J.M.; Adánez, J. Operation of a 10 kWth chemical-looping combustor during 200h with a CuO–Al2O3 oxygen carrier. Fuel 2007, 86, 1036–1045. [Google Scholar] [CrossRef]
- Adánez, J.; Gayán, P.; Celaya, J.; De Diego, L.F.; García-Labiano, F.; Abad, A. Chemical Looping Combustion in a 10 kWth Prototype Using a CuO/Al2O3 Oxygen Carrier: Effect of Operating Conditions on Methane Combustion. Ind. Eng. Chem. Res. 2006, 45, 6075–6080. [Google Scholar] [CrossRef]
- Pröll, T.; Bolhàr-Nordenkampf, J.; Kolbitsch, P.; Hofbauer, H. Syngas and a separate nitrogen/argon stream via chemical looping reforming—A 140 kW pilot plant study. Fuel 2010, 89, 1249–1256. [Google Scholar] [CrossRef]
- Ohlemüller, P.; Busch, J.-P.; Reitz, M.; Ströhle, J.; Epple, B. Chemical-Looping Combustion of Hard Coal: Autothermal Operation of a 1 MWth Pilot Plant. J. Energy Resour. Technol. 2016, 138, 042203. [Google Scholar] [CrossRef]
- Mallick, D.; Mahanta, P.; Moholkar, V.S. Co-gasification of coal and biomass blends: Chemistry and engineering. Fuel 2017, 204, 106–128. [Google Scholar] [CrossRef]
- Matthesius, G.A.; Morris, R.M.; Desai, M.J. Prediction of the volatile matter in coal from ultimate and proximate analyses. J. S. Afr. Inst. Min. Metall. 1987, 5, 157–161. [Google Scholar]
- Neves, D.; Thunman, H.; Matos, A.; Tarelho, L.; Gómez-Barea, A. Characterization and prediction of biomass pyrolysis products. Prog. Energy Combust. Sci. 2011, 37, 611–630. [Google Scholar] [CrossRef]
- Cuadrat, A.; Abad, A.; Gayán, P.; De Diego, L.F.; García-Labiano, F.; Adánez, J. Theoretical approach on the CLC performance with solid fuels: Optimizing the solids inventory. Fuel 2012, 97, 536–551. [Google Scholar] [CrossRef]
- Mendiara, T.; Pérez-Astray, A.; Izquierdo, M.T.; Abad, A.; De Diego, L.F.; García-Labiano, F.; Gayán, P.; Adánez, J. Chemical Looping Combustion of different types of biomass in a 0.5 kWth unit. Fuel 2018, 211, 868–875. [Google Scholar] [CrossRef]
- Leion, H.; Lyngfelt, A.; Johansson, M.; Jerndal, E.; Mattisson, T. The use of ilmenite as an oxygen carrier in chemical-looping combustion. Chem. Eng. Res. Des. 2008, 86, 1017–1026. [Google Scholar] [CrossRef]
- Pissot, S.; Vilches, T.B.; Maric, J.; Seemann, M. Chemical looping gasification in a 2–4 MWth dual fluidized bed gasifier. In Proceedings of the 23rd International Conference on Fluidized Bed Conversion, Seoul, South Korea, 13 May 2018. [Google Scholar]
- Li, K.; Zhang, R.; Bi, J. Experimental study on syngas production by co-gasification of coal and biomass in a fluidized bed. Int. J. Hydrogen Energy 2010, 35, 2722–2726. [Google Scholar] [CrossRef]
- Narváez, I.; Orío, A.; Aznar, M.P.; Corella, J. Biomass gasification with air in an atmospheric bubbling fluidized bed. effect of six operational variables on the quality of the produced raw gas. Ind. Eng. Chem. Res. 1996, 35, 2110–2120. [Google Scholar] [CrossRef]
- Abad, A.; Adánez, J.; Cuadrat, A.; García-Labiano, F.; Gayán, P.; De Diego, L.F. Kinetics of redox reactions of ilmenite for chemical-looping combustion. Chem. Eng. Sci. 2011, 66, 689–702. [Google Scholar] [CrossRef]
- Zafar, Q.; Abad, A.; Mattisson, T.; Gevert, B. Reaction kinetics of freeze-granulated NiO/MgAl2O4 oxygen carrier particles for chemical-looping combustion. Energy Fuels 2007, 21, 610–618. [Google Scholar] [CrossRef][Green Version]
- Mattisson, T.; Lyngfelt, A.; Cho, P. The use of iron oxide as an oxygen carrier in chemical-looping combustion of methane with inherent separation of CO2. Fuel 2001, 80, 1953–1962. [Google Scholar] [CrossRef]
- Ohlemüller, P.; Ströhle, J.; Epple, B. Chemical looping combustion of hard coal and torrefied biomass in a 1 MWth pilot plant. Int. J. Greenh. Gas Control 2017, 65, 149–159. [Google Scholar] [CrossRef]
- Dennis, J.S.; Scott, S.A. In situ gasification of a lignite coal and CO2 separation using chemical looping with a Cu-based oxygen carrier. Fuel 2010, 89, 1623–1640. [Google Scholar] [CrossRef]
- He, F.; Huang, Z.; Li, H.; Zhao, Z. Biomass Direct Chemical Looping Conversion in a Fluidized Bed Reactor with Natural Hematite as an Oxygen Carrier. In Proceedings of the Asia-Pacific Power and Energy Engineering Conference (IEEE), Wuhan, China, 28–31 March 2011; pp. 1–7. [Google Scholar]
- Zhao, H.; Guo, L.; Zou, X. Chemical-looping auto-thermal reforming of biomass using Cu-based oxygen carrier. Appl. Energy 2015, 157, 408–415. [Google Scholar] [CrossRef]
- Kunii, D.; Levenspiel, O. Fluidization Engineering, 2nd ed.; Butterworth-Heinemann: Boston, MA, USA, 1991. [Google Scholar]
- Song, Q.; Xiao, R.; Deng, Z.; Zhang, H.; Shen, L.; Xiao, J.; Zhang, M. Chemical-looping combustion of methane with CaSO4 oxygen carrier in a fixed bed reactor. Energy Convers. Manag. 2008, 49, 3178–3187. [Google Scholar] [CrossRef]
- Ryu, H.-J.; Bae, D.-H.; Jin, G.-T. Effect of temperature on reduction reactivity of oxygen carrier particles in a fixed bed chemical-looping combustor. Korean J. Chem. Eng. 2003, 20, 960–966. [Google Scholar] [CrossRef]
- Cuadrat, A.; Abad, A.; Adánez, J.; De Diego, L.F.; García-Labiano, F.; Gayán, P. Behavior of ilmenite as oxygen carrier in chemical-looping combustion. Fuel Process. Technol. 2012, 94, 101–112. [Google Scholar] [CrossRef]
- Cho, P.; Mattisson, T.; Lyngfelt, A. Carbon Formation on Nickel and Iron Oxide-Containing Oxygen Carriers for Chemical-Looping Combustion. Ind. Eng. Chem. Res. 2005, 44, 668–676. [Google Scholar] [CrossRef]
- Leion, H.; Lyngfelt, A.; Mattisson, T. Effects of Steam and CO2 in the Fluidizing Gas when Using Bituminous Coal in Chemical-Looping Combustion. In Proceedings of the 20th International Conference on Fluidized Bed Combustion, Xi’an, China, 18–21 May 2009; pp. 608–611. [Google Scholar]
- Leion, H.; Mattisson, T.; Lyngfelt, A. The use of petroleum coke as fuel in chemical-looping combustion. Fuel 2007, 86, 1947–1958. [Google Scholar] [CrossRef]
- Brown, T.A.; Dennis, J.S.; Scott, S.A.; Davidson, J.F.; Hayhurst, A.N. Gasification and chemical-looping combustion of a lignite char in a fluidized bed of iron oxide. Energy Fuels 2010, 24, 3034–3048. [Google Scholar] [CrossRef]
- Mendiara, T.; De Diego, L.F.; García-Labiano, F.; Gayán, P.; Abad, A.; Adánez, J. Behaviour of a bauxite waste material as oxygen carrier in a 500 Wth CLC unit with coal. Int. J. Greenh. Gas Control 2013, 17, 170–182. [Google Scholar] [CrossRef]
- Herdel, P.; Krause, D.; Peters, J.; Kolmorgen, B.; Ströhle, J.; Epple, B. Experimental investigations in a demonstration plant for fluidized bed gasification of multiple feedstock’s in 0.5 MWth scale. Fuel 2017, 205, 286–296. [Google Scholar] [CrossRef]
- Weidenfeller, D.J.; Kulik, R.; Rothenpieler, K.; Stückrath, K.; Hetzer, J. Design, Simulation and Practical Experience of the Largest Syngas Cooler in Operation for Coal Gasification. In Proceedings of the 8th International Freiberg Conference, Cologne, Germany, 12–16 June 2016. [Google Scholar]
- Schmidtsche Schack, ARVOS GmbH. Schmidtsche Schack® Solutions for Gasification Plants. Available online: https://www.schmidtsche-schack.com/products/syngas-cooler#c255 (accessed on 17 June 2020).
- Bischi, A.; Langørgen, Ø.; Morin, J.-X.; Bakken, J.; Ghorbaniyan, M.; Bysveen, M.; Bolland, O. Hydrodynamic viability of chemical looping processes by means of cold flow model investigation. Appl. Energy 2012, 97, 201–216. [Google Scholar] [CrossRef]
- Markström, P.; Lyngfelt, A. Designing and operating a cold-flow model of a 100 kW chemical-looping combustor. Powder Technol. 2012, 222, 182–192. [Google Scholar] [CrossRef]


| Ultimate Analysis | wt-% | Proximate Analysis | wt-% |
|---|---|---|---|
| C (d.a.f.) | 50.8 | Moisture | 6.5 |
| H (d.a.f.) | 6 | Ash (d.b.) | 0.7 |
| N (d.a.f.) | 0.07 | Volatile matter (d.b.) | 85.1 |
| O (d.a.f.) | 43.2 | Fixed carbon (d.b.) | 14.2 |
| S (d.a.f.) | 0.008 | ||
| Cl (d.a.f.) | 0.006 | ||
| Net calorific value [MJ/kg] | 17.96 |
| Component | wt-% | Component | wt-% |
|---|---|---|---|
| ASH | 0.65 | H2O | 14.06 |
| CO | 55.20 | N2 | 0.06 |
| C | 11.92 | CO2 | 3.11 |
| CH4 | 13.55 | H2S | 0.01 |
| H2 | 1.43 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dieringer, P.; Marx, F.; Alobaid, F.; Ströhle, J.; Epple, B. Process Control Strategies in Chemical Looping Gasification—A Novel Process for the Production of Biofuels Allowing for Net Negative CO2 Emissions. Appl. Sci. 2020, 10, 4271. https://doi.org/10.3390/app10124271
Dieringer P, Marx F, Alobaid F, Ströhle J, Epple B. Process Control Strategies in Chemical Looping Gasification—A Novel Process for the Production of Biofuels Allowing for Net Negative CO2 Emissions. Applied Sciences. 2020; 10(12):4271. https://doi.org/10.3390/app10124271
Chicago/Turabian StyleDieringer, Paul, Falko Marx, Falah Alobaid, Jochen Ströhle, and Bernd Epple. 2020. "Process Control Strategies in Chemical Looping Gasification—A Novel Process for the Production of Biofuels Allowing for Net Negative CO2 Emissions" Applied Sciences 10, no. 12: 4271. https://doi.org/10.3390/app10124271
APA StyleDieringer, P., Marx, F., Alobaid, F., Ströhle, J., & Epple, B. (2020). Process Control Strategies in Chemical Looping Gasification—A Novel Process for the Production of Biofuels Allowing for Net Negative CO2 Emissions. Applied Sciences, 10(12), 4271. https://doi.org/10.3390/app10124271






