Municipal Solid Waste Incineration Bottom Ash as Sole Precursor in the Alkali-Activated Binder Formulation
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
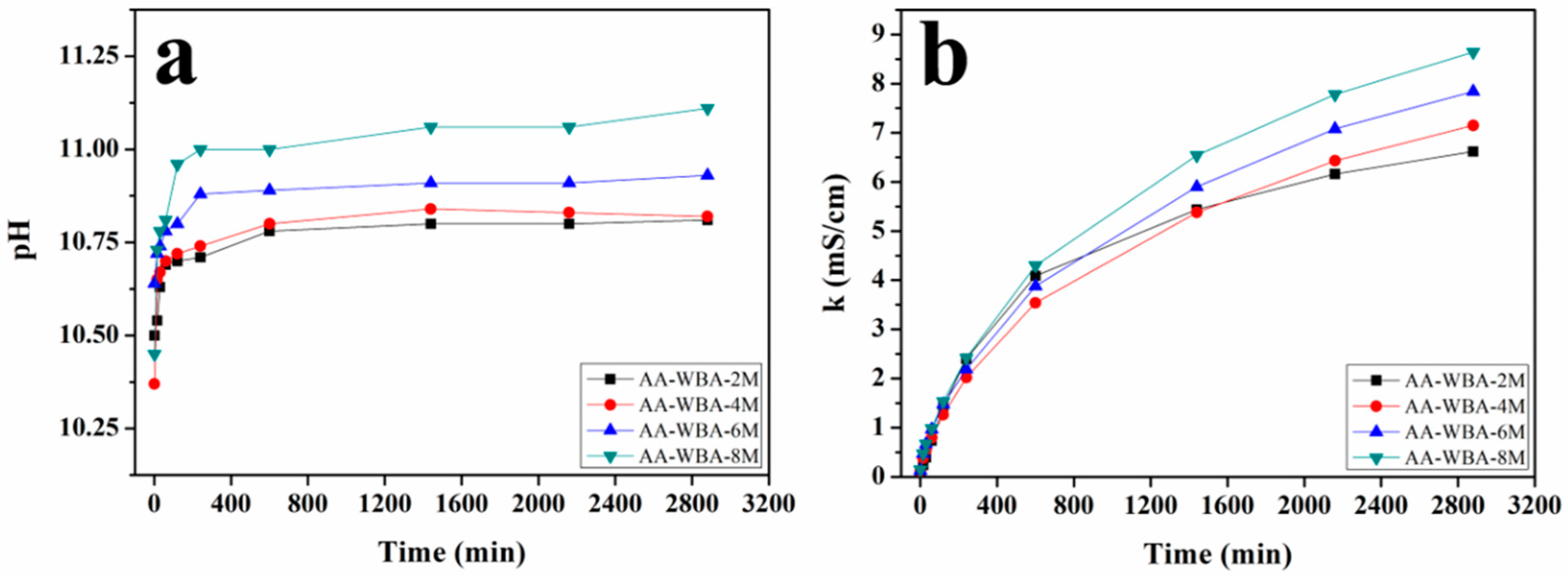
3.1. Hydrolytic Stability Test and Integrity Test
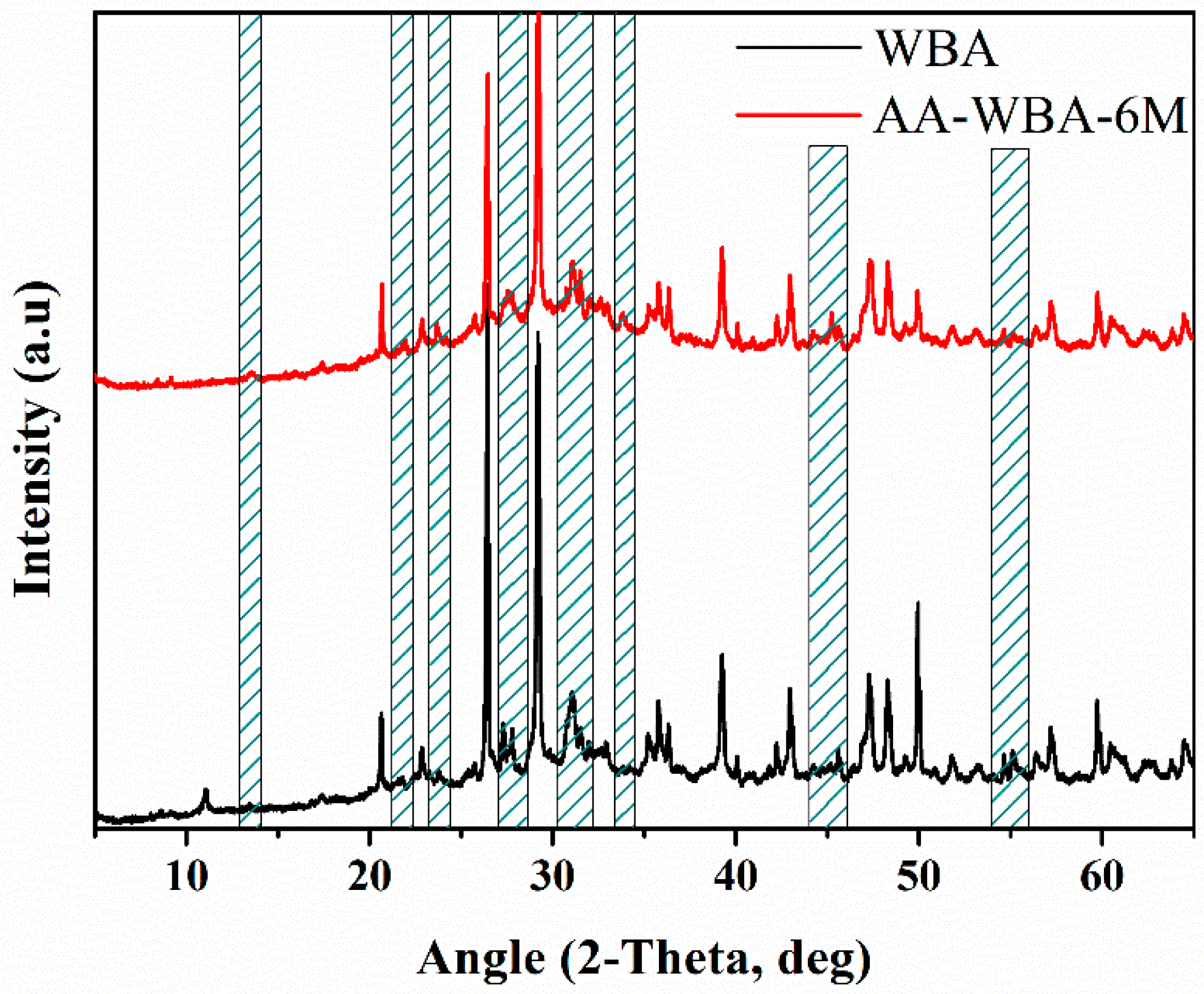
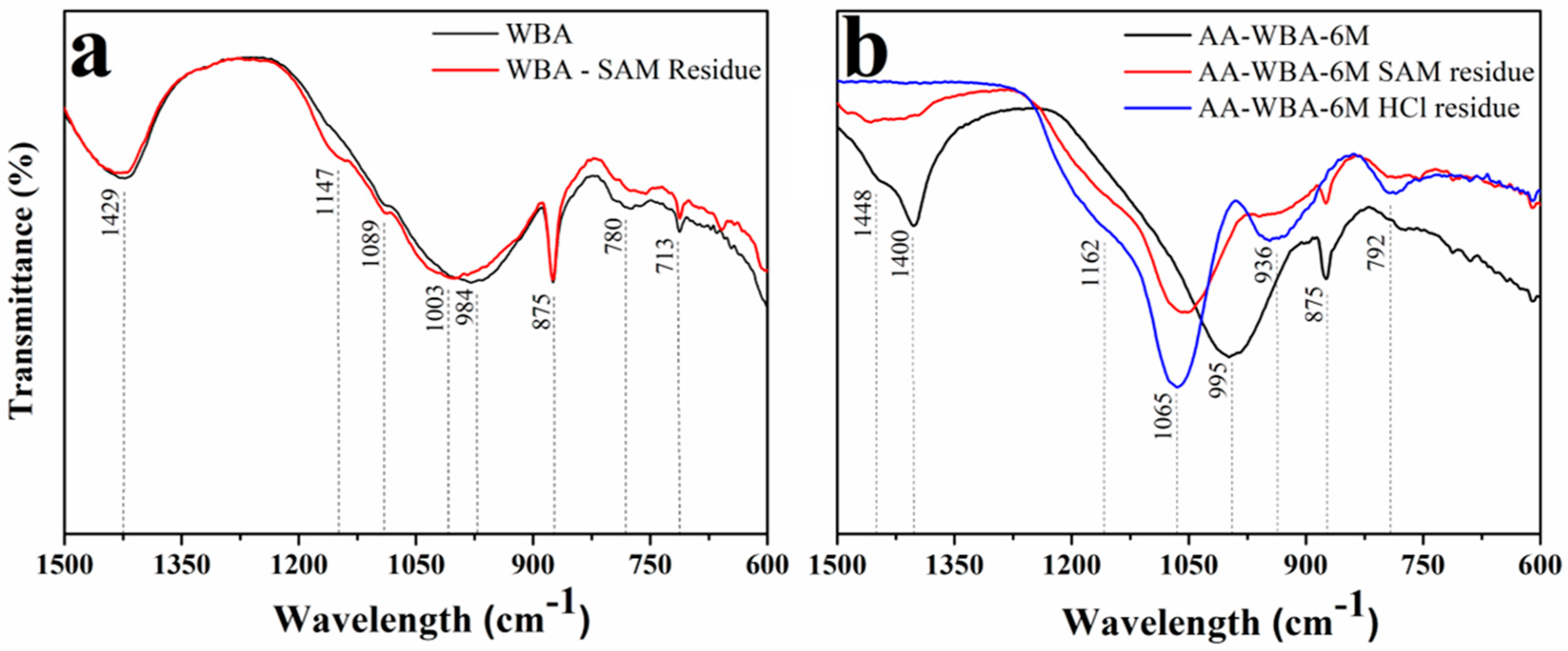
3.2. Selective Chemical Extractions
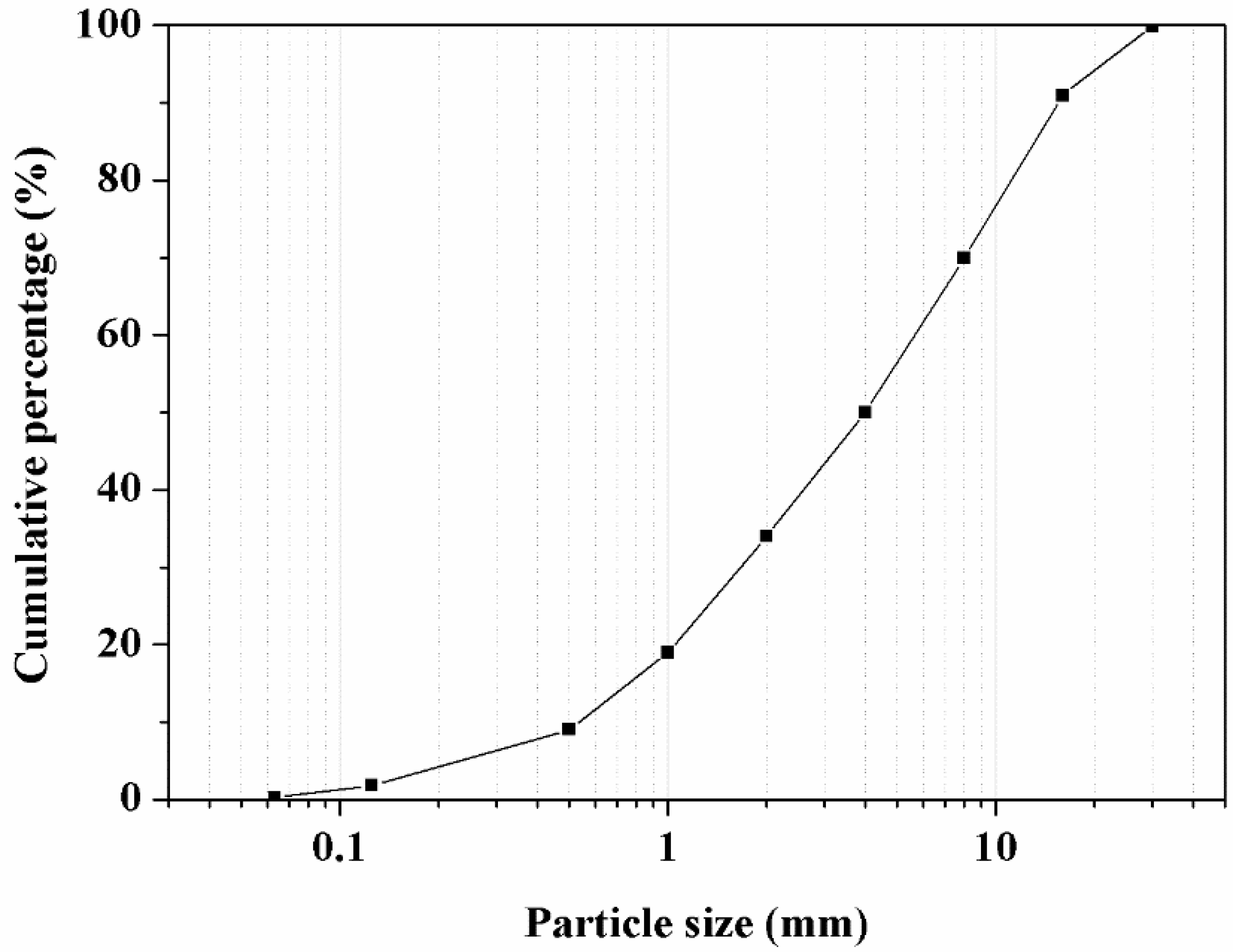
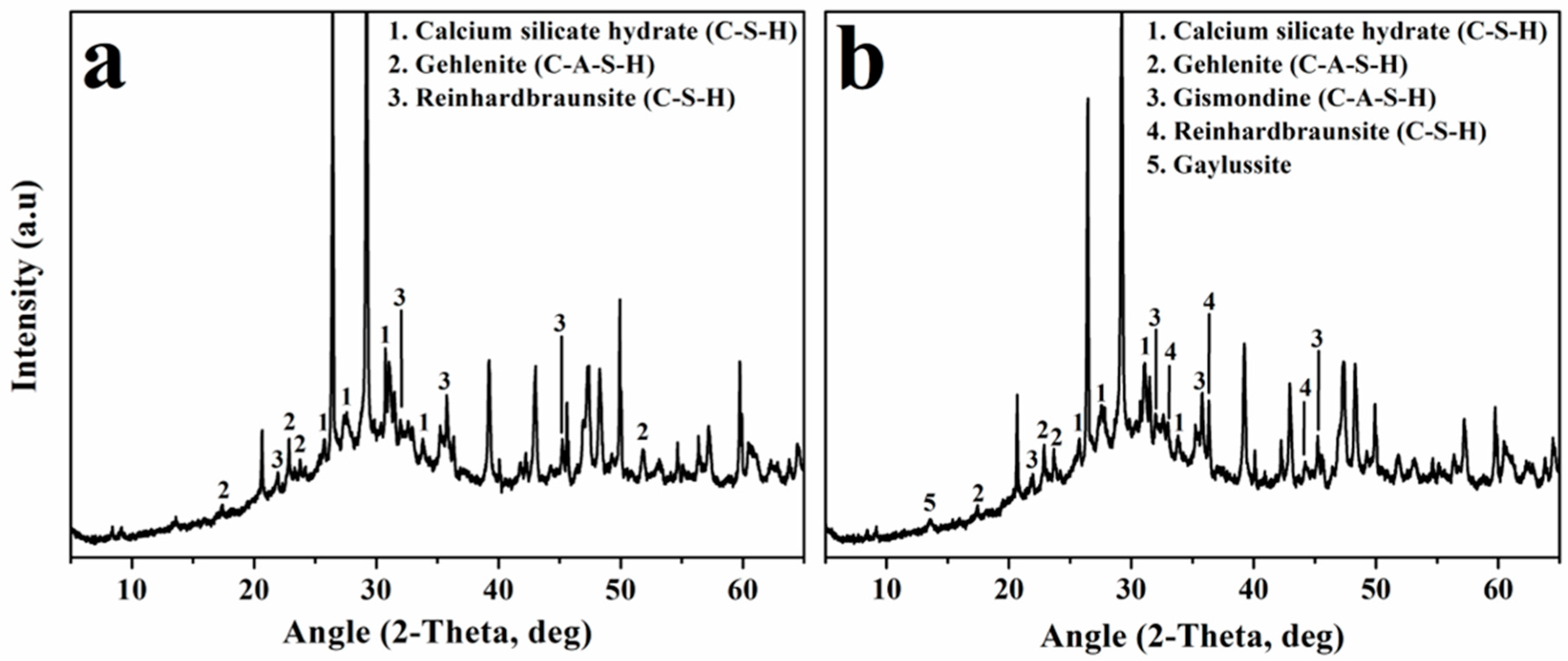
3.3. Physicochemical Characterization
3.4. Physical and Mechanical Characterization
3.5. Environmental Characterization
3.6. AA-WBA Formulation Costs
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- European Commission. The Role of Waste-to-Energy in the Circular Economy; European Commission: Brussels, Belgium, 2017; Available online: http://ec.europa.eu/priorities/energy-union-and-climate/state-energy-union_en%0Ahttp://ec.europa.eu/environment/waste/waste-to-energy.pdf (accessed on 11 June 2020).
- Cheng, H.; Hu, Y. Municipal solid waste (MSW) as a renewable source of energy: Current and future practices in China. Bioresour. Technol. 2010, 101, 3816–3824. [Google Scholar] [CrossRef] [PubMed]
- Confederation of European Waste-to-Energy Plants. Waste-to-Energy Plants in Europe in 2017; CEWEP: Brussels, Belgium, 2019; Available online: https://www.cewep.eu/waste-to-energy-plants-in-europe-in-2017/ (accessed on 11 June 2020).
- Bontempi, E.; Šyc, M.; Lederer, J.; Quina, M.J.; Blanc-Biscarat, D.; Bogush, A.; Bontempi, E.; Blondeau, J.; Chimenos, J.M.; Quina, M.J.; et al. Legal situation and current practice of waste incineration bottom ash utilisation in Europe. Waste Manag. 2020, 102, 868–883. [Google Scholar] [CrossRef]
- Eurostat—European Statistical Office. Municipal Waste Statistics, Luxembourg. 2019. Available online: https://ec.europa.eu/eurostat/statistics-explained/index.php?title=Municipal_waste_statistics (accessed on 11 June 2020).
- Del Valle-Zermeño, R.; Gómez-Manrique, J.; Paloma, J.G.; Formosa, J.; Simon, F.-G. Material characterization of the MSWI bottom ash as a function of particle size. Effects of glass recycling over time. Sci. Total Environ. 2017, 581, 897–905. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Shimaoka, T.; Saffarzadeh, A.; Takahashi, F. Mineralogical characterization of municipal solid waste incineration bottom ash with an emphasis on heavy metal-bearing phases. J. Hazard. Mater. 2011, 187, 534–543. [Google Scholar] [CrossRef] [PubMed]
- Chimenos, J.M.; Fernandez, A.I.; Nadal, R.; Espiell, F. Short-term natural weathering of MSWI bottom ash. J. Hazard. Mater. 2000, 79, 287–299. [Google Scholar] [CrossRef]
- Verbinnen, B.; Billen, P.; Van Caneghem, J.; Vandecasteele, C. Recycling of MSWI Bottom Ash: A Review of Chemical Barriers, Engineering Applications and Treatment Technologies. Waste Biomass Valoriz. 2016, 8, 1453–1466. [Google Scholar] [CrossRef]
- Silva, R.; De Brito, J.; Lynn, C.; Dhir, R.K. Use of municipal solid waste incineration bottom ashes in alkali-activated materials, ceramics and granular applications: A review. Waste Manag. 2017, 68, 207–220. [Google Scholar] [CrossRef]
- Shi, C.; Fernandez-Jiménez, A.; Palomo, A. New cements for the 21st century: The pursuit of an alternative to Portland cement. Cem. Concr. Res. 2011, 41, 750–763. [Google Scholar] [CrossRef]
- Provis, J.L.; Palomo, A.; Shi, C. Advances in understanding alkali-activated materials. Cem. Concr. Res. 2015, 78, 110–125. [Google Scholar] [CrossRef]
- Duxson, P.; Fernandez-Jiménez, A.; Provis, J.L.; Lukey, G.C.; Palomo, A.; Van Deventer, J.S.J. Geopolymer technology: The current state of the art. J. Mater. Sci. 2006, 42, 2917–2933. [Google Scholar] [CrossRef]
- Lodeiro, I.G.; Palomo, A.; Fernandez-Jiménez, A.; Macphee, D. Compatibility studies between N-A-S-H and C-A-S-H gels. Study in the ternary diagram Na2O–CaO–Al2O3–SiO2–H2O. Cem. Concr. Res. 2011, 41, 923–931. [Google Scholar] [CrossRef]
- Fernandez-Jiménez, A.; Palomo, A. Composition and microstructure of alkali activated fly ash binder: Effect of the activator. Cem. Concr. Res. 2005, 35, 1984–1992. [Google Scholar] [CrossRef]
- De Vargas, A.S.; Molin, D.C.D.; Vilela, A.; Da Silva, F.J.; Pavão, B.; Veit, H.M. The effects of Na2O/SiO2molar ratio, curing temperature and age on compressive strength, morphology and microstructure of alkali-activated fly ash-based geopolymers. Cem. Concr. Compos. 2011, 33, 653–660. [Google Scholar] [CrossRef]
- Alonso, S.; Vázquez, T.; Puertas, F.; Martínez-Ramírez, S. Alkali-activated fly ash/slag cement Strength behaviour and hydration products. Cem. Concr. Res. 2000, 30, 1625–1632. [Google Scholar]
- Bakharev, T. Resistance of geopolymer materials to acid attack. Cem. Concr. Res. 2005, 35, 658–670. [Google Scholar] [CrossRef]
- Provis, J.L.; van Deventer, J.S.J. Geopolymers: Structures, Processing, Properties and Industrial Applications; Elsevier: Amsterdam, The Netherlands, 2009. [Google Scholar]
- Van Deventer, J.S.J.; Provis, J.L.; Duxson, P. Technical and commercial progress in the adoption of geopolymer cement. Miner. Eng. 2012, 29, 89–104. [Google Scholar] [CrossRef]
- Bernal, S.A.; Rodriguez, E.D.; Kirchheim, A.P.; Provis, J.L. Management and valorisation of wastes through use in producing alkali-activated cement materials. J. Chem. Technol. Biotechnol. 2016, 91, 2365–2388. [Google Scholar] [CrossRef]
- Rivera, J.; Castro, F.; Fernández-Jiménez, A.; Cristelo, N. Alkali-Activated Cements from Urban, Mining and Agro-Industrial Waste: State-of-the-art and Opportunities. Waste Biomass Valoriz. 2020. [Google Scholar] [CrossRef]
- Provis, J.L. Alkali-activated materials. Cem. Concr. Res. 2018, 114, 40–48. [Google Scholar] [CrossRef]
- Lancellotti, I.; Kamseu, E.; Michelazzi, M.; Barbieri, L.; Corradi, A.; Leonelli, C. Chemical stability of geopolymers containing municipal solid waste incinerator fly ash. Waste Manag. 2010, 30, 673–679. [Google Scholar] [CrossRef]
- Galiano, Y.L.; Pereira, C.F.; Vale, J. Stabilization/solidification of a municipal solid waste incineration residue using fly ash-based geopolymers. J. Hazard. Mater. 2011, 185, 373–381. [Google Scholar] [CrossRef]
- Zheng, L.; Wang, C.; Wang, W.; Shi, Y.; Gao, X. Immobilization of MSWI fly ash through geopolymerization: Effects of water-wash. Waste Manag. 2011, 31, 311–317. [Google Scholar] [CrossRef] [PubMed]
- Komnitsas, K. Potential of geopolymer technology towards green buildings and sustainable cities. Procedia Eng. 2011, 21, 1023–1032. [Google Scholar] [CrossRef]
- European Comission. Towards a Circular Economy: A Zero Waste Programme for Europe; European Comission: Brussels, Belgium, 2014; Available online: https://eur-lex.europa.eu/resource.html?uri=cellar:aa88c66d-4553-11e4-a0cb-01aa75ed71a1.0022.03/DOC_1&format=PDF (accessed on 11 June 2020).
- Lancellotti, I.; Ponzoni, C.; Barbieri, L.; Leonelli, C. Alkali activation processes for incinerator residues management. Waste Manag. 2013, 33, 1740–1749. [Google Scholar] [CrossRef] [PubMed]
- Lodeiro, I.G.; Carcelen-Taboada, V.; Fernandez-Jiménez, A.; Palomo, A. Manufacture of hybrid cements with fly ash and bottom ash from a municipal solid waste incinerator. Constr. Build. Mater. 2016, 105, 218–226. [Google Scholar] [CrossRef]
- Huang, G.; Ji, Y.; Li, J.; Zhang, L.; Liu, X.; Liu, B. Effect of activated silica on polymerization mechanism and strength development of MSWI bottom ash alkali-activated mortars. Constr. Build. Mater. 2019, 201, 90–99. [Google Scholar] [CrossRef]
- Lancellotti, I.; Ponzoni, C.; Bignozzi, M.C.; Barbieri, L.; Leonelli, C. Incinerator Bottom Ash and Ladle Slag for Geopolymers Preparation. Waste Biomass Valorization 2014, 5, 393–401. [Google Scholar] [CrossRef]
- Saffarzadeh, A.; Arumugam, N.; Shimaoka, T. Aluminum and aluminum alloys in municipal solid waste incineration (MSWI) bottom ash: A potential source for the production of hydrogen gas. Int. J. Hydrog. Energy 2016, 41, 820–831. [Google Scholar] [CrossRef]
- Zhu, W.; Rao, X.H.; Liu, Y.; Yang, E.-H. Lightweight aerated metakaolin-based geopolymer incorporating municipal solid waste incineration bottom ash as gas-forming agent. J. Clean. Prod. 2018, 177, 775–781. [Google Scholar] [CrossRef]
- Huang, G.; Yuan, L.; Ji, Y.; Liu, B.; Xu, Z. Cooperative action and compatibility between Portland cement and MSWI bottom ash alkali-activated double gel system materials. Constr. Build. Mater. 2019, 209, 445–453. [Google Scholar] [CrossRef]
- Liu, Y.; Sidhu, K.S.; Chen, Z.; Yang, E.-H. Alkali-treated incineration bottom ash as supplementary cementitious materials. Constr. Build. Mater. 2018, 179, 371–378. [Google Scholar] [CrossRef]
- Chen, Z.; Yang, E.-H. Early age hydration of blended cement with different size fractions of municipal solid waste incineration bottom ash. Constr. Build. Mater. 2017, 156, 880–890. [Google Scholar] [CrossRef]
- Chen, Z.; Liu, Y.; Zhu, W.; Yang, E.-H. Incinerator bottom ash (IBA) aerated geopolymer. Constr. Build. Mater. 2016, 112, 1025–1031. [Google Scholar] [CrossRef]
- Zhu, W.; Chen, X.; Struble, L.J.; Yang, E.-H. Characterization of calcium-containing phases in alkali-activated municipal solid waste incineration bottom ash binder through chemical extraction and deconvoluted Fourier transform infrared spectra. J. Clean. Prod. 2018, 192, 782–789. [Google Scholar] [CrossRef]
- Lancellotti, I.; Cannio, M.; Bollino, F.; Catauro, M.; Barbieri, L.; Leonelli, C. Geopolymers: An option for the valorization of incinerator bottom ash derived “end of waste”. Ceram. Int. 2015, 41, 2116–2123. [Google Scholar] [CrossRef]
- Maldonado-Alameda, A.; Paloma, J.G.; Svobodova-Sedlackova, A.; Formosa, J.; Simon, F.-G. Municipal solid waste incineration bottom ash as alkali-activated cement precursor depending on particle size. J. Clean. Prod. 2020, 242, 118443. [Google Scholar] [CrossRef]
- Chimenos, J.M.; Segarra, M.; Fernández, M.; Espiell, F. Characterization of the bottom ash in municipal solid waste incinerator. J. Hazard. Mater. 1999, 64, 211–222. [Google Scholar] [CrossRef]
- Lodeiro, I.G.; Macphee, D.E.; Palomo, A.; Fernández-jiménez, A. Effect of alkalis on fresh C–S–H gels. FTIR analysis. Cem. Concr. Res. 2009, 39, 147–153. [Google Scholar] [CrossRef]
- Fernandez-Jiménez, A.; Palomo, A. Mid-infrared spectroscopic studies of alkali-activated fly ash structure. Microporous Mesoporous Mater. 2005, 86, 207–214. [Google Scholar] [CrossRef]
- Huang, G.; Ji, Y.; Zhang, L.L.; Li, J.; Hou, Z. The influence of curing methods on the strength of MSWI bottom ash-based alkali-activated mortars: The role of leaching of OH− and free alkali. Constr. Build. Mater. 2018, 186, 978–985. [Google Scholar] [CrossRef]
- Puligilla, S.; Mondal, P. Co-existence of aluminosilicate and calcium silicate gel characterized through selective dissolution and FTIR spectral subtraction. Cem. Concr. Res. 2015, 70, 39–49. [Google Scholar] [CrossRef]
- Zhuang, X.Y.; Chen, L.; Komarneni, S.; Zhou, C.; Tong, D.S.; Yang, H.M.; Yu, W.H.; Wang, H. Fly ash-based geopolymer: Clean production, properties and applications. J. Clean. Prod. 2016, 125, 253–267. [Google Scholar] [CrossRef]
- You, S.; Ho, S.W.; Li, T.; Maneerung, T.; Wang, C.-H. Techno-economic analysis of geopolymer production from the coal fly ash with high iron oxide and calcium oxide contents. J. Hazard. Mater. 2019, 361, 237–244. [Google Scholar] [CrossRef] [PubMed]
- Alonso, S.; Palomo, A. Calorimetric study of alkaline activation of calcium hydroxide–metakaolin solid mixtures. Cem. Concr. Res. 2001, 31, 25–30. [Google Scholar] [CrossRef]
- Taylor, H. Cement chemistry. Cem. Chem. 1997. [Google Scholar] [CrossRef]
- Kovtun, M.; Kearsley, E.; Shekhovtsova, J. Chemical acceleration of a neutral granulated blast-furnace slag activated by sodium carbonate. Cem. Concr. Res. 2015, 72, 1–9. [Google Scholar] [CrossRef]
- Puertas, F.; Fernández-Jiménez, A.; Blanco-Varela, M.T. Pore solution in alkali-activated slag cement pastes. Relation to the composition and structure of calcium silicate hydrate. Cem. Concr. Res. 2004, 34, 139–148. [Google Scholar] [CrossRef]
- Walkley, B.; Nicolas, R.S.; Sani, M.-A.; Rees, G.J.; Hanna, J.V.; Van Deventer, J.S.J.; Provis, J.L. Phase evolution of C-(N)-A-S-H/N-A-S-H gel blends investigated via alkali-activation of synthetic calcium aluminosilicate precursors. Cem. Concr. Res. 2016, 89, 120–135. [Google Scholar] [CrossRef]
- Criado, M.; Palomo, A.; Fernandez-Jiménez, A. Alkali activation of fly ashes. Part 1: Effect of curing conditions on the carbonation of the reaction products. Fuel 2005, 84, 2048–2054. [Google Scholar] [CrossRef]
- Ravikumar, D.; Neithalath, N. Effects of activator characteristics on the reaction product formation in slag binders activated using alkali silicate powder and NaOH. Cem. Concr. Compos. 2012, 34, 809–818. [Google Scholar] [CrossRef]
- Lodeiro, I.G.; Fernandez-Jiménez, A.; Blanco, M.T.; Palomo, A. FTIR study of the sol–gel synthesis of cementitious gels: C–S–H and N–A–S–H. J. Sol Gel Sci. Technol. 2007, 45, 63–72. [Google Scholar] [CrossRef]
- Zhu, W.; Chen, X.; Zhao, A.; Struble, L.J.; Yang, E.-H. Synthesis of high strength binders from alkali activation of glass materials from municipal solid waste incineration bottom ash. J. Clean. Prod. 2019, 212, 261–269. [Google Scholar] [CrossRef]
- Puertas, F.; Palacios, M.; Manzano, H.; Dolado, J.S.; Rico, A.; Rodriguez, J. A model for the C-A-S-H gel formed in alkali-activated slag cements. J. Eur. Ceram. Soc. 2011, 31, 2043–2056. [Google Scholar] [CrossRef]
- Council of the European Union. 2003/33/EC, Council Decision establishing criteria and procedures for the acceptance of waste at landfills pursuant to Article 16 of and Annex II to Directive 1999/31/EC. Off. J. Eur. Communities 2003, 11, 27–49. [Google Scholar]
- Apostoli, P.; Giusti, S.; Bartoli, D.; Perico, A.; Bavazzano, P.; Alessio, L. Multiple exposure to arsenic, antimony, and other elements in art glass manufacturing. Am. J. Ind. Med. 1998, 34, 65–72. [Google Scholar] [CrossRef]
- McLellan, B.; Williams, R.; Lay, J.; Van Riessen, A.; Corder, G. Costs and carbon emissions for geopolymer pastes in comparison to ordinary portland cement. J. Clean. Prod. 2011, 19, 1080–1090. [Google Scholar] [CrossRef]




| Major Elements (wt.%) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| SiO2 | CaO | Al2O3 | Na2O | K2O | Fe2O3 | MgO | TiO2 | Cl− | SO3 | 1 LOI |
| 45.44 | 17.55 | 10.38 | 5.04 | 1.54 | 6.08 | 2.66 | 0.65 | 1.42 | 2.57 | 5.78 |
| Reference | L | ||||||
|---|---|---|---|---|---|---|---|
| 1 NaOH (wt.%) | 1 Na2SiO3 (wt.%) | 1 Na2O (wt.%) | 2 SiO2/Na2O | ||||
| 2M | 4M | 6M | 8M | ||||
| AA-WBA-2M | 20 | 80 | 7.7 | 3.6 | |||
| AA-WBA-4M | 20 | 80 | 8.7 | 3.2 | |||
| AA-WBA-6M | 20 | 80 | 9.7 | 2.9 | |||
| AA-WBA-8M | 20 | 80 | 10.7 | 2.6 | |||
| Mass Dissolved by SAM (wt.%) | Mass Dissolved by HCl (wt.%) | |
|---|---|---|
| Portland cement paste | 89.8 | - |
| WBA powder | 15.9 | - |
| AA-WBA-2M | 16.6 | 56.0 |
| AA-WBA-4M | 24.2 | 57.6 |
| AA-WBA-6M | 33.5 | 59.9 |
| AA-WBA-8M | 29.7 | 61.5 |
| Sample | As | Ba | Cd | Cr | Cu | Hg | Mo | Pb | Ni | Sb | Zn | pH |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Non-milled WBA | 0.02 | 0.42 | <0.01 | 0.21 | 3.27 | 0.05 | 0.71 | 0.07 | <0.03 | 0.23 | 0.90 | 9.66 |
| WBA powder | 0.04 | 0.37 | <0.01 | 0.51 | 3.33 | 0.01 | 1.26 | 0.03 | <0.03 | 0.35 | 0.44 | 11.33 |
| AA-WBA-2M | 1.29 | 0.05 | 0.01 | 0.47 | 3.46 | <0.01 | 0.94 | 0.41 | 0.23 | 1.61 | 2.30 | 11.26 |
| AA-WBA-4M | 1.44 | 0.07 | 0.02 | 0.34 | 2.80 | <0.01 | 0.85 | 0.51 | 0.27 | 1.50 | 3.26 | 11.39 |
| AA-WBA-6M | 1.51 | 0.08 | 0.00 | 0.27 | 3.25 | 0.01 | 0.88 | 0.54 | 0.29 | 1.62 | 3.44 | 11.62 |
| AA-WBA-8M | 1.65 | 0.08 | 0.00 | 0.37 | 3.91 | 0.12 | 0.89 | 0.40 | 0.12 | 1.93 | 3.09 | 11.77 |
| 1 Inert waste (mg·kg−1) | 0.5 | 20 | 0.04 | 0.5 | 2 | 0.01 | 0.5 | 0.5 | 0.4 | 0.06 | 4 | |
| 1 Non-hazardous waste (mg·kg−1) | 2 | 100 | 1 | 10 | 50 | 0.2 | 10 | 10 | 10 | 0.7 | 50 | |
| 1 Hazardous waste (mg·kg−1) | 25 | 300 | 5 | 70 | 100 | 2 | 30 | 50 | 40 | 5 | 200 |
| OPC | AA-WBA-2M | AA-WBA-4M | AA-WBA-6M | AA-WBA-8M |
|---|---|---|---|---|
| 106.2 [61] | 137.6 | 143.3 | 148.8 | 153.9 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maldonado-Alameda, À.; Giro-Paloma, J.; Alfocea-Roig, A.; Formosa, J.; Chimenos, J.M. Municipal Solid Waste Incineration Bottom Ash as Sole Precursor in the Alkali-Activated Binder Formulation. Appl. Sci. 2020, 10, 4129. https://doi.org/10.3390/app10124129
Maldonado-Alameda À, Giro-Paloma J, Alfocea-Roig A, Formosa J, Chimenos JM. Municipal Solid Waste Incineration Bottom Ash as Sole Precursor in the Alkali-Activated Binder Formulation. Applied Sciences. 2020; 10(12):4129. https://doi.org/10.3390/app10124129
Chicago/Turabian StyleMaldonado-Alameda, Àlex, Jessica Giro-Paloma, Anna Alfocea-Roig, Joan Formosa, and Josep Maria Chimenos. 2020. "Municipal Solid Waste Incineration Bottom Ash as Sole Precursor in the Alkali-Activated Binder Formulation" Applied Sciences 10, no. 12: 4129. https://doi.org/10.3390/app10124129
APA StyleMaldonado-Alameda, À., Giro-Paloma, J., Alfocea-Roig, A., Formosa, J., & Chimenos, J. M. (2020). Municipal Solid Waste Incineration Bottom Ash as Sole Precursor in the Alkali-Activated Binder Formulation. Applied Sciences, 10(12), 4129. https://doi.org/10.3390/app10124129







