1. Introduction
Over the past decades, transparent conducting electrodes (TCEs) have played a significant role in developing electronic and optoelectronic devices, such as solar cells [
1,
2], thin-film transistors [
3], and field emission displays [
4]. In particular, low-dimensional materials, such as Ag nanowires (NWs) and graphene, have recently been researched to replace traditional TCE materials, i.e., indium-tin-oxide (ITO) thin films, owing to their possible advantages, such as higher electrical conductivity and optical transmittance and improved flexibility. Thin films with a network of Ag NWs exhibit a sheet resistance (
Rs) less than 100 Ω/sq with an optical transmittance higher than 90% in the visible range. However, Ag NW thin films have inevitable weaknesses, such as substantial diffused scattering and light absorption [
5]. In contrast, graphene has been investigated as a potential flexible TCE as well, specifically owing to its superior optical properties and unique electrical transport properties, which include the room-temperature quantum Hall effect and the mass-less Dirac-fermion behavior of its charge carriers [
6,
7]. However, the lack of stability and low electrical conductivity of graphene thin films have been major obstacles to their industrial applications [
2,
6,
7]. Other polymeric transparent electrodes based on conjugated polymers and polypyrrole/polyol-borate networks were recently suggested as well [
8,
9]. Meanwhile, ruthenium (Ru)-based oxide (RuO
2) nanosheets (NSs) were suggested as a promising candidate for their application in flexible TCEs owing to their ability to exhibit relatively high electrical conductivity and optical transmittance [
10,
11,
12,
13]. Additionally, oxide NSs are reported to be chemically stable under ambient conditions and can be synthesized on a large scale using the chemical exfoliation process; all of these are highly advantageous for their implementation on an industrial scale. Despite the advantages of RuO
2 NSs, the major obstacle to their TCE application is their poor electrical conductivity after fabricating thin films [
14,
15]. In our prior work [
10], the Ag-doping effect and layer number dependence in individual RuO
2 NSs devices, which were fabricated via electron-beam lithography methods, were the main factors investigated to enhance electrical conductivity in individual RuO
2 NSs. However, the Ag-doping method is not suitable for large-scale NS films due to difficulties in the doping process. Moreover, low electrical transport properties in oxide NS films are primarily caused by the contact resistance between the surfaces of the NSs due to residual organic materials [
14,
15]. Considering this, research on methods for decreasing the contact resistance becomes crucial for increasing the possibility of industrial applications of RuO
2 NSs.
Herein, we propose a facile treatment that can improve the electrical contact and percolation between oxide NSs during the fabrication of transparent and electrically conducting RuO2 NS films by removing the residual organic materials from their surface. Between every coating procedure of the RuO2 NSs, ultraviolet-ozone (UVO) irradiation was executed, and it was found that this industrially friendly, easy process significantly improved the sheet resistance Rs of the fabricated multilayer films of the RuO2 NSs, as well as their optical transmittance.
2. Experimental
First, potassium ruthenates (KRuO2) were synthesized through a solid-state reaction method. Then, K2CO3 (>99.0%, Sigma Aldrich, St. Louis, United States) and bulk RuO2 (99.9%, Sigma Aldrich, St. Louis, United States) were calcined at 500 °C for 6 h. Later, a mixture of K2CO3 and RuO2 was prepared in a molar ratio of 5:8 and annealed at 900 °C under N2 gas for 24 h. The annealed compounds of K2CO3 and RuO2 were milled into a fine powdered form using hand grinding, which was later washed with 1 L of deionized water using magnetic stirring at room temperature for 24 h. The deionized water-washed compounds were then immersed in 1 M hydrochloric acid (HCl, 35%, Samchun Chemicals, Seoul, Korea) for ion-exchanging with the protons for 72 h. Furthermore, these acid-treated potassium ruthenates KRuO2 were exfoliated using a tetrabutylammonium hydroxide (TBAOH, 99.0%, Aldrich) solution of 40 wt% in deionized water. After completion of the exfoliation process, the RuO2 NSs were finally collected through the centrifugation process. Subsequently, scanning electron microscopy (SEM, FEI Nova NanoSEM 450, Thermo Fisher Scientific, Waltham, MA, USA).), high-resolution transmission electron microscopy (HR-TEM, FEI Titan Cubed), and atomic force microscopy (AFM, NanoWizard, JPK Instruments, Berlin, Germany) were employed to observe the microstructure of the RuO2 NSs.
For the fabrication of conducting films from the RuO2 NSs, the Langmuir–Blodgett (LB) coating method was utilized (MiniMicro LB System, KSV Instruments Ltd., Helsinki, Finland) on a glass substrate. Prior to the LB coating, the glass substrates were cleaned using acetone, ethanol, and deionized water. After washing, the glass surface was rendered hydrophilic through UVO exposure for 10 min under standard atmospheric pressure and room temperature. During the UVO exposure process, the pressure of O2 gas flow was controlled to be maintained at 10 sccm. For the generation of ultraviolet (UV) light, low-pressure mercury discharge tubes with wavelengths of 184 nm and 253 nm were employed. In general, it is known that 184 nm UV light decomposes oxygen molecules and synthesizes ozone. Moreover, 253 nm UV light decomposes ozone to produce activated oxygen with high energy, which plays a significant role in decomposing organic materials. This UVO exposure was repeated after each LB coating of single-layered RuO2 NSs. Deionized water (210 mL) was poured into the LB trough, and diluted RuO2 NS solutions were dropped slowly on the surface of the water using a syringe. After 10 min of stabilization, RuO2 was coated at the speed of 5 mm/min. During the coating, the surface pressure was controlled at 12 mN/m. The electrical and optical properties of the RuO2 NS films on glass substrates were separately observed using a non-contact resistance meter (EC-80P, Napson, Tokyo, Japan) and an optical haze meter (NDH 7000, Nippon Denshoku Industries Co., Tokyo, Japan), respectively. Moreover, a white light-emitting diode (LED) was employed as the light source for the optical haze meter.
3. Results and Discussion
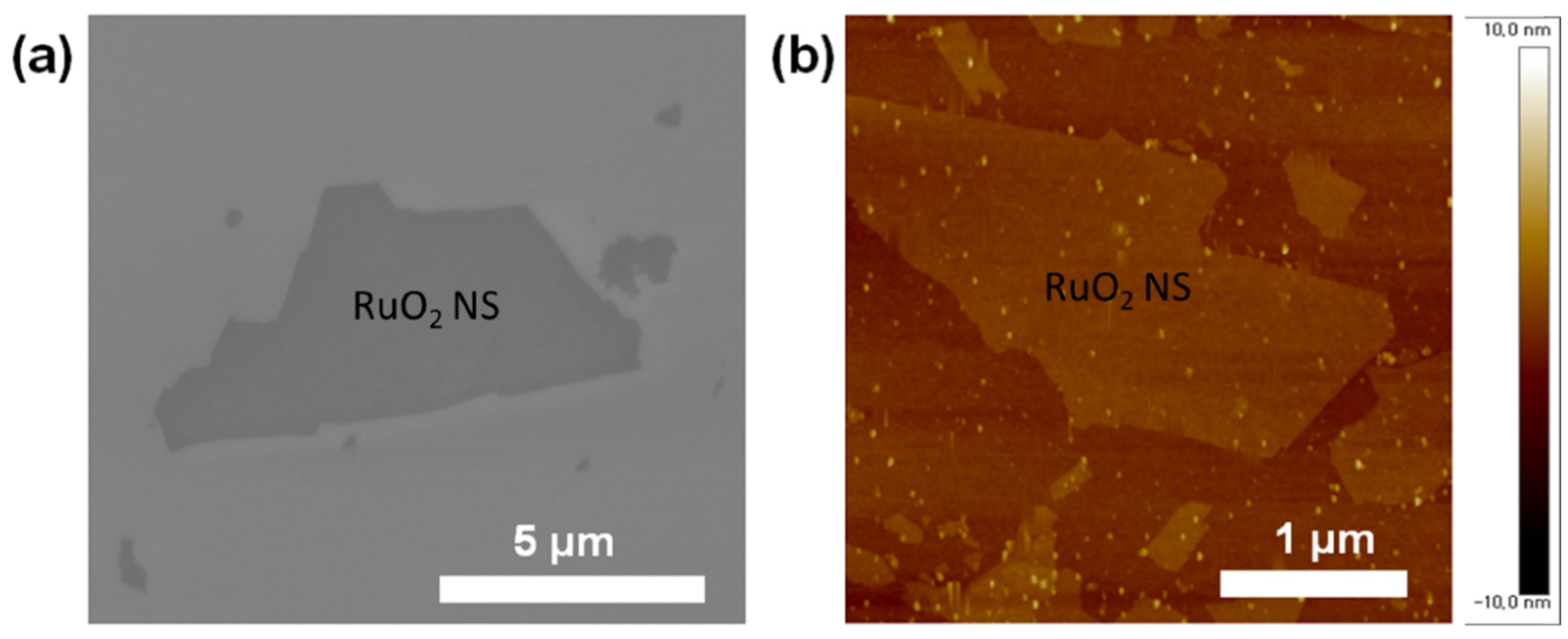
The SEM and AFM images of individual RuO
2 NSs dispersed on a glass substrate are illustrated in
Figure 1a,b, respectively.
Figure 1a depicts the smooth surface of the NSs, and
Figure 1b clearly reveals exfoliated RuO
2 NSs, which have a smooth and flat surface with an almost uniform thickness. The thicknesses of the monolayer RuO
2 NSs were measured to be ~1.2 nm, which is the same as their theoretical thickness [
11]. Notably, in the SEM and AFM images depicted in
Figure 1, the exfoliated RuO
2 NSs exhibit varying lateral sizes, which aids in the fabrication of conducting films from the RuO
2 NSs by enhancing the percolation between the NSs within a coating.
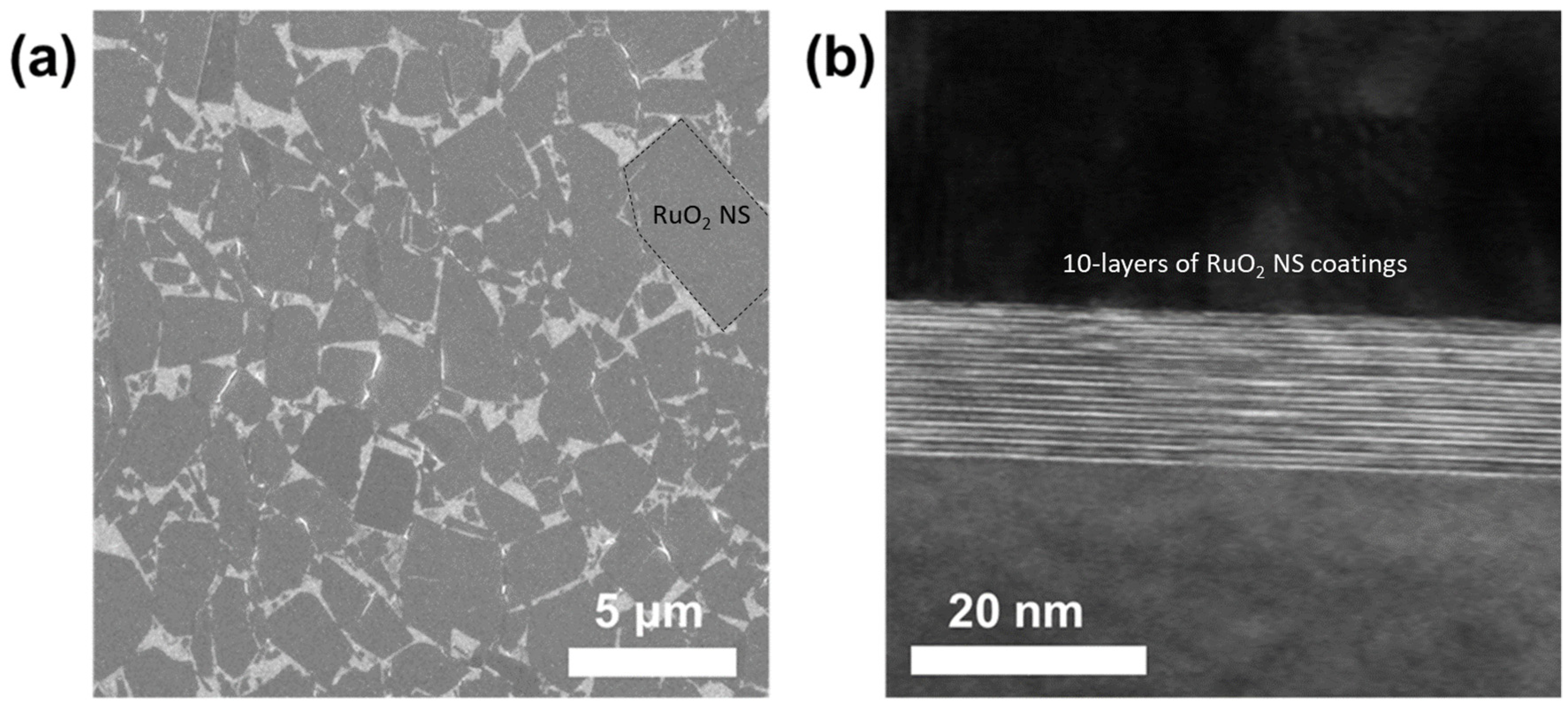
Figure 2a depicts an SEM image of the conducting transparent films after the coating of a single layer of the RuO
2 NSs. The packing density was controlled by fine-tuning the surface pressure of the air–water interface during the LB coating process to ensure that it was approximately 90%. LB coating was repeatedly performed 5–10 times to form the electrical conducting path across the multi-layered NS films. Furthermore, HR-TEM was employed for investigating the lateral deposition structure of the conducting transparent RuO
2 NS films.
Figure 2b illustrates the cross-sectional HR-TEM image of the 10-time-deposited RuO
2 NS films on a glass substrate, which exhibits a close-packed, uniform, and flat structure. These features are known to be advantageous for the fabrication of electrical-conducting pathways through these films [
13].
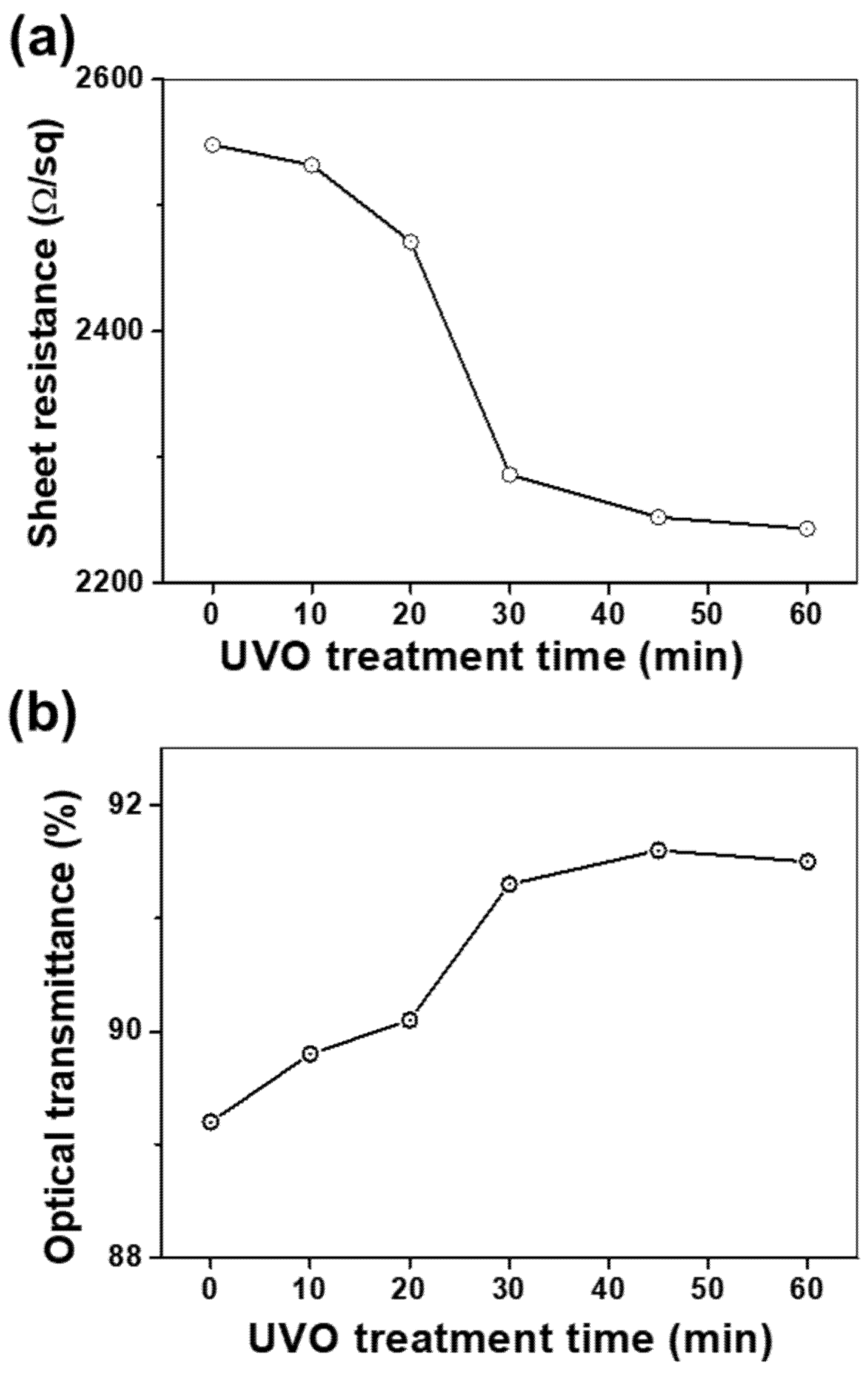
To investigate the influence of UVO treatment time on the electrical as well as optical properties of the RuO
2 NS films, UVO exposure times were varied to a maximum of 60 min for the five-layered RuO
2 NS films. The observations depicted in
Figure 3a indicate that
Rs decreased from 2548 to 2243 Ω/sq, by 14%, as time progressed. In particular, the decline in the value of
Rs was rather significant in the first 30 min, demonstrating a decrease of ~10% to 2286 Ω/sq. The UVO is able to decompose the tetrabutylammonium cations (TBA
+), which cover the surface of RuO
2 NS [
16,
17]. Lee et al. reported that ultraviolet and thermal treatment was effective for enhancing the electrical properties of nanosheets films by removing the TBA
+ [
16]. However, beyond 30 min, the
Rs decrease became much smaller, as observed in the 45 min and 60 min UVO exposure samples. Therefore, we found that the effect of UVO treatment was marginal when its duration was longer than 30 min. The variation in the optical transmittance with the treatment time is depicted in
Figure 3b. The enhancement of electrical conductivities was maintained for more than 24 h. This chemical stableness of RuO
2 NS films caused by the imperfect removal of TBAOH after UVO irradiation was reported by Lee et al. [
16]. It seems that the residual TBAOH after UVO irradiation serves as a stabilizer. The optical transmittance increased from 89.2 to 91.5% by 2.6% as time progressed. Moreover, we observed that after 30 min of treatment, the rate of increase in the optical transmittance was apparently saturated. From these observations, it can be concluded that a moderate amount of UVO improves the electrical and optical properties of the RuO
2 NS films simultaneously by eliminating the residual organic contaminations on the surfaces of the RuO
2 NS layers. Specifically, 30 min of UVO treatment reduced the
Rs by 10% and increased the optical transmittance by 2.4%. This may have an ameliorating effect due to the establishment of a stronger electrical network and the enhancement of optical properties of the films through the removal of TBAOH residues on the surface of the RuO
2 NSs [
18,
19,
20].
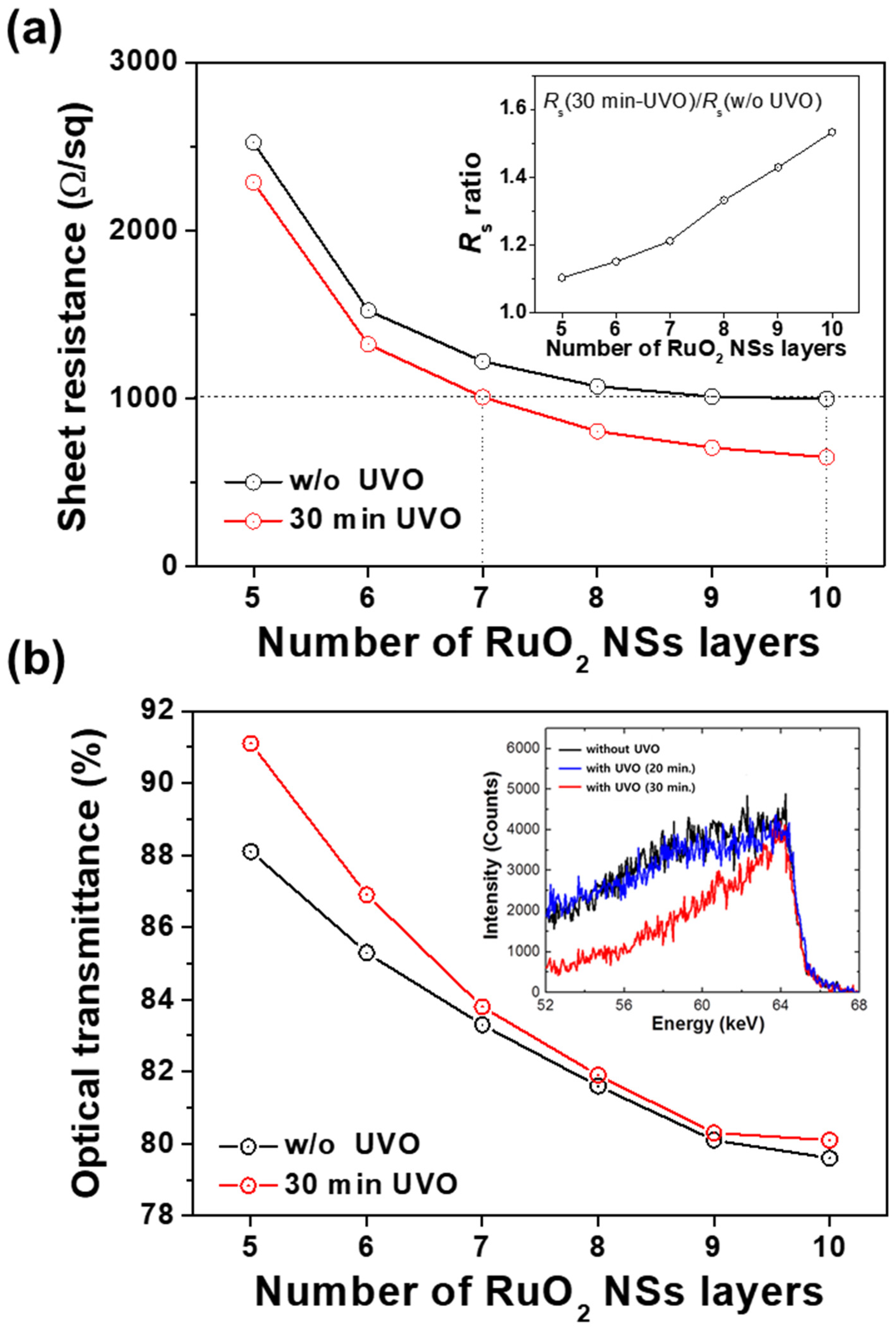
We investigated the
Rs and optical transmittance of the conducting films with varying numbers (5–10 times) of LB coatings. Based on the obtained results for the relation between
Rs, optical transmittance, and UVO treatment time, the optimized UVO exposure time for the RuO
2 NS conducting films was fixed at 30 min.
Figure 4a depicts the
Rs of the RuO
2 NS films depending on the number of layers with and without UVO exposure. Owing to the UVO treatment, a reduction in the
Rs values was observed in all samples. Without the UVO treatment, it was found that the reduction of
Rs was saturated after eight layers of deposition, whereas further
Rs reduction was expected for the UVO-treated layers even after 10 layers of deposition. Additionally, the reduction ratio increased with the number of NS layers. The inset of
Figure 4a depicts the
Rs ratio between the films without UVO treatment and that between the films with 30 min of UVO treatment. As shown,
Rs increased from 1.1 for the 5-layered films to 1.5 for the 10-layered films. This implies that the electrical connection between the RuO
2 NS layers becomes further improved as the number of layers increases. For example, an
Rs of 1000 Ω/sq was achieved at 10 layers in the samples without UVO treatment, whereas this value was achieved at only 7 layers in the 30-min UVO-treated samples. This result indicates that UVO exposure is highly efficient for enhancing the electrical percolation between coatings of RuO
2 NSs by removing the residual TBAOH from the NS surfaces.
Figure 4b depicts the variation in the optical transmittance of the RuO
2 NS films with the number of layers with and without UVO exposure. Specifically, this exhibits the improved optical transmittance after UVO treatment. However, the improvement becomes marginal as the number of NS layers increases.
To confirm the elimination of the residual organic TBAOH by the UVO treatment, the contents of hydrogen atoms in the LB-deposited film were investigated using Rutherford backscattering spectrometry (RBS). As shown in the inset of
Figure 4b, the intensity of the hydrogen peak near 64–65 keV in the 30-min UVO-treated films was slightly lower in comparison with that in the UVO-untreated films, revealing that the residual TBAOH was effectively removed in the UVO-treated sample. In addition, the intensity of the hydrogen peak in the 20-min UVO-treated sample was almost the same as that in the UVO-untreated sample, indicating that the residual TBAOH on the surface of NSs was not sufficiently removed until after 20 min of UVO exposure.
These results are consistent with those provided in
Figure 3a, i.e., the relation between UVO treatment time and sheet resistance. The reduction in
Rs was saturated at eight layers of coating in normal films due to the competition between the reduction in electron surface scattering in thicker films and the increased barrier that inhibits the transport of electrons, which was caused by the residual TBAOH on the NS surfaces. In contrast, in the UVO-exposed films, the saturation of
Rs was not observed until after 10 layers of coating. As a result, the gap of
Rs values between UVO-exposed films and normal films grows with the number of NS layers. The optical transmittance significantly decreased with the increasing number of LB layers in both UVO-exposed as well as untreated films, as depicted in
Figure 3b. In the five-layered films, the difference in optical transmittance was ~1.5% between the UVO-exposed films and untreated films. The difference was smaller in the case of more layers of NS coating. Moreover, the optical transmittance was almost constant after eight layers of NS coating. Considering that the optical transmittance of an individual monolayer RuO
2 NS was measured to be approximately 98% in our previous work [
11], it appears that the original optical transmittance of the RuO
2 NSs is a dominant factor in obtaining the total optical transmittance in the transparent conducting films.
Additionally, the sheet resistance changes with time were investigated to reveal the aging effect of RuO
2 NSs films, as shown in
Table 1. In this experiment, five-layered RuO
2 NS films with or without 30-min UVO treatment were employed. Each RuO
2 NS film was stored in the dark in an air environment. As shown in
Table 1,
Rs was slightly degraded by about 5% after 24 h. In the case of 30-min UVO-treated NS films, the degradation of
Rs was saturated at 48 h. On the other hand, the
Rs of RuO
2 NS films without UVO treatment increased monotonically with time, resulting in an increase in
Rs by more than 10% after 120 h. This suggests that the chemical stability of NS films was more enhanced through the removal of chemical residues by UVO treatment.