Histopathological Signatures of the Femoral Head in Patients with Osteonecrosis and Potential Applications in a Multi-Targeted Approach: A Pilot Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Data and Surgical Procedure
2.2. Histological Assessment
2.3. Immunohistochemical Analyses
2.4. Statistical Analysis
3. Results
3.1. Radiographic Assessment
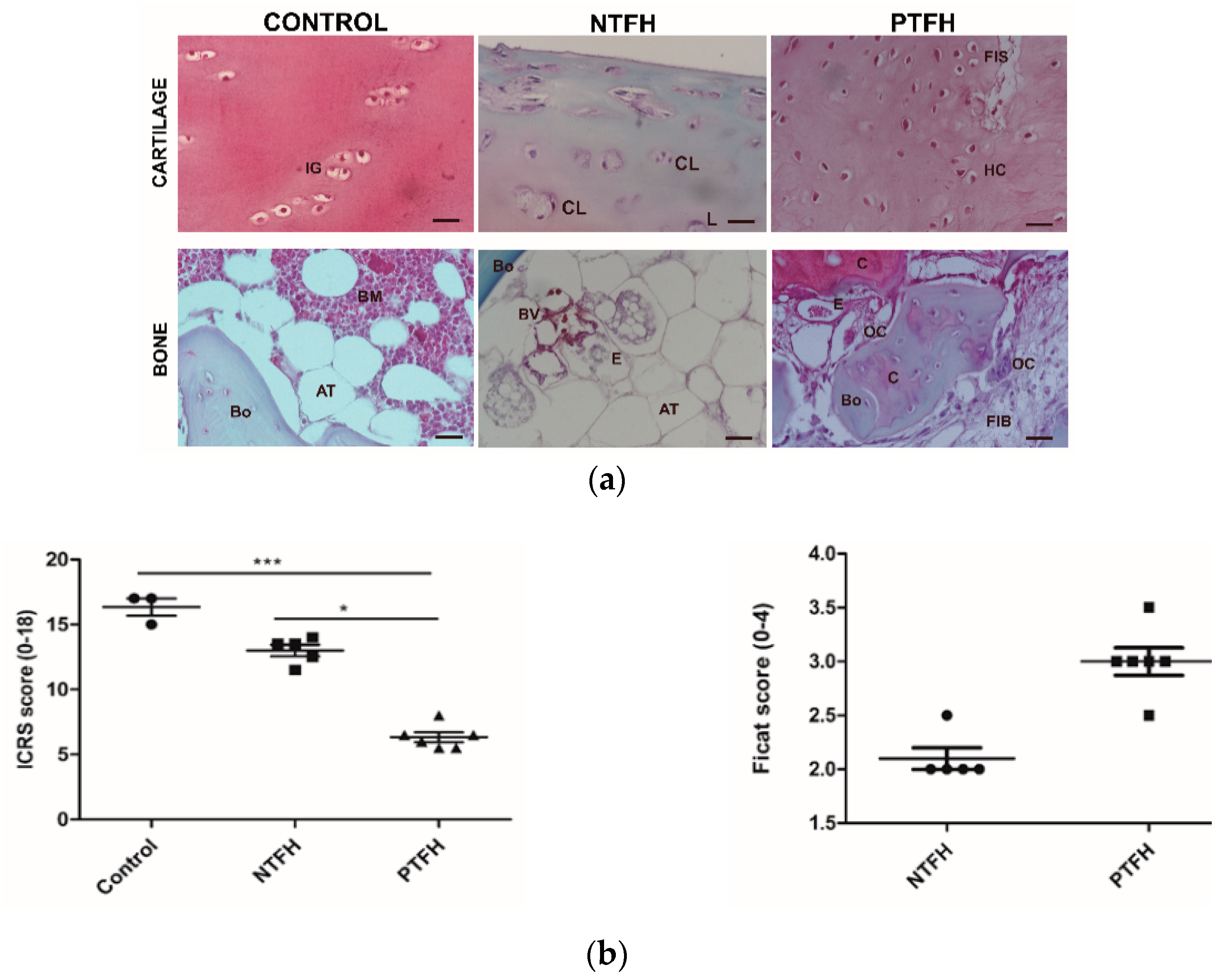
3.2. NTFH and PTFH Groups Displayed Different Histological Features in Cartilage and Bone
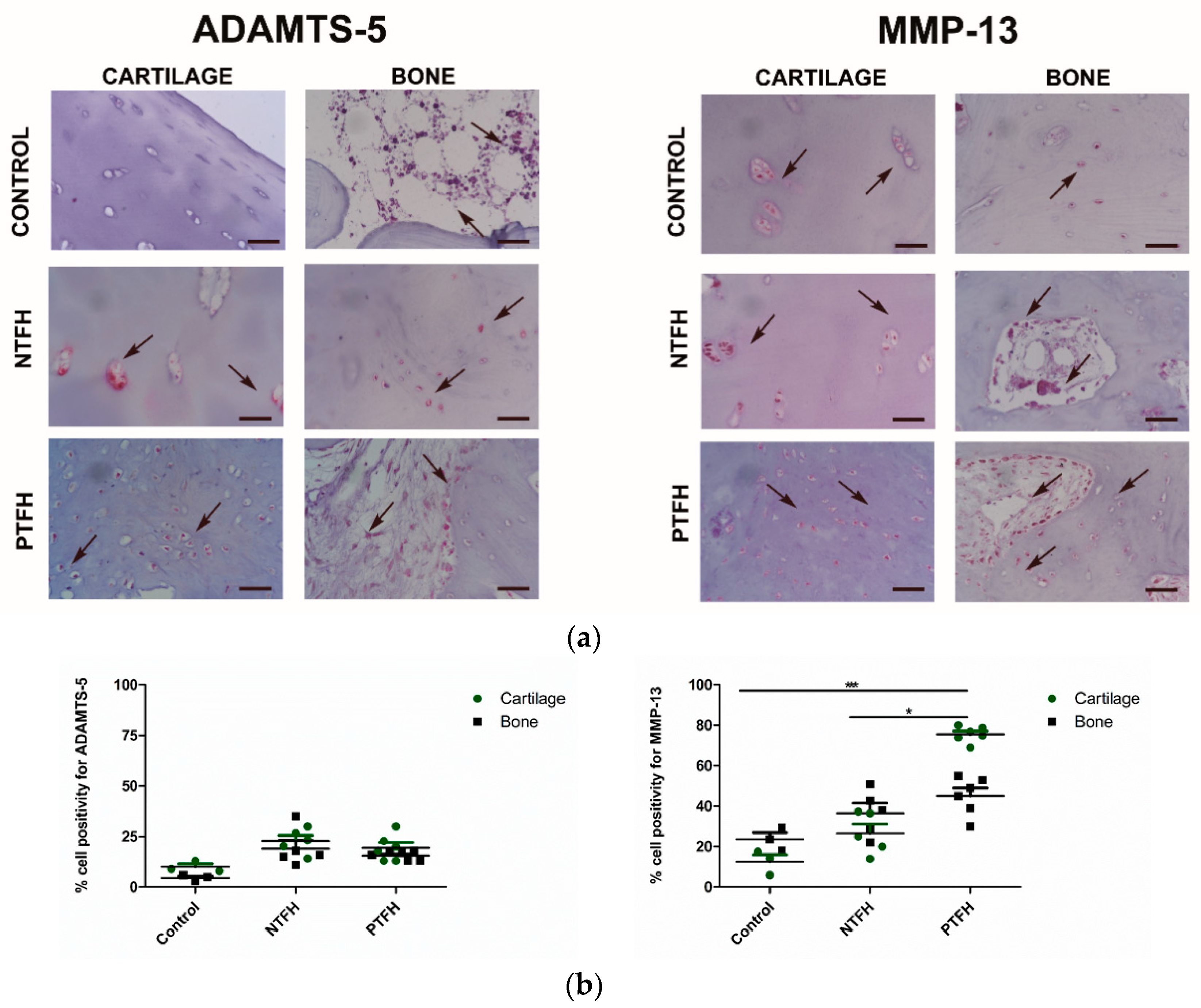
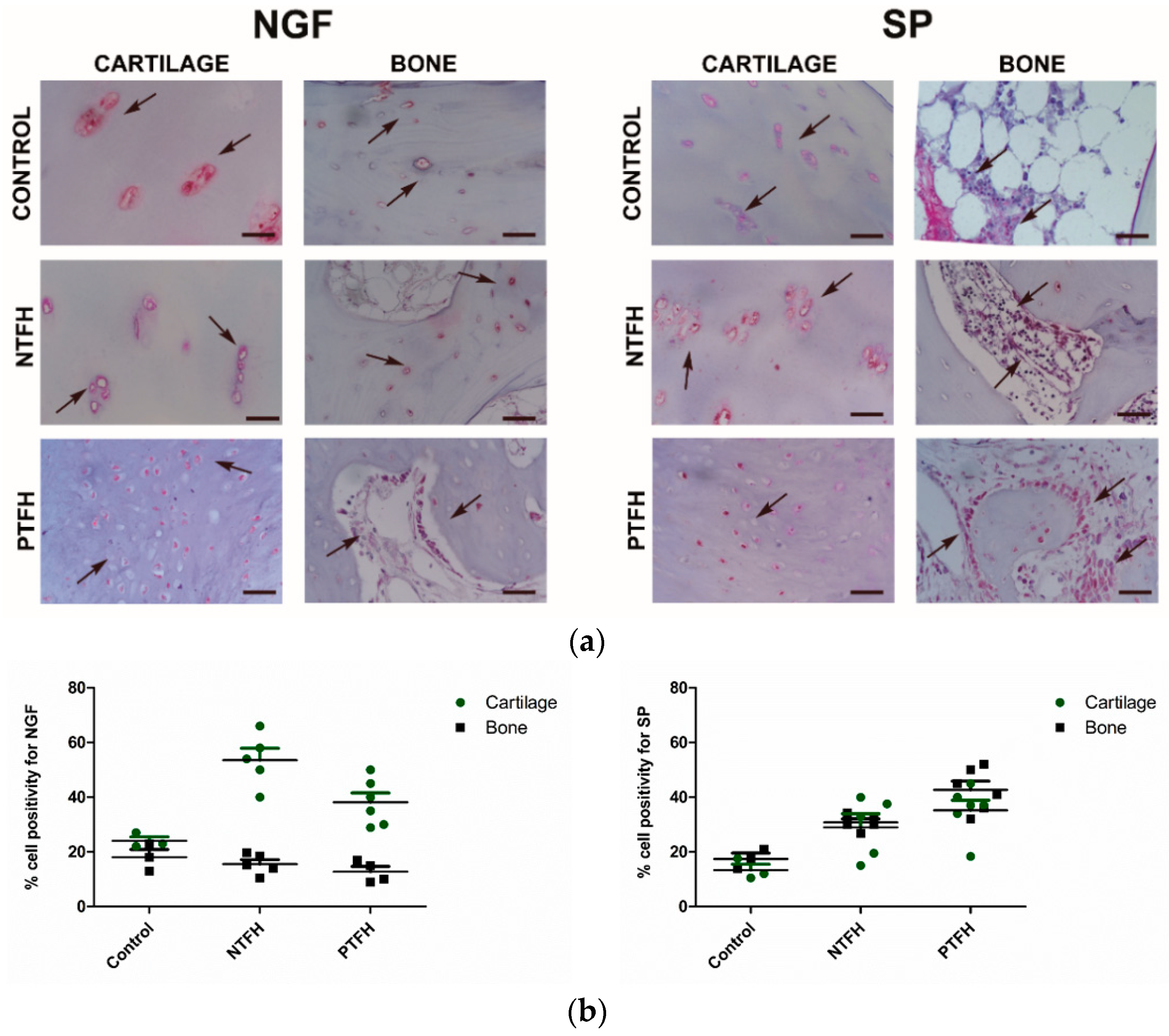
3.3. PTFH Group Displayed a Higher Expression of Fibrotic Markers than NTFH Specimens
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Zalavras, C.G.; Lieberman, J.R. Osteonecrosis of the femoral head. J. Am. Acad. Orthop. Surg. 2014, 22, 455–464. [Google Scholar] [CrossRef]
- Banerjee, S.; Issa, K.; Pivec, R.; Kapadia, B.H.; Khanuja, H.S.; Mont, M.A. Osteonecrosis of the hip. Orthop. Clin. North Am. 2013, 44, 463–476. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wang, J.; Zhang, K.; Wang, Y.; Bao, X. Bayesian network meta-analysis of the effectiveness of various interventions for nontraumatic osteonecrosis of the femoral head. Biomed Res. Int. 2018, 2018, 2790163. [Google Scholar] [CrossRef] [PubMed]
- Parsons, S.J.; Steele, N. Osteonecrosis of the femoral head: Part 1-Aetiology, pathogenesis, investigation, classification. Curr. Orthop. 2007, 21, 457–463. [Google Scholar] [CrossRef]
- Mah, W.; Sonkusare, S.K.; Wang, T.; Azeddine, B.; Pupavac, M.; Carrot-Zhang, J.; Hong, K.; Majewski, J.; Harvey, E.J.; Russell, L.; et al. Gain-of-function mutation in TRPV4 identified in patients with osteonecrosis of the femoral head. J. Med. Genet. 2016, 53, 705–709. [Google Scholar] [CrossRef]
- Khan, A.M.; Choi, J.; Freiberg, R.A.; Glueck, C.J.; Goldenberg, N.; Wang, P. T786C Mutation in the endothelial nitric oxide synthase gene in patients with primary osteonecrosis. Orthopedics 2017, 40, e898–e903. [Google Scholar] [CrossRef]
- Assouline-Dayan, Y.; Chang, C.; Greenspan, A.; Shoenfeld, Y.; Gershwin, M.E. Pathogenesis and natural history of osteonecrosis. In Seminars in Arthritis and Rheumatism; WB Saunders: Philadelphia, PA, USA, 2002. [Google Scholar]
- Zhao, D.W.; Yu, M.; Hu, K.; Wang, W.; Yang, L.; Wang, B.J.; Gao, X.H.; Guo, Y.M.; Xu, Y.Q.; Wei, Y.S.; et al. Prevalence of nontraumatic osteonecrosis of the femoral head and its associated risk factors in the Chinese population: Results from a nationally representative survey. Chin. Med. J. Engl. 2015, 128, 2843–2850. [Google Scholar] [CrossRef]
- Hwang, Y.; Park, J.; Choi, S.H.; Kim, G. Traumatic and Non-traumatic Osteonecrosis in the Femoral Head of a Rabbit Model. Lab. Anim. Res. 2011, 27, 127. [Google Scholar] [CrossRef]
- Pivec, R.; Johnson, A.J.; Harwin, S.F.; Mont, M.A. Differentiation, Diagnosis, and Treatment of Osteoarthritis, Osteonecrosis, and Rapidly Progressive Osteoarthritis. Orthopedics 2013, 36, 118–125. [Google Scholar] [CrossRef]
- Rajpura, A.; Wright, A.C.; Board, T.N. Medical management of osteonecrosis of the hip: A review. HIP Int. 2011, 21, 385–392. [Google Scholar] [CrossRef]
- Malizos, K.N.; Karantanas, A.H.; Varitimidis, S.E.; Dailiana, Z.H.; Bargiotas, K.; Maris, T. Osteonecrosis of the femoral head: Etiology, imaging and treatment. Eur. J. Radiol. 2007, 63, 16–28. [Google Scholar] [CrossRef]
- Tripathy, S.K.; Goyal, T.; Sen, R.K. Management of femoral head osteonecrosis: Current concepts. Indian J. Orthop. 2015, 49, 28–45. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Wang, X.; Xu, X.L.; Yuan, X.L.; Gou, W.L.; Wang, A.Y.; Guo, Q.Y.; Peng, J.; Lu, S.B. Bone microstructure and regional distribution of osteoblast and osteoclast activity in the osteonecrotic femoral head. PLoS ONE 2014, 9, e96361. [Google Scholar] [CrossRef] [PubMed]
- Miao, Q.; Hao, S.; Li, H.; Sun, F.; Wang, X. Expression of osteoprotegerin, RNAK and RANKL genes in femoral head avascular necrosis and related signaling pathway. Int. J. Clin. Exp. Pathol. 2015, 8, 10460–10467. [Google Scholar] [PubMed]
- Dimitrova, N.; Zamudio, J.R.; Jong, R.M.; Soukup, D.; Resnick, R.; Sarma, K.; Ward, A.J.; Raj, A.; Lee, J.; Sharp, P.A.; et al. Regulation of bone mass, bone loss and osteoclast activity by cannabinoid receptors. PLoS ONE 2017, 32, 736–740. [Google Scholar] [CrossRef]
- Ofek, O.; Karsak, M.; Leclerc, N.; Fogel, M.; Frenkel, B.; Wright, K.; Tam, J.; Attar-Namdar, M.; Kram, V.; Shohami, E.; et al. Peripheral cannabinoid receptor, CB2, regulates bone mass. Proc. Natl. Acad. Sci. USA 2006, 103, 696–701. [Google Scholar] [CrossRef]
- Whyte, L.S.; Ford, L.; Ridge, S.A.; Cameron, G.A.; Rogers, M.J.; Ross, R.A. Cannabinoids and bone: Endocannabinoids modulate human osteoclast function in vitro. Br. J. Pharmacol. 2012, 165, 2584–2597. [Google Scholar] [CrossRef]
- Xiang, S.; Li, Z.; Weng, X. Changed cellular functions and aberrantly expressed miRNAs and circRNAs in bone marrow stem cells in osteonecrosis of the femoral head. Int. J. Mol. Med. 2020, 45, 805–815. [Google Scholar] [CrossRef]
- Li, J.; Cheng, L.; Zhao, Y.; Guo, Y.; Liu, Y.; Zhang, W.; Wang, S.; Zhang, Y.; Pan, X.; Nie, L. ADAMTS-7 exhibits elevated expression in cartilage of osteonecrosis of femoral head and has a positive correlation with TNF-α and NF-κ B P65. Mediat. Inflamm. 2015, 2015, 196702. [Google Scholar] [CrossRef]
- Magnussen, R.A.; Guilak, F.; Vail, T.P. Articular cartilage degeneration in post-collapse osteonecrosis of the femoral head. Radiographic staging, macroscopic grading, and histologic changes. J. Bone Jt. Surg Am. 2005, 87, 1272–1277. [Google Scholar] [CrossRef]
- Houpt, J.B.; Pritzker, K.P.; Alpert, B.; Greyson, N.D.; Gross, A.E. Natural history of spontaneous osteonecrosis of the knee (SONK): A Review. In Seminars in Arthritis and Rheumatism; WB Saunders: Philadelphia, PA, USA, 1983; Volume 13. [Google Scholar]
- Sonoda, K.; Motomura, G.; Kawanami, S.; Takayama, Y.; Honda, H.; Yamamoto, T.; Nakashima, Y. Degeneration of articular cartilage in osteonecrosis of the femoral head begins at the necrotic region after collapse: A preliminary study using T1 rho MRI. Skeletal Radiol. 2017, 46, 463–467. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.H.; Chang, J.K.; Lai, K.A.; Hou, S.M.; Chang, C.H.; Wang, G.J. Alendronate in the prevention of collapse of the femoral head in nontraumatic osteonecrosis: A two-year multicenter, prospective, randomized, double-blind, placebo-controlled study. Arthritis Rheum. 2012, 64, 1572–1578. [Google Scholar] [CrossRef] [PubMed]
- Lai, K.A.; Shen, W.J.; Yang, C.Y.; Shao, C.J.; Hsu, J.T.; Lin, R.M. The use of alendronate to prevent early collapse of the femoral head in patients with nontraumatic osteonecrosis. J. Bone Jt. Surg Am. 2005, 87, 2155–2159. [Google Scholar] [CrossRef]
- Chan, K.; Mok, C. Glucocorticoid-Induced Avascular Bone Necrosis: Diagnosis and Management. Open Orthop. J. 2012, 6, 449–457. [Google Scholar] [CrossRef]
- Choi, H.R.; Steinberg, M.E.; Cheng, E. Osteonecrosis of the femoral head: Diagnosis and classification systems. Curr. Rev. Musculoskelet Med. 2015, 8, 210–220. [Google Scholar] [CrossRef]
- Jones, L.C.; Hungerford, D.S. Osteonecrosis: Etiology, diagnosis, and treatment. Curr. Opin. Rheumatol. 2004, 16, 443–449. [Google Scholar] [CrossRef]
- Hernigou, P.; Beaujean, F. Treatment of osteonecrosis with autologous bone marrow grafting. Clin. Orthop. Relat. Res. 2002, 405, 14–23. [Google Scholar] [CrossRef]
- Hernigou, P.; Trousselier, M.; Roubineau, F.; Bouthors, C.; Chevallier, N.; Rouard, H.; Flouzat-Lachaniette, C.H. Stem cell therapy for the treatment of hip osteonecrosis: A 30-year review of progress. CiOS Clin. Orthop. Surg. 2016, 8, 1–8. [Google Scholar] [CrossRef]
- Sacco, R.; Leeson, R.; Nissan, J.; Olate, S.; de Castro, C.H.B.C.; Acocella, A.; Lalli, A. A systematic review of oxygen therapy for the management of Medication-Related Osteonecrosis of the Jaw (MRONJ). Appl. Sci. 2019, 9, 1026. [Google Scholar] [CrossRef]
- Wang, Y.; Ma, X.; Chai, W.; Tian, J. Multiscale stem cell technologies for osteonecrosis of the femoral head. Stem Cells Int. 2019, 2019, 8914569. [Google Scholar] [CrossRef]
- Mobasheri, A.; Csaki, C.; Clutterbuck, A.L.; Rahmanzadeh, M.; Shakibaei, M. Mesenchymal stem cells in connective tissue engineering and regenerative medicine: Applications in cartilage repair and osteoarthritis therapy. Histol. Histopathol. 2009, 24, 347–366. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Petrigliano, F.A.; Ba, K.; Lee, S.; Bogdanov, J.; McAllister, D.R.; Lin, Y. Lysophosphatidic acid mediates fibrosis in injured joints by regulating collagen type I biosynthesis. Osteoarthr. Cartil. 2015, 23, 308–318. [Google Scholar] [CrossRef] [PubMed]
- Flammier, S.; Peyruchaud, O.; Bourguillault, F.; Duboeuf, F.; Davignon, J.; Norman, D.D.; Isaac, S.; Marotte, H.; Tigyi, G.; Machuca-Gayet, I. Osteoclast—Derived autotaxin, a distinguishing factor for inflammatory bone loss. Arthritis Rheumatol. 2019, 71, 1801–1811. [Google Scholar] [CrossRef]
- Troeberg, L.; Nagase, H. Proteases involved in cartilage matrix degradation in osteoarthritis. Biochim. Biophys. Acta Proteins Proteom. 2012, 1824, 133–145. [Google Scholar] [CrossRef]
- Fan, L.; Li, J.; Yu, Z.; Dang, X.; Wang, K. Hypoxia-inducible factor prolyl hydroxylase inhibitor prevents steroid-associated osteonecrosis of the femoral head in rabbits by promoting angiogenesis and inhibiting apoptosis. PLoS ONE 2014, 9, e107774. [Google Scholar] [CrossRef] [PubMed]
- Dunn, S.L.; Wilkinson, J.M.; Crawford, A.; Bunning, R.A.D.; Le Maitre, C.L. Expression of cannabinoid receptors in human osteoarthritic cartilage: Implications for future therapies. Cannabis Cannabinoid Res. 2016, 1, 3–15. [Google Scholar] [CrossRef]
- Shang, X.; Wang, Z.; Tao, H. Mechanism and therapeutic effectiveness of nerve growth factor in osteoarthritis pain. Ther. Clin. Risk Manag. 2017, 13, 951–956. [Google Scholar] [CrossRef]
- Li, F.X.Z.; Xu, F.; Lin, X.; Wu, F.; Zhong, J.Y.; Wang, Y.; Guo, B.; Zheng, M.H.; Shan, S.K.; Yuan, L.Q. The role of substance P in the regulation of bone and cartilage metabolic activity. Front. Endocrinol. 2020, 11, 77. [Google Scholar] [CrossRef]
- Gardeniers, J. A new international classification of osteonecrosis of the ARCO-committee on terminology and classification. ARCO Newsl. 1992, 4, 41–46. [Google Scholar]
- Desando, G.; Bartolotti, I.; Vannini, F.; Cavallo, C.; Castagnini, F.; Buda, R.; Giannini, S.; Mosca, M.; Mariani, E.; Grigolo, B. Repair potential of matrix-induced bone marrow aspirate concentrate and matrix-induced autologous chondrocyte implantation for talar osteochondral repair: Patterns of some catabolic, inflammatory, and pain mediators. Cartilage 2017, 8, 50–60. [Google Scholar] [CrossRef]
- Hoemann, C.; Kandel, R.; Roberts, S.; Saris, D.B.F.; Creemers, L.; Mainil-Varlet, P.; Méthot, S.; Hollander, A.P.; Buschmann, M.D. International cartilage repair society (ICRS) recommended guidelines for histological endpoints for cartilage repair studies in animal models and clinical trials. Cartilage 2011, 2, 153–172. [Google Scholar] [CrossRef] [PubMed]
- Jawad, M.U.; Haleem, A.A.; Scully, S.P. In brief: Ficat classification: Avascular necrosis of the femoral head. Clin. Orthop. Relat. Res. 2012, 470, 2636–2639. [Google Scholar] [CrossRef] [PubMed]
- Goldring, S.R.; Goldring, M.B. Changes in the osteochondral unit during osteoarthritis: Structure, function and cartilage-bone crosstalk. Nat. Rev. Rheumatol. 2016, 12, 632–644. [Google Scholar] [CrossRef] [PubMed]
- Knowlden, S.; Georas, S.N. The autotaxin-LPA axis emerges as a novel regulator of lymphocyte homing and inflammation. J. Immunol. 2014, 192, 851–857. [Google Scholar] [CrossRef]
- Thirunavukkarasu, K.; Swearingen, C.A.; Oskins, J.L.; Lin, C.; Bui, H.H.; Jones, S.B.; Pfeifer, L.A.; Norman, B.H.; Mitchell, P.G.; Chambers, M.G. Identification and pharmacological characterization of a novel inhibitor of autotaxin in rodent models of joint pain. Osteoarthr. Cartil. 2017, 25, 935–942. [Google Scholar] [CrossRef]
- Murphy, G.; Knäuper, V.; Atkinson, S.; Butler, G.; English, W.; Hutton, M.; Stracke, J.; Clark, I. Matrix metalloproteinases in arthritic disease. Arthritis Res. 2002, 4, S39. [Google Scholar] [CrossRef]
- Grässel, S.; Beckmann, J.; Rath, B.; Vogel, M.; Grifka, J.; Tingart, M. Expression profile of matrix metalloproteinase-2 and -9 and their endogenous tissue inhibitors in osteonecrotic femoral heads. Int. J. Mol. Med. 2010, 26, 127–133. [Google Scholar] [CrossRef][Green Version]
- Herm, L.; Haxhia, A.; de Alcantara Camejo, F.; Tayebi, L.; Almeida, L.E. Matrix metalloproteinases and temporomandibular joint disorder: A review of the literature. Appl. Sci. 2019, 9, 4508. [Google Scholar] [CrossRef]
- Vandenbroucke, R.E.; Libert, C. Is there new hope for therapeutic matrix metalloproteinase inhibition? Nat. Publ. Gr. 2014, 13, 904–927. [Google Scholar] [CrossRef]
- Jiang, S.; Zagozdzon, R.; Jorda, M.A.; Parmar, K.; Fu, Y.; Williams, J.S.; Groopman, J.E. Endocannabinoids are expressed in bone marrow stromal niches and play a role in interactions of hematopoietic stem and progenitor cells with the bone marrow microenvironment. J. Biol. Chem. 2010, 285, 35471–35478. [Google Scholar] [CrossRef]
- Roemer, F.W.; Neogi, T.; Nevitt, M.C.; Felson, D.T.; Zhu, Y.; Zhang, Y.; Lewis, C.E. Subchondral bone marrow lesions are highly associated with, and predict subchondral bone attrition longitudinally: The MOST study. Osteoarthr. Cart. 2010, 18, 47. [Google Scholar] [CrossRef] [PubMed]
- Bellido, M.; Lugo, L.; Roman-Blas, J.A.; Castañeda, S.; Calvo, E.; Largo, R.; Herrero-Beaumont, G. Improving subchondral bone integrity reduces progression of cartilage damage in experimental osteoarthritis preceded by osteoporosis. Osteoarthr. Cartil. 2011, 19, 1228–1236. [Google Scholar] [CrossRef]
- Campbell, T.M.; Churchman, S.M.; Gomez, A.; McGonagle, D.; Conaghan, P.G.; Ponchel, F.; Jones, E. Mesenchymal stem cell alterations in bone marrow lesions in patients with hip osteoarthritis. Arthritis Rheumatol. 2016, 68, 1648–1659. [Google Scholar] [CrossRef] [PubMed]
- Karsdal, M.A.; Bay-Jensen, A.C.; Lories, R.J.; Abramson, S.; Spector, T.; Pastoureau, P.; Christiansen, C.; Attur, M.; Henriksen, K.; Goldring, S.R.; et al. The coupling of bone and cartilage turnover in osteoarthritis: Opportunities for bone antiresorptives and anabolics as potential treatments? Ann. Rheum. Dis. 2014, 73, 336–348. [Google Scholar] [CrossRef]
- Desando, G.; Cavallo, C.; Sartoni, F.; Martini, L.; Parrilli, A.; Veronesi, F.; Fini, M.; Giardino, R.; Facchini, A.; Grigolo, B. Intra-articular delivery of adipose derived stromal cells attenuates osteoarthritis progression in an experimental rabbit model Intra-articular delivery of adipose derived stromal cells attenuates osteoarthritis progression in an experimental rabbit model. Arthritis Res. Ther. 2013, 15, R22. [Google Scholar] [CrossRef]
- Perdisa, F.; Gostyńska, N.; Roffi, A.; Filardo, G.; Marcacci, M.; Kon, E. Adipose-derived mesenchymal stem cells for the treatment of articular cartilage: A systematic review on preclinical and clinical evidence. Stem Cells Int. 2015, 2015, 597652. [Google Scholar] [CrossRef]
- Murphy, M.B.; Moncivais, K.; Caplan, A.I. Mesenchymal stem cells: Environmentally responsive therapeutics for regenerative medicine. Exp. Mol. Med. 2013, 45, e54. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.; Cui, D.; Wang, B.; Tian, F.; Guo, L.; Yang, L.; Liu, B.; Yu, X. Treatment of early stage osteonecrosis of the femoral head with autologous implantation of bone marrow-derived and cultured mesenchymal stem cells. Bone 2012, 50, 325–330. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, S.; Su, X. Core decompression and implantation of bone marrow mononuclear cells with porous hydroxylapatite composite filler for the treatment of osteonecrosis of the femoral head. Arch. Orthop. Trauma Surg. 2013, 133, 125–133. [Google Scholar] [CrossRef]
- Jiang, Y.; Hu, C.; Yu, S.; Yan, J.; Peng, H.; Ouyang, H.W.; Tuan, R.S. Cartilage stem/progenitor cells are activated in osteoarthritis via interleukin-1β/nerve growth factor signaling. Arthritis Res. Ther. 2015, 17, 327. [Google Scholar] [CrossRef]
- Chartier, S.R.; Mitchell, S.A.T.; Majuta, L.A.; Mantyh, P.W. Immunohistochemical localization of nerve growth factor, tropomyosin receptor kinase A, and p75 in the bone and articular cartilage of the mouse femur. Mol. Pain 2017, 13, 1744806917745465. [Google Scholar] [CrossRef] [PubMed]


© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Desando, G.; Roseti, L.; Bartolotti, I.; Dallari, D.; Stagni, C.; Grigolo, B. Histopathological Signatures of the Femoral Head in Patients with Osteonecrosis and Potential Applications in a Multi-Targeted Approach: A Pilot Study. Appl. Sci. 2020, 10, 3945. https://doi.org/10.3390/app10113945
Desando G, Roseti L, Bartolotti I, Dallari D, Stagni C, Grigolo B. Histopathological Signatures of the Femoral Head in Patients with Osteonecrosis and Potential Applications in a Multi-Targeted Approach: A Pilot Study. Applied Sciences. 2020; 10(11):3945. https://doi.org/10.3390/app10113945
Chicago/Turabian StyleDesando, Giovanna, Livia Roseti, Isabella Bartolotti, Dante Dallari, Cesare Stagni, and Brunella Grigolo. 2020. "Histopathological Signatures of the Femoral Head in Patients with Osteonecrosis and Potential Applications in a Multi-Targeted Approach: A Pilot Study" Applied Sciences 10, no. 11: 3945. https://doi.org/10.3390/app10113945
APA StyleDesando, G., Roseti, L., Bartolotti, I., Dallari, D., Stagni, C., & Grigolo, B. (2020). Histopathological Signatures of the Femoral Head in Patients with Osteonecrosis and Potential Applications in a Multi-Targeted Approach: A Pilot Study. Applied Sciences, 10(11), 3945. https://doi.org/10.3390/app10113945








