1. Introduction
Since its first development in the 1960s in the U.S. as a promising solution to problems regarding accumulated spent fuel, pyroprocessing, which recycles spent fuel as a fuel material and reduces the amount and toxicity of the spent fuel, has also been studied at KAERI (Korea Atomic Energy Research Institute) [
1,
2,
3,
4]. Pyroprocessing begins with the head-end process, which recovers fuel oxide from the spent fuel and converts it into the form of a UO
2 porous pellet. As a starting process of the pyroprocessing, the head-end consists of sequential subprocesses: de-cladding, mixing, pelletizing, and sintering [
5,
6,
7,
8].
As noted in a previous paper [
9], a reduction process has been adopted between the pelletizing and sintering processes to protect metallic crucibles from oxidation attack during sintering. Molybdenum has been used as the metallic crucible material due to its excellent chemical and physical integrity, in addition to its outstanding thermal shock resistance up to high temperature. However, unless reduction heat treatment is preceded, the metallic crucible will be oxidized during sintering. The uranium oxide in the form of UO
2+x gives off oxygen accompanied by a reduction to the UO
2 phase during sintering in a reducing atmosphere. The oxygen forms an oxidative atmosphere temporarily in the vicinity of the pellets and thus attacks the metallic crucible, even under the reducing atmosphere [
9]. To inhibit this oxidation attack, U
3O
8 pellets are pre-reduced to UO
2 by high-temperature heat treatment to eliminate the oxygen source before sintering. This allows the use of a commercial metallic sintering crucible without any other treatment. In other words, without an appropriate alternative material for the sintering crucible, reduction heat treatment is inevitably required.
However, such heat treatment requires high temperature, ~1000 °C [
9], and a long processing time, detrimentally affecting processing economics and safety. Therefore, once a successful alternative material is developed for the crucible, the reduction process should be excluded. Requirements for the alternative material are very strict: it should possess oxidation and reduction resistance, as well as excellent thermal shock resistance up to high temperature. This is why studies for the development of an alternative crucible material should be carried out in parallel to related research. Modifications of existing materials, such as surface coatings, may be promising routes. As a general approach, a coating of a promising material satisfying the requirements for a metallic crucible could be a possibility. However, it would be strictly limited when the scale is enlarged under a severe temperature gradient [
10]. In addition, if the shape of the coated material is irregular or complex, the coating method will hardly form a uniform passivation layer over the surface. Unfortunately, the sintering crucible in pyroprocessing presents difficulties in terms of size and shape. To meet processing yield requirements, the size of the crucible reaches about 300 mm in diameter [
9], and penetration holes facilitating the flow of atmospheric gas should be made in its bottom surface. In other words, a promising method of surface passivation of the metallic crucible is required to overcome the problems related to size and shape.
Previous studies highlighted the formation of an oxidation-resistant Hf
6Ta
2O
17 superstructure in Hf-Ta alloy systems as a favorable method for passivation [
11,
12]. Basically, the Hf-Ta alloys have sufficiently higher melting temperature, ~2000 °C, than the sintering temperature used in pyroprocessing, ~1450 °C. Moreover, a uniform oxide can be formed because its formation is due to solid/vapor reaction-induced oxidation of the metallic surface. By securing solid/vapor contact, a uniform superstructure layer can be formed even on the inner surface of penetration holes. In addition, the oxide is known for its excellent oxidation resistance up to high temperature with excellent adhesion, and it is referred to as a superstructure [
12]. If the superstructure is confirmed to be reduction resistant and to maintain physical integrity under the sintering conditions in pyroprocessing, it will be appropriate for sintering crucibles. This study confirms the uniform formation of the superstructure and evaluates its applicability by a thermal test with the same sintering conditions as those used in the head-end process in pyroprocessing. To this end, a direction of compositional design is suggested based on the experimental results to enhance the usefulness of the superstructure.
3. Results and Discussion
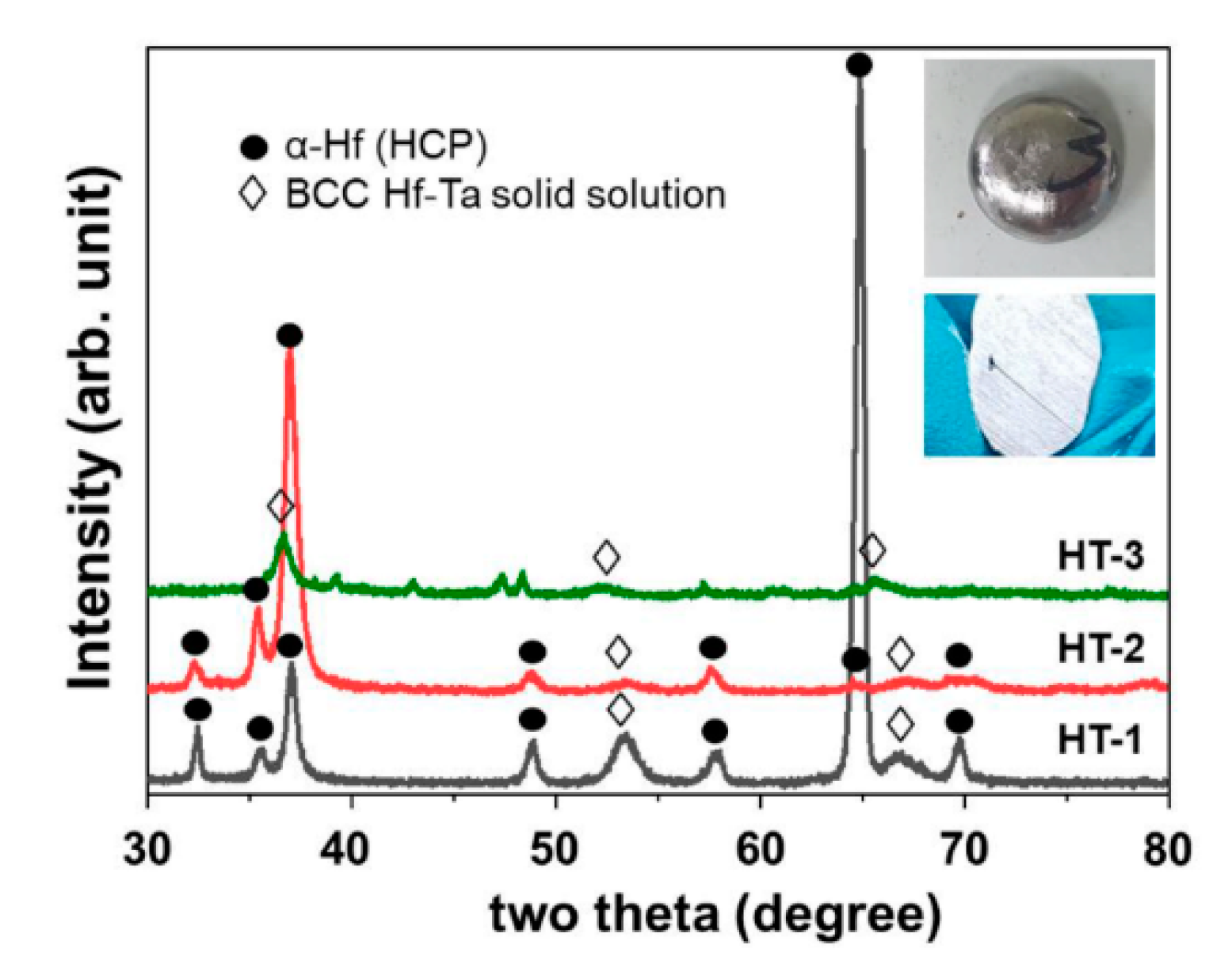
Figure 1 shows the XRD patterns of the as-cast ingot samples with different chemical composition, as listed in
Table 1. The HT-1 and HT-2 samples containing relatively higher Hf (lower Ta) content were revealed as mixtures of the hexagonal close-packed (HCP) α-Hf and body-centered cubic (BCC) Hf-Ta phases. Except for a peculiar peak observed near 65°, the HT-1 sample showed the same tendency as the HT-2 sample. On the other hand, in the case of the HT-3 sample, no peak of the HCP (α-Hf) phase was observed and only the BCC (Hf-Ta) phase was identified. Despite incomplete identification due to the unknown peaks, it is clearly shown that a phase transition occurred between the HT-2 and HT-3 samples. The same tendency was also observed in a previous paper: constituent phases changed from a mixture of both α-Hf and Hf-Ta into Hf-Ta phase alone with increasing Ta content from 19.7 to 26.7 [
12]. Meanwhile, the critical chemical composition leading to phase transition appears to lie between 27.2 and 35.2 (% Ta) in this experiment, considering the chemical compositions of the HT-2 and HT-3 samples. This discrepancy can be attributed to a locally different chemical composition that arises during the arc melting process, even when the cross section appears to be uniform without an unmelted phase, as shown in the inset photograph in
Figure 1. Nevertheless, the well-known Hf
6Ta
2O
17 phase as a superstructure was expected from the HT-2 and HT-3 samples because its formation range is known to be sufficiently broad in terms of chemical composition [
12]. To form the superstructure, the same method of oxidation was applied.
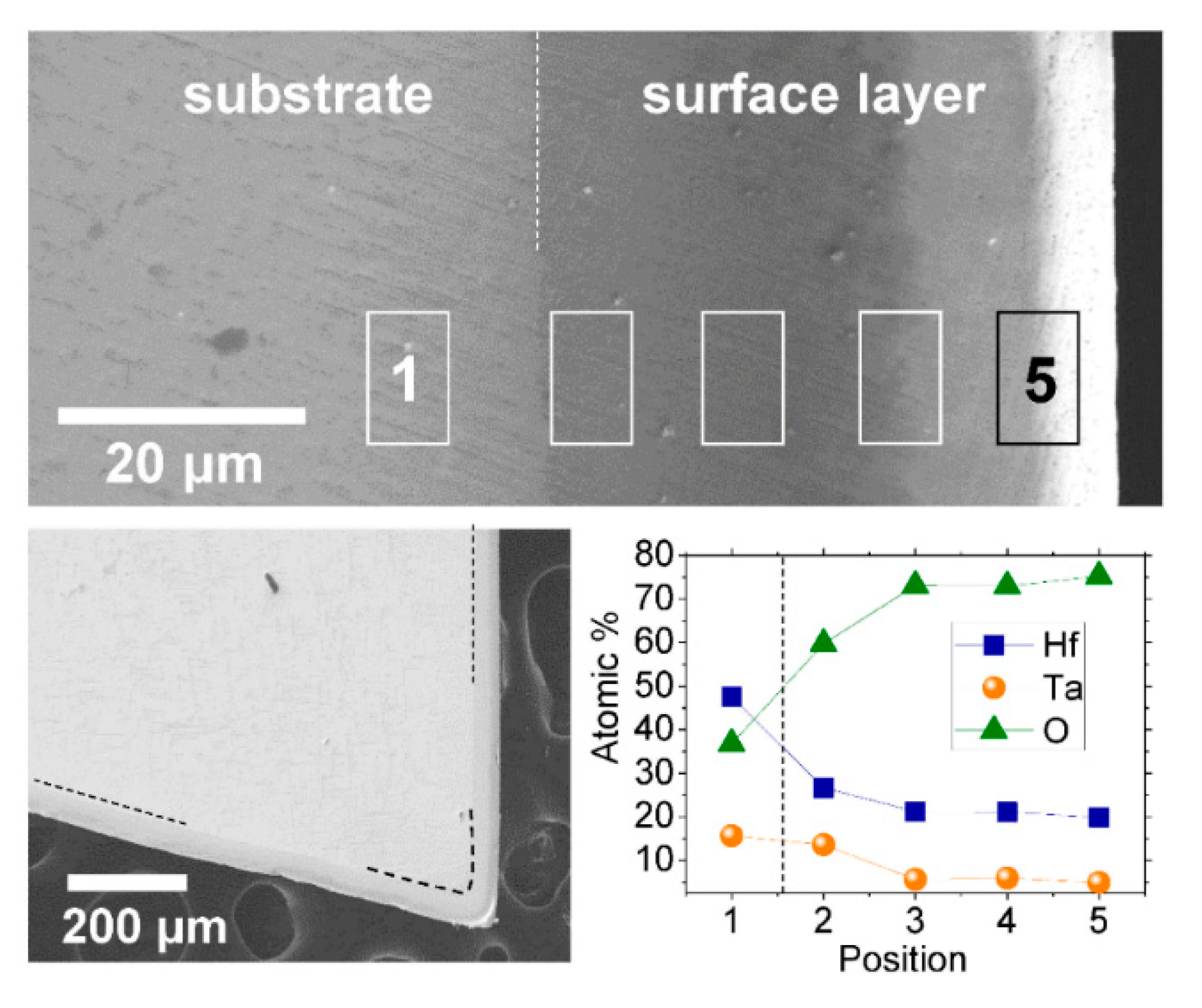
Figure 2 shows the SEM and EDS results on a cross section of the HT-1 sample after oxidation heat treatment. The cross-sectional images reveal the outermost layer (surface layer), which is distinguished by its contrast from the substrate region. In a low-magnification micrograph, the thickness of the surface layer appears to be quite uniform along the surface. In both SEM images in
Figure 2, the position of the interface between the substrate and surface layer is denoted by dashed lines. Taking into account the applicability of this oxidized alloy as a crucible material in pyroprocessing, the uniformity provides practical benefits. This is attributed to the even diffusion depth of oxygen by the solid/vapor reaction from the surface. As long as solid/vapor contact is secured between the alloy and gaseous oxygen, the oxide scale can be formed uniformly regardless of the shape of the sample. This gives practical significance where other well-known passivation coating methods are not effective due to the irregular shape of the materials to be passivated.
Across the well-defined interface between the substrate and surface layer, an EDS scan was carried out, as shown in
Figure 2. It provides the compositional distribution of Hf, Ta, and O across the interface. To minimize any local fluctuation that may result from local heterogeneity, the EDS signal was collected from a specific area. As a result, every element shows typical diffusional profiles: the oxygen content drastically increased and then became saturated near the surface, while both Hf and Ta gradually decreased and then became saturated towards the surface layer.
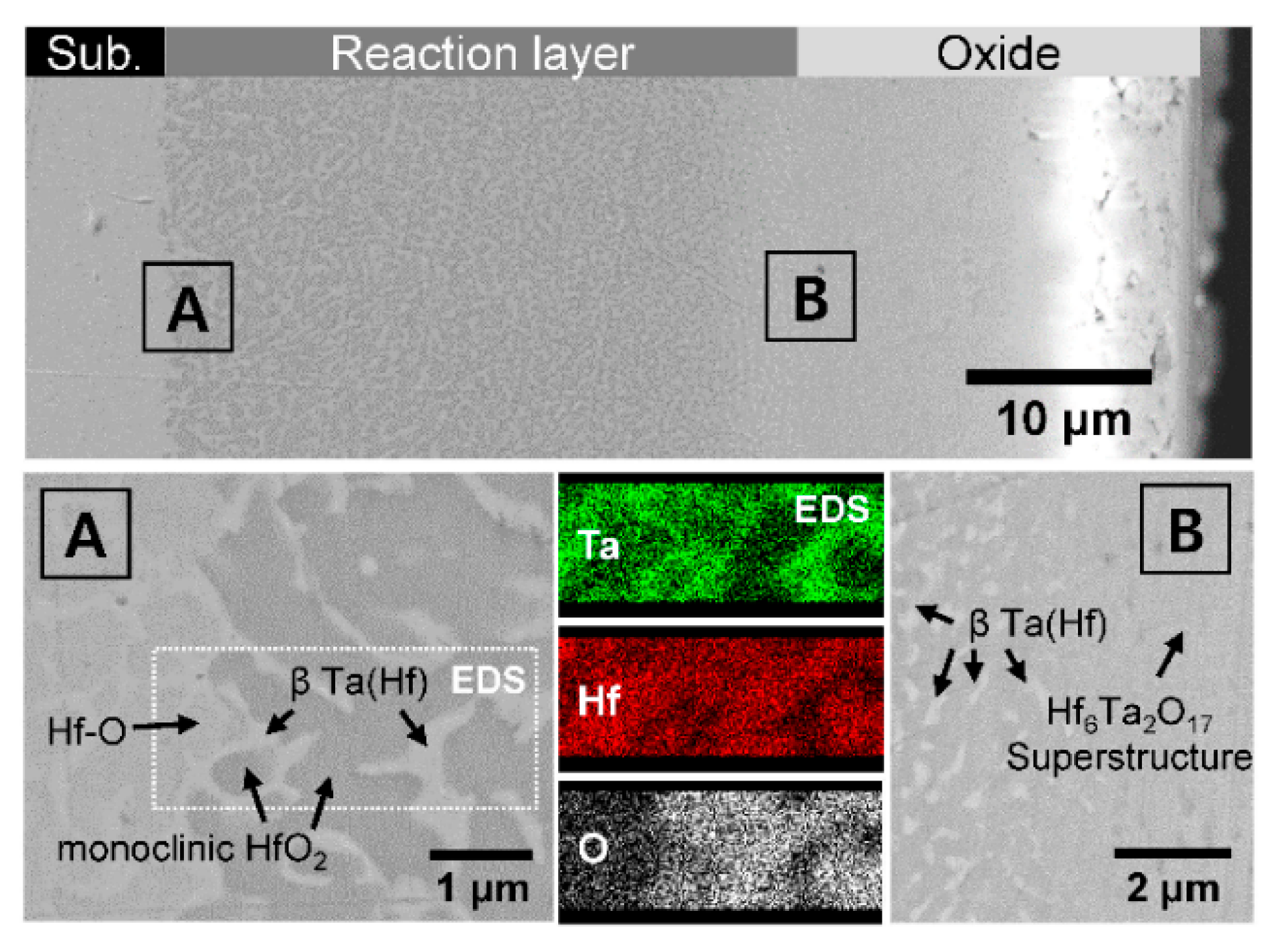
The newly formed oxide scale was examined more precisely from the HT-3 sample by SEM and EDS, as shown in
Figure 3. The HT-3 sample, containing a relatively high amount of Ta, revealed a thicker layer than did the other samples with a lower amount of Ta. Despite the edge effect of SEM (abnormally bright contrast), the micrographs with higher magnifications clarify two different layers distinguished by their morphology. The inner layer in the vicinity of the substrate reveals a heterogeneous phase, while the outer layer appears as a uniform phase. The regions near the interface between the substrate and the inner layer and that between the inner and outer layers (denoted by “A” and “B”, respectively) are enlarged in
Figure 3. In addition, the spatial compositional distribution was revealed by EDS mapping at interface “A”. At both “A” and “B”, relatively higher Ta content was observed from the irregular shape with bright contrast. On the other hand, the dark phase contained a larger amount of Hf than Ta. Considering the morphology and the compositional distribution together with the previous analysis results [
12], the inner layer is regarded as a “reaction layer”, which is composed of hafnium oxides and β-Ta(Hf) phases, whereas the outer layer is an “oxide layer” of Hf
6Ta
2O
17 superstructure. An EDS point scan revealed the local composition of the hafnium oxides to be 28.58Hf-3.21Ta-68.21O, that of β-Ta(Hf) to be 19.57Hf-33.93Ta-46.50O, and that of the Hf
6Ta
2O
17 superstructure to be 22.85Hf-7.08Ta-70.07O. It is noted that the atomic ratio of the oxide layer closely matches the previous observation [
12]. In the reaction layer, from the area near the substrate to the oxide layer, the size of the β-Ta(Hf) phase and its connectivity decreased simultaneously. Thus, the Ta content gradually decreased outwards, resulting in the compositional profile shown in
Figure 2. However, the chemical composition was similar regardless of the position; i.e., a similar composition was observed at the “A” interface to that at the “B” interface. All these observations are quite similar to those in a previous study that revealed the same β-Ta(Hf) phase in the reaction layer by XRD [
12].
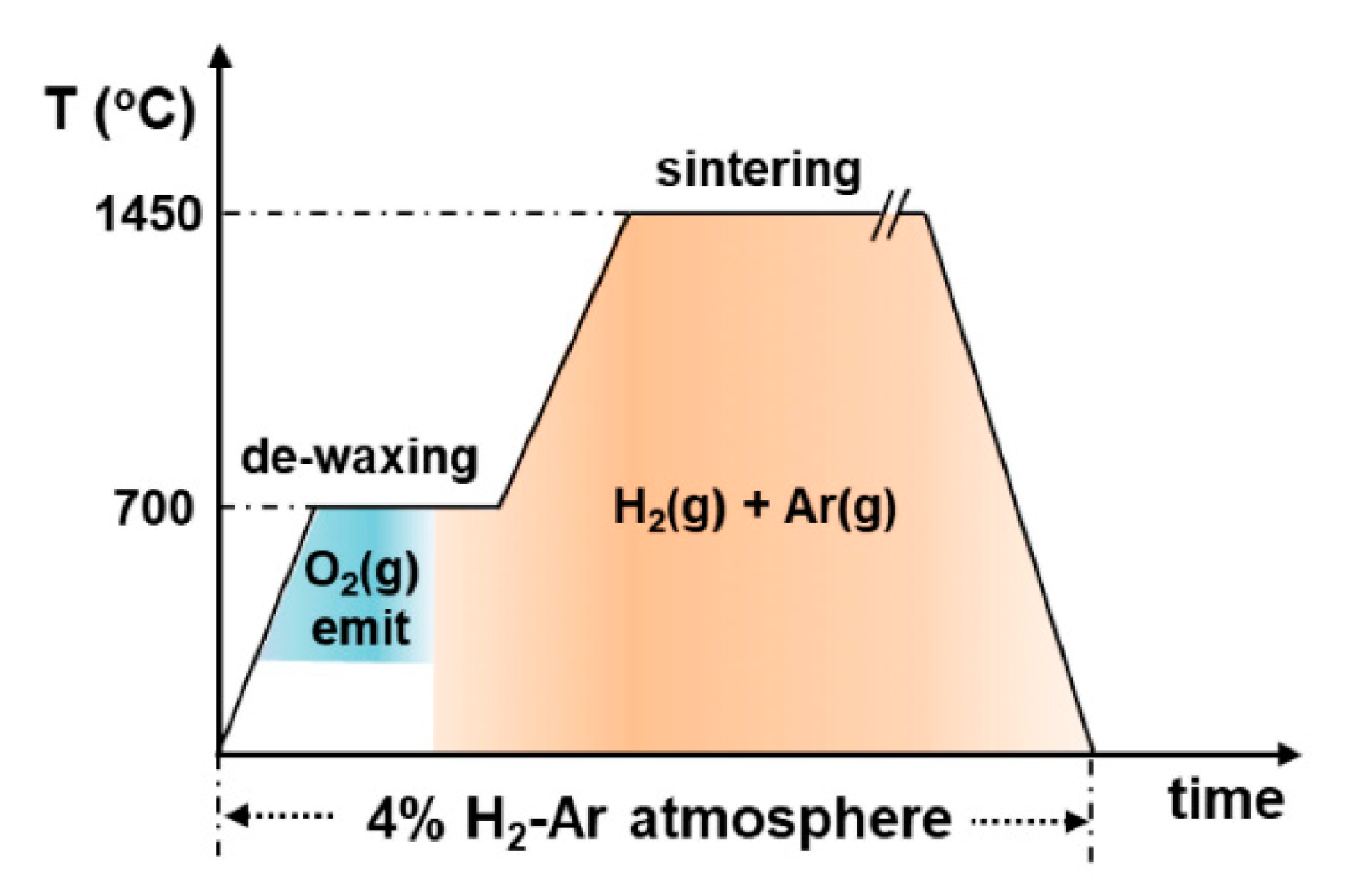
Figure 4 shows the thermal profile of the sintering process in the head-end of pyroprocessing [
8], where it is speculated that the Hf
6Ta
2O
17 superstructure can be applied as a crucible material. The crucible material should endure both oxidative and reducing atmospheres during repeated thermal cycles. At the initial stage, during ramping, the uranium oxide contained in a crucible starts to be reduced, giving off oxygen gases resulting in the oxidation of metallic crucibles. Reduction of U
3O
8 in a 4% H
2-Ar balanced atmosphere is known to start and finish at about 500 °C and 900 °C, respectively [
9]. During ramping through this temperature range, the crucible material must remain intact against the oxidation attack. The oxygen generated from the reduction of uranium oxide creates a locally oxidative atmosphere despite abundant hydrogen gas in the atmosphere. Although the oxidation attack would initially focus on the surface, it can be widespread, and moreover, the degree of damage would be severe as the processing scale is enlarged [
9]. Consequently, during the initial stage of sintering (denoted as “O
2(g) emit” in
Figure 4), the crucible material should be oxidation resistant. Soon after, the crucible material is surrounded by a H
2-containing reducing atmosphere, and it should endure the reducing attack up to 1450 °C, which is the highest temperature during sintering in the head-end process. From a thermodynamic viewpoint, the oxygen partial pressure (
) increases as the temperature increases; thus, the reduction attack rate would be mitigated, whereas its kinetics can be thermally stimulated.
A candidate material for the crucible should thus be reduction resistant in addition to being oxidation resistant.
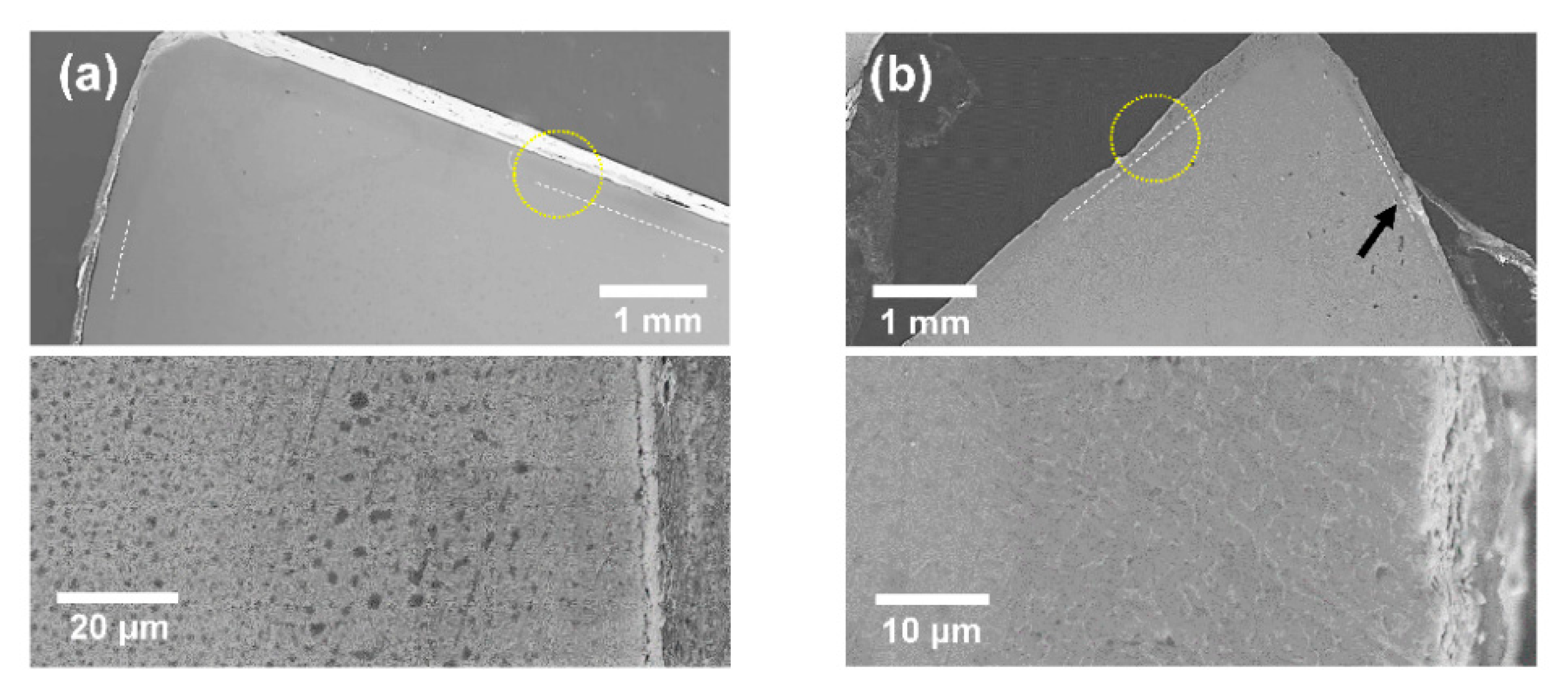
Figure 5 shows SEM micrographs of the HT-1 and HT-3 samples after the thermal cycle shown in
Figure 4. From both macroscopic- and microscopic-scale observations, consistent results were obtained. In the HT-1 sample, the surface layer shown in
Figure 2 (reaction layer + oxide layer) was intact with a sharp edge line, while a rough surface layer was observed in the HT-3 sample. In a high-magnification observation of the HT-3 sample, the outermost surface layer appears to have peeled off partially or mostly (indicated by an arrow in
Figure 5b). However, the interfaces between the substrates and reaction layers were maintained as straight lines in all samples, suggesting that the thermal treatment hardly gives rise to additional oxygen diffusion into the substrates. In other words, oxidative attack during ramping was negligible in the Hf
6Ta
2O
17 superstructure sample.
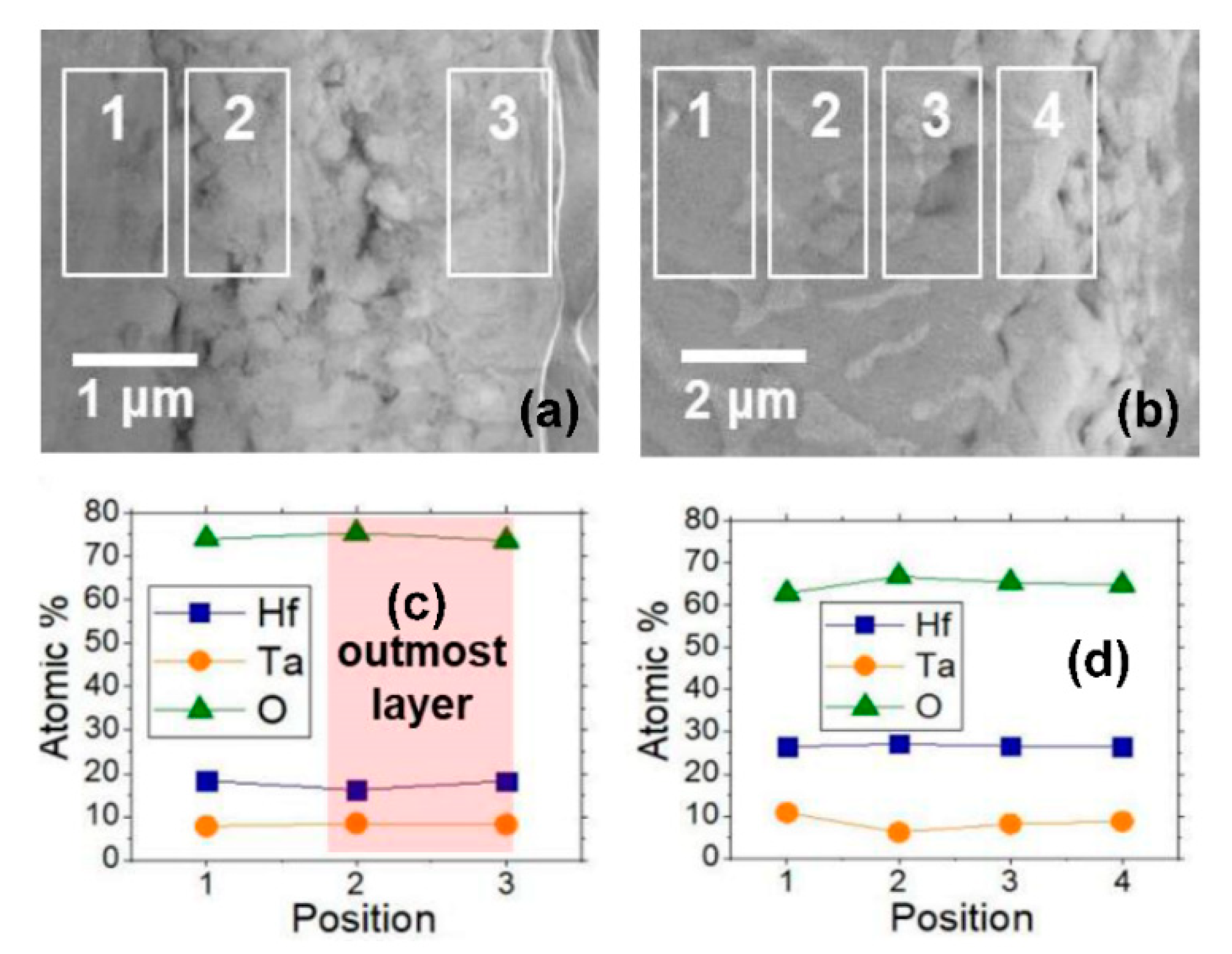
To clarify the cause of the different peeling behaviors among the samples with different Ta contents, the chemical composition was observed by SEM/EDS at the samples’ surface regions.
Figure 6 shows the SEM/EDS line scan results on the cross-sectional surfaces of the HT-1 and HT-3 samples after the thermal cycle. The average atomic ratios of the constituent elements were measured as 18.6Hf–8.2Ta–74.2O for the HT-1 sample and as 27.2Hf–8.1Ta–64.7O for the HT-3 sample in the vicinity of the surface. However, the measured region was different depending on whether or not the sample surface was conserved after the thermal cycle. The chemical composition could be examined at the outermost oxide layer only in the HT-1 sample with no severe peeling. In contrast, the EDS results for the HT-3 sample show the chemical composition near the interface between the substrate and the reaction layer. According to the position, the atomic ratios in the HT-1 and HT-3 samples are similar to those at Position 5 and at Position 2 in the EDS results shown in
Figure 2. Therefore, no evidence for the cause of the surface peeling behavior was drawn from the EDS measurements. Meanwhile, the atomic ratio of Ta slightly increased in both samples compared to that in the sample before the thermal cycle. This is attributed to Ta diffusion from the substrate to the surface region during the thermal cycle at a high temperature of ~1450 °C. The Ta diffusion would be more dynamic in the HT-3 sample than in the HT-1 sample because of the higher Ta content in the substrate region. Nevertheless, the outward diffusion of Ta and Hf may be insignificant in both samples considering the slight compositional changes in the samples before and after the thermal cycle. This secures chemical integrity, which is a benefit in the practical use of the material in a sintering crucible.
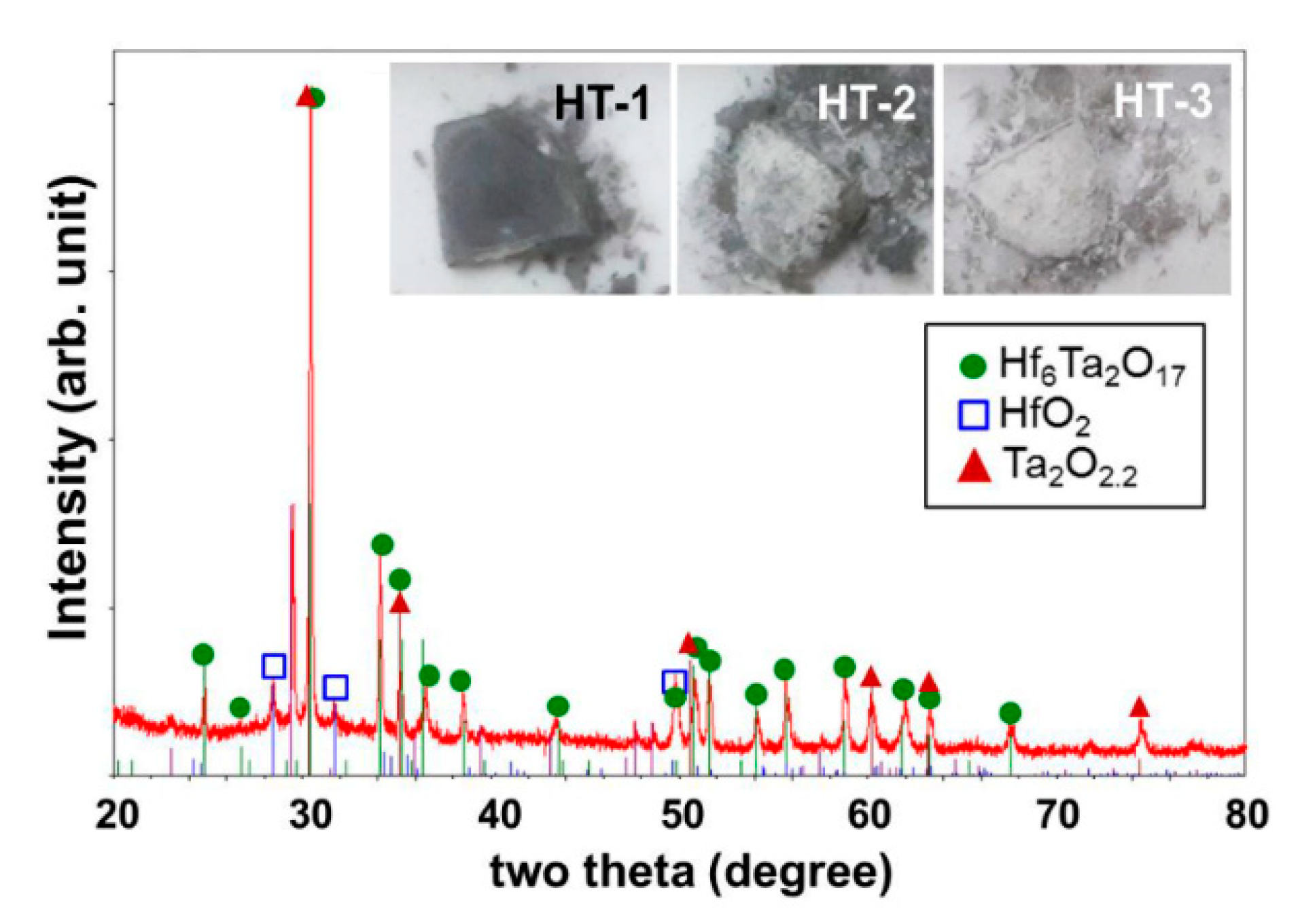
Figure 7 shows the XRD pattern of the residual powder collected from the HT-3 sample after the thermal cycle. The inset micrographs show changes in the appearance of the samples. Note that only a small amount of residual powder was formed from the HT-1 sample with little change of color. On the other hand, a larger amount of residual powder was observed in the HT-2 and HT-3 samples with significant color changes of the sample surface. In contrast to the HT-2 sample with partial color change, the HT-3 sample color was uniformly white. This color change behavior may be evidence of phase transformation from the original Hf
6Ta
2O
17 phase to other phases of brighter color. To examine the constituent phases, the residual powder was collected from the HT-3 sample and analyzed by XRD, as shown in
Figure 7. The measured pattern reveals that the residual powder contained Hf
6Ta
2O
17 as a major constituent; that is, the superstructure still remained after the thermal cycle at 1450 °C. However, Hf and Ta were observed as separate phases distinguished by other peaks. This gave evidence of a phase transformation from the Hf-Ta-O superstructure to each separated oxide form, such as Hf-O and Ta-O. The color changes also support this phase separation, considering that the colors of HfO
2 and Ta
2O
5 are bright (e.g., white-like), as revealed in the inset photographs in
Figure 7.
In addition, this phase change would cause the different peeling behaviors of the samples shown in
Figure 5 and
Figure 6. It is worth noting that the chemical form of Ta oxide is Ta
2O
2.2 rather than Ta
2O
5, as presented in the XRD results. This reduction should occur by hydrogen during the thermal cycle. Provided that phase separation occurs from the Hf
6Ta
2O
17 superstructure, the residual powders are expected to be in the form of HfO
2 and Ta
2O
5. However, in the XRD analysis, the Ta oxide appears to be slightly reduced to Ta
2O
2.2, while the Hf oxide form is maintained. Due to the lack of thermodynamic information on Ta oxides, it is difficult to precisely calculate the oxygen potential leading to the reduction process. Instead, the observed reduction form empirically shows that the Ta oxide can be reduced under the sintering conditions. More importantly, the reduction of Ta oxide would give rise to breakage of the crystal structure, resulting in the different peeling behavior. The crystal structure of Ta
2O
5 is known to be hexagonal, while Ta
2O
2.2 is known to be monoclinic [
13]. This difference in crystal structure leading to different lattice constants would result in severe lattice distortion and may finally cause destruction of the lattice; i.e., phase-transformation-induced peeling behavior was expected to occur. Accordingly, the amount of reduced Ta oxide affects the degree of lattice distortion; in other words, a higher Ta content facilitates lattice destruction due to reduction. It is therefore expected that the degree of lattice distortion should be mild in the HT-1 sample compared to the distortion in the HT-2 and HT-3 samples. This is supported empirically by the peeling behaviors: note that the outermost layer was maintained at the surface of the HT-1 sample, whereas it peeled off in the HT-2 and HT-3 samples. Based on this, to minimize phase-transformation-induced destruction during thermal cycling in a reducing atmosphere, either the Ta content should be lowered or Ta reduction should be suppressed. As in the case of the sintering in pyroprocessing, if the oxygen potential during thermal cycling is expected to be sufficiently low, resulting in a reduction of Ta oxide into a lower oxidation state (e.g., Ta
2O
2.2), lowering the initial Ta content should be effective. In this case, the Ta content should be in the range allowing the Hf
6Ta
2O
17 superstructure to be formed.