Exopolysaccharides from Cyanobacteria: Strategies for Bioprocess Development
Abstract
1. Introduction
2. EPS from Cyanobacteria
2.1. Ecology
2.2. Chemodiversity
3. Cyanobacterial Bioprocess (Cyano-EPS) Development
3.1. Strain Selection
3.2. Production and Optimization
3.2.1. Culture Medium
3.2.2. Process Conditions
3.2.3. Modes of Cultivation and PBR Design
3.2.4. Design of Experiments
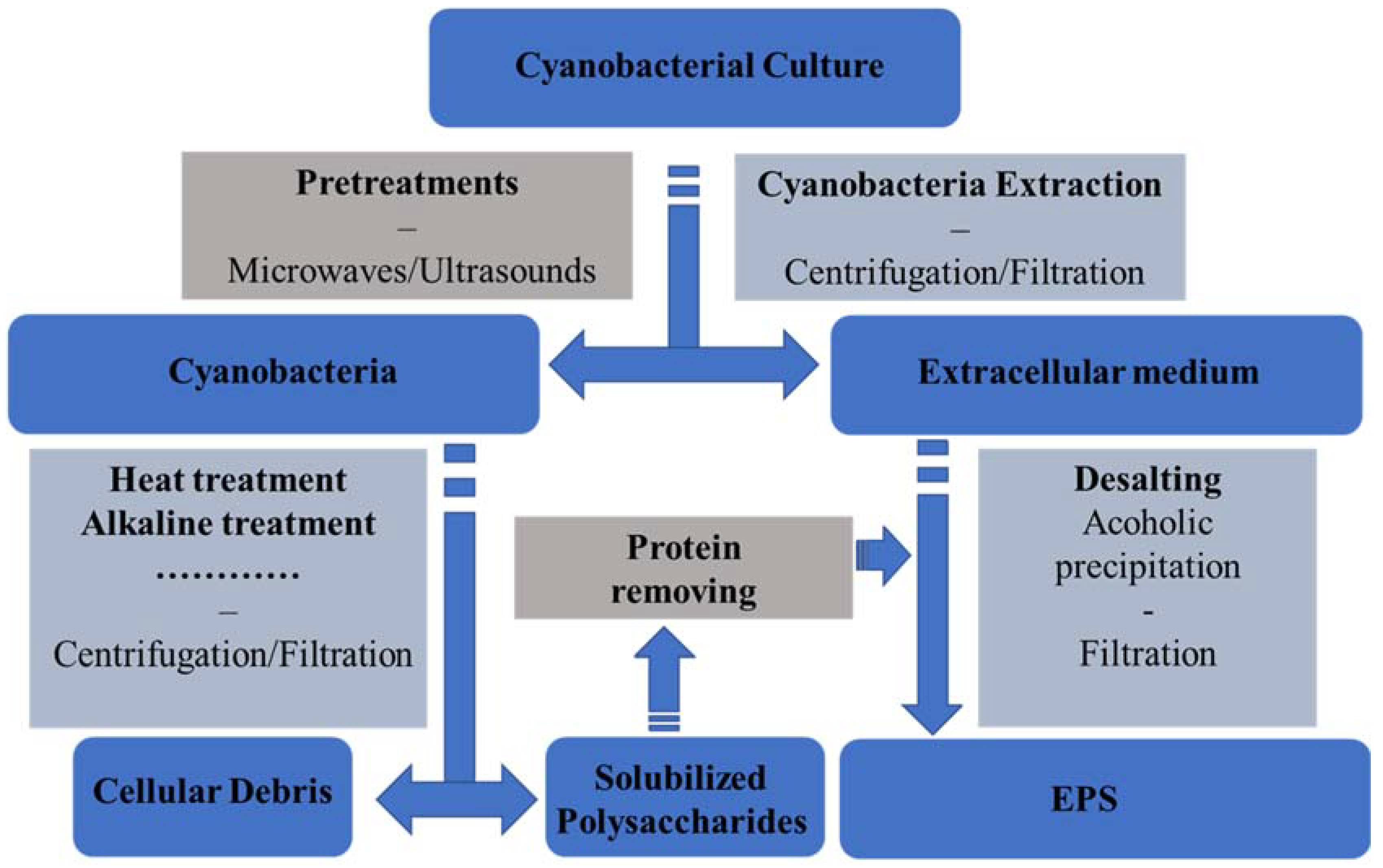
3.3. Downstream Processes and Cyano-EPS Global Market
4. Considerations for Cyano-EPS Development
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Michaud, P. Polysaccharides from Microalgae, what’s future? Adv. Biotechnol. Microbiol. 2018, 8. [Google Scholar] [CrossRef]
- Freitas, F.; Torres, C.A.V.; Reis, M.A.M. Engineering aspects of microbial exopolysaccharide production. Bioresour. Technol. 2017, 245, 1674–1683. [Google Scholar] [CrossRef] [PubMed]
- Barcelos, M.C.S.; Vespermann, K.A.C.; Pelissari, F.M.; Molina, G. Current status of biotechnological production and applications of microbial exopolysaccharides. Crit. Rev. Food Sci. Nutr. 2019, 60, 1–21. [Google Scholar]
- Kamravamanesh, D.; Lackner, M.; Herwig, C. Bioprocess engineering aspects of sustainable polyhydroxyalkanoate production in Cyanobacteria. Bioengineering 2018, 5, 111. [Google Scholar] [CrossRef] [PubMed]
- Wijffels, R.H.; Barbosa, M.J. An outlook on microalgal biofuels. Science 2010, 329, 796–799. [Google Scholar] [CrossRef]
- Okajima, M.K.; Sornkamnerd, S.; Kaneko, T. Development of functional bionanocomposites using cyanobacterial polysaccharides. Chem. Rec. 2018, 18, 1167–1177. [Google Scholar] [CrossRef]
- Ramos, V.; Morais, J.; Castelo-Branco, R.; Pinheiro, Â.; Martins, J.; Regueiras, A.; Pereira, A.L.; Lopes, V.R.; Frazão, B.; Gomes, D. Cyanobacterial diversity held in microbial biological resource centers as a biotechnological asset: The case study of the newly established LEGE culture collection. J. Appl. Phycol. 2018, 30, 1437–1451. [Google Scholar]
- Hays, S.G.; Ducat, D.C. Engineering cyanobacteria as photosynthetic feedstock factories. Photosynth. Res. 2015, 123, 285–295. [Google Scholar] [CrossRef]
- Pereira, S.; Zille, A.; Micheletti, E.; Moradas-Ferreira, P.; De Philippis, R.; Tamagnini, P. Complexity of cyanobacterial exopolysaccharides: Composition, structures, inducing factors and putative genes involved in their biosynthesis and assembly. FEMS Microbiol. Rev. 2009, 33, 917–941. [Google Scholar]
- Delattre, C.; Pierre, G.; Laroche, C.; Michaud, P. Production, extraction and characterization of microalgal and cyanobacterial exopolysaccharides. Biotechnol. Adv. 2016, 34, 1159–1179. [Google Scholar] [CrossRef]
- Han, P.; Sun, Y.; Wu, X.; Yuan, Y.; Dai, Y.; Jia, S. Emulsifying, flocculating, and physicochemical properties of exopolysaccharide produced by cyanobacterium Nostoc flagelliforme. Appl. Biochem. Biotechnol. 2014, 172, 36–49. [Google Scholar] [CrossRef] [PubMed]
- Roncero-Ramos, B.; Román, J.R.; Gómez-Serrano, C.; Cantón, Y.; Acién, F.G. Production of a biocrust-cyanobacteria strain (Nostoc commune) for large-scale restoration of dryland soils. J. Appl. Phycol. 2019, 31, 2217–2230. [Google Scholar]
- Xu, Y.; Rossi, F.; Colica, G.; Deng, S.; De Philippis, R.; Chen, L. Use of cyanobacterial polysaccharides to promote shrub performances in desert soils: A potential approach for the restoration of desertified areas. Biol. Fertil. Soils 2013, 49, 143–152. [Google Scholar] [CrossRef]
- Wang, H.-B.; Wu, S.-J.; Liu, D. Preparation of polysaccharides from cyanobacteria Nostoc commune and their antioxidant activities. Carbohydr. Polym. 2014, 99, 553–555. [Google Scholar]
- Gudmundsdottir, A.B.; Omarsdottir, S.; Brynjolfsdottir, A.; Paulsen, B.S.; Olafsdottir, E.S.; Freysdottir, J. Exopolysaccharides from cyanobacterium aponinum from the Blue Lagoon in Iceland increase IL-10 secretion by human dendritic cells and their ability to reduce the IL-17 + RORγt + /IL-10 + FoxP3 + ratio in CD4 + T cells. Immunol. Lett. 2015, 163, 157–162. [Google Scholar] [CrossRef]
- Hussein, M.H.; Abou-ElWafa, G.S.; Shaaban-Dessuuki, S.A.; Hassan, N.I. Characterization and antioxidant activity of exopolysaccharide secreted by Nostoc carneum. Int. J. Pharm. 2015, 11, 432–439. [Google Scholar]
- Li, H.; Su, L.; Chen, S.; Zhao, L.; Wang, H.; Ding, F.; Chen, H.; Shi, R.; Wang, Y.; Huang, Z. Physicochemical characterization and functional analysis of the Polysaccharide from the edible microalga nostoc sphaeroides. Molecules 2018, 23, 508. [Google Scholar] [CrossRef]
- Bellini, E.; Ciocci, M.; Savio, S.; Antonaroli, S.; Seliktar, D.; Melino, S.; Congestri, R. Trichormus variabilis (Cyanobacteria) biomass: From the nutraceutical products to novel EPS-cell/protein carrier systems. Mar. Drugs 2018, 16, 298. [Google Scholar]
- Leite, J.P.; Mota, R.; Durão, J.; Neves, S.C.; Barrias, C.C.; Tamagnini, P.; Gales, L. Cyanobacterium-derived extracellular carbohydrate polymer for the controlled delivery of functional proteins. Macromol. Biosci. 2017, 17, 1600206. [Google Scholar] [CrossRef]
- Bhunia, B.; Uday, U.S.P.; Oinam, G.; Mondal, A.; Bandyopadhyay, T.K.; Tiwari, O.N. Characterization, genetic regulation and production of cyanobacterial exopolysaccharides and its applicability for heavy metal removal. Carbohydr. Polym. 2018, 179, 228–243. [Google Scholar] [CrossRef]
- Chamizo, S.; Mugnai, G.; Rossi, F.; Certini, G.; De Philippis, R. Cyanobacteria inoculation improves soil stability and fertility on different textured soils: Gaining insights for applicability in soil restoration. Front. Environ. Sci. 2018, 6, 49. [Google Scholar]
- Li, P.; Harding, S.E.; Liu, Z. Cyanobacterial exopolysaccharides: Their nature and potential biotechnological applications. Biotechnol. Genet. Eng. Rev. 2001, 18, 375–404. [Google Scholar] [CrossRef] [PubMed]
- Rossi, F.; De Philippis, R. Role of cyanobacterial exopolysaccharides in phototrophic biofilms and in complex microbial mats. Life 2015, 5, 1218–1238. [Google Scholar] [PubMed]
- Cui, L.; Xu, H.; Zhu, Z.; Gao, X. The effects of the exopolysaccharide and growth rate on the morphogenesis of the terrestrial filamentous cyanobacterium Nostoc flagelliforme. Biol. Open 2017, 6, 1329–1335. [Google Scholar]
- Sciuto, K.; Moro, I. Cyanobacteria: The bright and dark sides of a charming group. Biodivers. Conserv. 2015, 24, 711–738. [Google Scholar]
- Myklestad, S.M. Release of extracellular products by phytoplankton with special emphasis on polysaccharides. Sci. Total Environ. 1995, 165, 155–164. [Google Scholar] [CrossRef]
- Rossi, F.; De Philippis, R. Exocellular polysaccharides in Microalgae and Cyanobacteria: Chemical features, role and enzymes and genes involved in their biosynthesis. In the Physiology of Microalgae; Springer: Berlin/Heidelberg, Germany, 2016; pp. 565–590. [Google Scholar]
- Ahmed, M.; Moerdijk-Poortvliet, T.C.W.; Wijnholds, A.; Stal, L.J.; Hasnain, S. Isolation, characterization and localization of extracellular polymeric substances from the cyanobacterium Arthrospira platensis strain MMG-9. Eur. J. Phycol. 2014, 49, 143–150. [Google Scholar]
- Pathak, J.; Rajneesh, R.; Sonker, A.S.; Kannaujiya, V.K.; Sinha, R.P. Cyanobacterial extracellular polysaccharide sheath pigment, scytonemin: A novel multipurpose pharmacophore. In Marine Glycobiology; CRC Press: Boca Raton, FL, USA, 2016; pp. 343–358. [Google Scholar]
- Rossi, F.; Mugnai, G.; De Philippis, R. Complex role of the polymeric matrix in biological soil crusts. Plant Soil 2018, 429, 1–16. [Google Scholar]
- Mota, R.; Vidal, R.; Pandeirada, C.; Flores, C.; Adessi, A.; De Philippis, R.; Nunes, C.; Coimbra, M.A.; Tamagnini, P. Cyanoflan: A cyanobacterial sulfated carbohydrate polymer with emulsifying properties. Carbohydr. Polym. 2019, 229, 115525. [Google Scholar] [CrossRef]
- Ngatu, N.R.; Okajima, M.K.; Yokogawa, M.; Hirota, R.; Eitoku, M.; Muzembo, B.A.; Dumavibhat, N.; Takaishi, M.; Sano, S.; Kaneko, T. Anti-inflammatory effects of sacran, a novel polysaccharide from Aphanothece sacrum, on 2, 4, 6-trinitrochlorobenzene–induced allergic dermatitis in vivo. Ann. Allergy Asthma Immunol. 2012, 108, 117–122. [Google Scholar] [CrossRef]
- Chi, Z.; Su, C.D.; Lu, W.D. A new exopolysaccharide produced by marine Cyanothece sp. 113. Bioresour. Technol. 2007, 98, 1329–1332. [Google Scholar] [CrossRef] [PubMed]
- Shah, V.; Ray, A.; Garg, N.; Madamwar, D. Characterization of the extracellular polysaccharide produced by a marine cyanobacterium, Cyanothece sp. ATCC 51142, and its exploitation toward metal removal from solutions. Curr. Microbiol. 2000, 40, 274–278. [Google Scholar] [CrossRef] [PubMed]
- Helm, R.F.; Huang, Z.; Edwards, D.; Leeson, H.; Peery, W.; Potts, M. Structural characterization of the released polysaccharide of desiccation-tolerant Nostoc communeDRH-1. J. Bacteriol. 2000, 182, 974–982. [Google Scholar] [CrossRef] [PubMed]
- Kanekiyo, K.; Lee, J.-B.; Hayashi, K.; Takenaka, H.; Hayakawa, Y.; Endo, S.; Hayashi, T. Isolation of an antiviral polysaccharide, nostoflan, from a terrestrial cyanobacterium, Nostoc f lagelliforme. J. Nat. Prod. 2005, 68, 1037–1041. [Google Scholar] [CrossRef] [PubMed]
- Volk, R.-B.; Venzke, K.; Blaschek, W. Structural investigation of a polysaccharide released by the cyanobacterium Nostoc insulare. J. Appl. Phycol. 2007, 19, 255. [Google Scholar] [CrossRef]
- Liu, Y.; Su, P.; Xu, J.; Chen, S.; Zhang, J.; Zhou, S.; Wang, Y.; Tang, Q.; Wang, Y. Structural characterization of a bioactive water-soluble heteropolysaccharide from Nostoc sphaeroids kütz. Carbohydr. Polym. 2018, 200, 552–559. [Google Scholar] [CrossRef]
- Hayashi, T.; Hayashi, K.; Maeda, M.; Kojima, I. Calcium spirulan, an inhibitor of enveloped virus replication, from a blue-green alga Spirulina platensis. J. Nat. Prod. 1996, 59, 83–87. [Google Scholar] [CrossRef]
- De Philippis, R.; Faraloni, C.; Margheri, M.C.; Sili, C.; Herdman, M.; Vincenzini, M. Morphological and biochemical characterization of the exocellular investments of polysaccharide-producing Nostoc strains from the pasteur culture collection. World J. Microbiol. Biotechnol. 2000, 16, 655–661. [Google Scholar] [CrossRef]
- Gaignard, C.; Laroche, C.; Pierre, G.; Dubessay, P.; Delattre, C.; Gardarin, C.; Gourvil, P.; Probert, I.; Dubuffet, A.; Michaud, P. Screening of marine microalgae: Investigation of new exopolysaccharide producers. Algal Res. 2019, 44, 101711. [Google Scholar] [CrossRef]
- Xu, H.; Cai, H.; Yu, G.; Jiang, H. Insights into extracellular polymeric substances of cyanobacterium Microcystis aeruginosa using fractionation procedure and parallel factor analysis. Water Res. 2013, 47, 2005–2014. [Google Scholar] [CrossRef]
- Mancuso-Nichols, C.A.; Nairn, K.M.; Glattauer, V.; Blackburn, S.I.; Ramshaw, J.A.M.; Graham, L.D. Screening microalgal cultures in search of microbial exopolysaccharides with potential as adhesives. J. Adhes. 2009, 85, 97–125. [Google Scholar] [CrossRef]
- Singh, S.; Das, S. Screening, production, optimization and characterization of cyanobacterial Polysaccharide. World J. Microbiol. Biotechnol. 2011, 27, 1971–1980. [Google Scholar]
- Uhliariková, I.; Chválová, B.; Matulová, M.; Cepák, V.; Lukavský, J.; Capek, P. Extracellular biopolymers produced by freshwater cyanobacteria: A screening study. Chem. Pap. 2019, 73, 771–776. [Google Scholar] [CrossRef]
- Zhang, Y.; Chi, Z.; Lu, W. Exopolysaccharide production by four cyanobacterial isolates and preliminary identification of these isolates. J. Ocean Univ. China 2007, 6, 147–152. [Google Scholar] [CrossRef]
- Tiwari, O.N.; Khangembam, R.; Shamjetshabam, M.; Sharma, A.S.; Oinam, G.; Brand, J.J. Characterization and optimization of bioflocculant exopolysaccharide production by Cyanobacteria Nostoc sp. BTA97 and Anabaena sp. BTA990 in culture conditions. Appl. Biochem. Biotechnol. 2015, 176, 1950–1963. [Google Scholar] [CrossRef] [PubMed]
- Khangembam, R.; Tiwari, O.; Kalita, M. Production of exopolysaccharides by the cyanobacterium Anabaena sp. BTA992 and application as bioflocculants. J. Appl. Biol. Biotechnol. 2016, 4, 8–11. [Google Scholar]
- Richert, L.; Golubic, S.; Le Guédès, R.; Ratiskol, J.; Payri, C.; Guezennec, J. Characterization of exopolysaccharides produced by Cyanobacteria isolated from Polynesian microbial mats. Curr. Microbiol. 2005, 51, 379–384. [Google Scholar] [CrossRef]
- Parikh, A.; Madamwar, D. Partial characterization of extracellular polysaccharides from Cyanobacteria. Bioresour. Technol. 2006, 97, 1822–1827. [Google Scholar]
- Di Pippo, F.; Ellwood, N.T.W.; Gismondi, A.; Bruno, L.; Rossi, F.; Magni, P.; De Philippis, R. Characterization of exopolysaccharides produced by seven biofilm-forming cyanobacterial strains for biotechnological applications. J. Appl. Phycol. 2013, 25, 1697–1708. [Google Scholar] [CrossRef]
- Ozturk, S.; Aslim, B. Modification of exopolysaccharide composition and production by three cyanobacterial isolates under salt stress. Environ. Sci. Pollut. Res. 2010, 17, 595–602. [Google Scholar] [CrossRef]
- Otero, A.; Vincenzini, M. Extracellular polysaccharide synthesis by Nostoc strains as affected by N source and light intensity. J. Biotechnol. 2003, 102, 143–152. [Google Scholar] [PubMed]
- Nicolaus, B.; Panico, A.; Lama, L.; Romano, I.; Manca, M.C.; De Giulio, A.; Gambacorta, A. Chemical composition and production of exopolysaccharides from representative members of heterocystous and non-heterocystous Cyanobacteria. Phytochemistry 1999, 52, 639–647. [Google Scholar] [CrossRef]
- Seviour, R.J.; McNeil, B.; Fazenda, M.L.; Harvey, L.M. Operating bioreactors for microbial exopolysaccharide production. Crit. Rev. Biotechnol. 2011, 31, 170–185. [Google Scholar]
- Mota, R.; Guimarães, R.; Büttel, Z.; Rossi, F.; Colica, G.; Silva, C.J.; Santos, C.; Gales, L.; Zille, A.; De Philippis, R. Production and characterization of extracellular carbohydrate polymer from Cyanothece sp. CCY 0110. Carbohydr. Polym. 2013, 92, 1408–1415. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Jia, S.; Dai, Y. Accumulation of exopolysaccharides in liquid suspension culture of Nostoc flagelliforme cells. Appl. Biochem. Biotechnol. 2010, 160, 552–560. [Google Scholar] [PubMed]
- Lama, L.; Nicolaus, B.; Calandrelli, V.; Manca, M.C.; Romano, I.; Gambacorta, A. Effect of growth conditions on endo-and exopolymer biosynthesis in Anabaena cylindrica 10 C. Phytochemistry 1996, 42, 655–659. [Google Scholar] [CrossRef]
- Khattar, J.I.S.; Singh, D.P.; Jindal, N.; Kaur, N.; Singh, Y.; Rahi, P.; Gulati, A. Isolation and characterization of exopolysaccharides produced by the cyanobacterium limnothrix redekei PUPCCC 116. Appl. Biochem. Biotechnol. 2010, 162, 1327–1338. [Google Scholar] [CrossRef]
- Jindal, N.; Singh, D.P.; Khattar, J.I.S. Kinetics and physico-chemical characterization of exopolysaccharides produced by the cyanobacterium Oscillatoria formosa. World J. Microbiol. Biotechnol. 2011, 27, 2139–2146. [Google Scholar]
- Han, P.; Shen, S.; Wang, H.-Y.; Yao, S.; Tan, Z.; Zhong, C.; Jia, S. Applying the strategy of light environment control to improve the biomass and polysaccharide production of Nostoc flagelliforme. J. Appl. Phycol. 2017, 29, 55–65. [Google Scholar] [CrossRef]
- Borah, D.; Nainamalai, S.; Gopalakrishnan, S.; Rout, J.; Alharbi, N.S.; Alharbi, S.A.; Nooruddin, T. Biolubricant potential of exopolysaccharides from the cyanobacterium Cyanothece epiphytica. Appl. Microbiol. Biotechnol. 2018, 102, 3635–3647. [Google Scholar]
- Han, P.; Yao, S.; Guo, R.; Yan, R.; Wu, Y.; Shen, S.; Jia, S. Influence of culture conditions on extracellular polysaccharide production and the activities of enzymes involved in the polysaccharide synthesis of Nostoc flagelliforme. RSC Adv. 2017, 7, 45075–45084. [Google Scholar] [CrossRef]
- Phlips, E.J.; Zeman, C.; Hansen, P. Growth, photosynthesis, nitrogen fixation and carbohydrate production by a unicellular cyanobacterium, Synechococcus sp.(Cyanophyta). J. Appl. Phycol. 1989, 1, 137–145. [Google Scholar] [CrossRef]
- Su, C.; Chi, Z.; Lu, W. Optimization of medium and cultivation conditions for enhanced exopolysaccharide yield by marine Cyanothece sp. 113. Chin. J. Oceanol. Limnol. 2007, 25, 411–417. [Google Scholar]
- Vergnes, J.-B.; Gernigon, V.; Guiraud, P.; Formosa-Dague, C. Bicarbonate concentration induces production of exopolysaccharides by Arthrospira platensis that mediate bioflocculation and enhance flotation harvesting efficiency. Acs Sustain. Chem. Eng. 2019, 7, 13796–13804. [Google Scholar]
- Shen, S.; Jia, S.; Wu, Y.; Yan, R.; Lin, Y.-H.; Zhao, D.; Han, P. Effect of culture conditions on the physicochemical properties and antioxidant activities of polysaccharides from Nostoc flagelliforme. Carbohydr. Polym. 2018, 198, 426–433. [Google Scholar] [CrossRef]
- Trabelsi, L.; Ouada, H.B.; Zili, F.; Mazhoud, N.; Ammar, J. Evaluation of Arthrospira platensis extracellular polymeric substances production in photoautotrophic, heterotrophic and mixotrophic conditions. Folia Microbiol. 2013, 58, 39–45. [Google Scholar] [CrossRef]
- Bemal, S.; Anil, A.C. Effects of salinity on cellular growth and exopolysaccharide production of freshwater Synechococcus strain CCAP1405. J. Plankton Res. 2017, 40, 46–58. [Google Scholar]
- Moreno, J.; Vargas, M.A.; Olivares, H.; Rivas, J.; Guerrero, M.G. Exopolysaccharide production by the cyanobacterium Anabaena sp. ATCC 33047 in batch and continuous culture. J. Biotechnol. 1998, 60, 175–182. [Google Scholar] [CrossRef]
- Kamennaya, N.A.; Zemla, M.; Mahoney, L.; Chen, L.; Holman, E.; Holman, H.-Y.; Auer, M.; Ajo-Franklin, C.M.; Jansson, C. High p CO2-induced exopolysaccharide-rich ballasted aggregates of planktonic cyanobacteria could explain paleoproterozoic carbon burial. Nat. Commun. 2018, 9, 2116. [Google Scholar]
- Tan, N.; Jia, S.R.; Han, P.P.; Guo, W.; Dai, Y.J. The open culture of Nostoc flagelliforme with a 25 L open pond. In Advanced Materials Research; Trans Tech Publications Ltd.: Bäch, Switzerland, 2012; Volume 554, pp. 1009–1012. [Google Scholar]
- Xiao, Y.; Li, Z.; Li, C.; Zhang, Z.; Guo, J. Effect of small-scale turbulence on the physiology and morphology of two bloom-forming cyanobacteria. PLoS ONE 2016, 11, e0168925. [Google Scholar] [CrossRef]
- Fadlallah, H.; Jarrahi, M.; Herbert, E.; Ferrari, R.; Mejean, A.; Peerhossaini, H. Effects of shear stress on the growth rate of micro-organisms in agitated reactors. In Proceedings of the ASME 2016 Fluids Engineering Division Summer Meeting collocated with the ASME 2016 Heat Transfer Summer Conference and the ASME 2016 14th International Conference on Nanochannels, Microchannels, and Minichannels, Washington, DC, USA, 10–14 July 2016. [Google Scholar]
- Trabelsi, L.; M’sakni, N.H.; Ouada, H.B.; Bacha, H.; Roudesli, S. Partial characterization of extracellular polysaccharides produced by cyanobacterium Arthrospira platensis. Biotechnol. Bioprocess Eng. 2009, 14, 27–31. [Google Scholar] [CrossRef]
- Gris, B.; Sforza, E.; Morosinotto, T.; Bertucco, A.; La Rocca, N. Influence of light and temperature on growth and high-value molecules productivity from Cyanobacterium aponinum. J. Appl. Phycol. 2017, 29, 1781–1790. [Google Scholar]
- Kvíderová, J.; Kumar, D.; Lukavský, J.; Kaštánek, P.; Adhikary, S.P. Estimation of growth and exopolysaccharide production by two soil Cyanobacteria, Scytonema tolypothrichoides and Tolypothrix bouteillei as determined by cultivation in irradiance and temperature crossed gradients. Eng. Life Sci. 2019, 19, 184–195. [Google Scholar] [CrossRef]
- Werner, A.; Broeckling, C.D.; Prasad, A.; Peebles, C.A.M. A comprehensive time-course metabolite profiling of the model cyanobacterium Synechocystis sp. PCC 6803 under diurnal light: Dark cycles. Plant J. 2019, 99, 379–388. [Google Scholar] [PubMed]
- Han, P.; Sun, Y.; Jia, S.; Zhong, C.; Tan, Z. Effects of light wavelengths on extracellular and capsular polysaccharide production by Nostoc flagelliforme. Carbohydr. Polym. 2014, 105, 145–151. [Google Scholar] [CrossRef]
- Han, P.; Shen, S.; Wang, H.-Y.; Sun, Y.; Dai, Y.; Jia, S. Comparative metabolomic analysis of the effects of light quality on polysaccharide production of cyanobacterium Nostoc flagelliforme. Algal Res. 2015, 9, 143–150. [Google Scholar] [CrossRef]
- Han, P.; Yao, S.; Guo, R.; Shen, S.; Yan, R.; Tan, Z.; Jia, S. The relationship between monosaccharide composition of extracellular polysaccharide and activities of related enzymes in Nostoc flagelliforme under different culture conditions. Carbohydr. Polym. 2017, 174, 111–119. [Google Scholar]
- Han, P.; Guo, R.; Shen, S.; Yan, R.; Wu, Y.; Yao, S.; Wang, H.; Jia, S. Proteomic profiling of Nostoc flagelliforme reveals the common mechanism in promoting polysaccharide production by different light qualities. Biochem. Eng. J. 2018, 132, 68–78. [Google Scholar]
- Han, P.; Shen, S.; Guo, R.; Zhao, D.; Lin, Y.-H.; Jia, S.; Yan, R.; Wu, Y. ROS Is a Factor regulating the increased polysaccharide production by light quality in the edible cyanobacterium Nostoc flagelliforme. J. Agric. Food Chem. 2019, 67, 2235–2244. [Google Scholar] [CrossRef]
- Shen, S.-G.; Lin, Y.-H.; Zhao, D.-X.; Wu, Y.-K.; Yan, R.-R.; Zhao, H.-B.; Tan, Z.-L.; Jia, S.-R.; Han, P.-P. Comparisons of functional properties of polysaccharides from Nostoc flagelliforme under three culture conditions. Polymers 2019, 11, 263. [Google Scholar] [CrossRef]
- Ge, H.; Xia, L.; Zhou, X.; Zhang, D.; Hu, C. Effects of light intensity on components and topographical structures of extracellular polysaccharides from the cyanobacteria Nostoc sp. J. Microbiol. 2014, 52, 179–183. [Google Scholar] [CrossRef] [PubMed]
- Phélippé, M.; Gonçalves, O.; Thouand, G.; Cogne, G.; Laroche, C. Characterization of the polysaccharides chemical diversity of the Cyanobacteria Arthrospira platensis. Algal Res. 2019, 38, 101426. [Google Scholar] [CrossRef]
- Ho, S.-H.; Liao, J.-F.; Chen, C.-Y.; Chang, J.-S. Combining light strategies with recycled medium to enhance the economic feasibility of phycocyanin production with Spirulina platensis. Bioresour. Technol. 2018, 247, 669–675. [Google Scholar] [CrossRef] [PubMed]
- Pagels, F.; Lopes, G.; Vasconcelos, V.; Guedes, A.C. White and red LEDs as two-phase batch for cyanobacterial pigments production. Bioresour. Technol. 2020, 123105. [Google Scholar] [CrossRef] [PubMed]
- Chentir, I.; Hamdi, M.; Doumandji, A.; HadjSadok, A.; Ouada, H.B.; Nasri, M.; Jridi, M. Enhancement of extracellular polymeric substances (EPS) production in Spirulina (Arthrospira sp.) by two-step cultivation process and partial characterization of their polysaccharidic moiety. Int. J. Biol. Macromol. 2017, 105, 1412–1420. [Google Scholar] [CrossRef]
- De Jesus, C.S.; de Jesus-Assis, D.; Rodriguez, M.B.; Menezes Filho, J.A.; Costa, J.A.V.; de Souza Ferreira, E.; Druzian, J.I. Pilot-scale isolation and characterization of extracellular polymeric substances (EPS) from cell-free medium of Spirulina sp. LEB-18 cultures under outdoor conditions. Int. J. Biol. Macromol. 2019, 124, 1106–1114. [Google Scholar] [CrossRef]
- Abe, A.; Ohashi, E.; Ren, H.; Hayashi, T.; Endo, H. Isolation and characterization of a cold-induced nonculturable suppression mutant of Vibrio vulnificus. Microbiol. Res. 2007, 162, 130–138. [Google Scholar] [CrossRef]
- Chen, H.-W.; Yang, T.-S.; Chen, M.-J.; Chang, Y.-C.; Lin, C.-Y.; Eugene, I.; Wang, C.; Ho, C.-L.; Huang, K.-M.; Yu, C.-C. Application of power plant flue gas in a photobioreactor to grow Spirulina algae, and a bioactivity analysis of the algal water-soluble polysaccharides. Bioresour. Technol. 2012, 120, 256–263. [Google Scholar] [CrossRef]
- Tredici, M.R. Photobiology of microalgae mass cultures: Understanding the tools for the next green revolution. Biofuels 2010, 1, 143–162. [Google Scholar] [CrossRef]
- Tiwari, O.N.; Chakraborty, S.; Devi, I.; Mondal, A.; Bhunia, B.; Boxiong, S. Bioprocess parameters of production of cyanobacterial exopolysaccharide: Biomass production and product recovery. In Handbook of Algal Technologies and Phytochemicals; CRC Press: Boca Raton, FL, USA, 2019; pp. 25–32. [Google Scholar]
- Pierre, G.; Delattre, C.; Dubessay, P.; Jubeau, S.; Vialleix, C.; Cadoret, J.-P.; Probert, I.; Michaud, P. What is in store for EPS Microalgae in the next decade? Molecules 2019, 24, 4296. [Google Scholar] [CrossRef]
- Fischer, D.; Schlösser, U.G.; Pohl, P. Exopolysaccharide production by cyanobacteria grown in closed photobioreactors and immobilized using white cotton towelling. J. Appl. Phycol. 1997, 9, 205–213. [Google Scholar] [CrossRef]
- Araujo, P.W.; Bereton, R.G. Experimental design II: Optimization. TrAC Trend. Anal. Chem. 1996, 15, 5–6. [Google Scholar] [CrossRef]
- Trabelsi, L.; Ouada, H.B.; Bacha, H.; Ghoul, M. Combined effect of temperature and light intensity on growth and extracellular polymeric substance production by the cyanobacterium Arthrospira platensis. J. Appl. Phycol. 2009, 21, 405–412. [Google Scholar] [CrossRef]
- De Philippis, R.; Sili, C.; Vincenzini, M. Response of an exopolysaccharide-producing heterocystous cyanobacterium to changes in metabolic carbon flux. J. Appl. Phycol. 1996, 8, 275–281. [Google Scholar]
- Ge, H.; Zhang, J.; Zhou, X.; Xia, L.; Hu, C. Effects of light intensity on components and topographical structures of extracellular polymeric substances from Microcoleus vaginatus (Cyanophyceae). Phycologia 2014, 53, 167–173. [Google Scholar]
- Wijffels, R.H.; Kruse, O.; Hellingwerf, K.J. Potential of industrial biotechnology with cyanobacteria and eukaryotic microalgae. Curr. Opin. Biotechnol. 2013, 24, 405–413. [Google Scholar] [CrossRef]
- De Philippis, R.; Vincenzini, M. Outermost polysaccharidic investments of cyanobacteria: Nature, significance and possible applications. Recent Res. Dev. Microbiol. 2003, 7, 13–22. [Google Scholar]
- Adhikary, S.P. Polysaccharides from mucilaginous envelope layers of Cyanobacteria and their ecological significance. J. Sci. Ind. Res. 1998, 57, 454–466. [Google Scholar]
- Bertocchi, C.; Navarini, L.; Cesàro, A.; Anastasio, M. Polysaccharides from Cyanobacteria. Carbohydr. Polym. 1990, 12, 127–153. [Google Scholar]
- Kumar, D.; Kastanek, P.; Adhikary, S.P. Exopolysaccharides from cyanobacteria and microalgae and their commercial application. Curr. Sci. 2018, 115, 234. [Google Scholar]
- Patel, A.K.; Laroche, C.; Marcati, A.; Ursu, A.V.; Jubeau, S.; Marchal, L.; Petit, E.; Djelveh, G.; Michaud, P. Separation and fractionation of exopolysaccharides from Porphyridium cruentum. Bioresour. Technol. 2013, 145, 345–350. [Google Scholar] [CrossRef] [PubMed]
- Dubois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.T.; Smith, F. Colorimetric method for determination of sugars and related substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar]
- De Philippis, R.; Vincenzini, M. Exocellular polysaccharides from Cyanobacteria and their possible applications. Fems Microbiol. Rev. 1998, 22, 151–175. [Google Scholar] [CrossRef]
- Kurd, F.; Samavati, V. Water soluble polysaccharides from Spirulina platensis: Extraction and in vitro anti-cancer activity. Int. J. Biol. Macromol. 2015, 74, 498–506. [Google Scholar] [CrossRef]
- De Silva, A.S.; de Magalhaes, W.T.; Moreira, L.M.; Rocha, M.V.P.; Bastos, A.K.P. Microwave-assisted extraction of polysaccharides from Arthrospira (Spirulina) platensis using the concept of green chemistry. Algal Res. 2018, 35, 178–184. [Google Scholar] [CrossRef]
- Rodriguez, S.; Torres, F.G.; López, D. Preparation and characterization of polysaccharide films from the cyanobacteria Nostoc commune. Polym. Renew. Resour. 2017, 8, 133–150. [Google Scholar]
- Pignolet, O.; Jubeau, S.; Vaca-Garcia, C.; Michaud, P. Highly valuable microalgae: Biochemical and topological aspects. J. Ind. Microbiol. Biotechnol. 2013, 40, 781–796. [Google Scholar]
- Fattom, A.; Shilo, M. Production of emulcyan by phormidium J-1: Its activity and function. FEMS Microbiol. Lett. 1985, 31, 3–9. [Google Scholar] [CrossRef]
- Kanekiyo, K.; Hayashi, K.; Takenaka, H.; Lee, J.-B.; Hayashi, T. Anti-herpes simplex virus target of an acidic polysaccharide, nostoflan, from the edible blue-green alga Nostoc flagelliforme. Biol. Pharm. Bull. 2007, 30, 1573–1575. [Google Scholar] [CrossRef]
- Patel, S.; Goyal, A. Current and prospective insights on food and pharmaceutical applications of spirulina. Curr. Trends Biotechnol. Pharm. 2013, 7, 681–695. [Google Scholar]
- Fresewinkel, M.; Rosello, R.; Wilhelm, C.; Kruse, O.; Hankamer, B.; Posten, C. Integration in microalgal bioprocess development: Design of efficient, sustainable, and economic processes. Eng. Life Sci. 2014, 14, 560–573. [Google Scholar]
- Schipper, K.; Al Muraikhi, M.; Alghasal, G.S.H.S.; Saadaoui, I.; Bounnit, T.; Rasheed, R.; Dalgamouni, T.; Al Jabri, H.M.S.J.; Wijffels, R.H.; Barbosa, M.J. Potential of novel desert microalgae and cyanobacteria for commercial applications and CO2 sequestration. J. Appl. Phycol. 2019, 1–13. [Google Scholar]
- Schulze, P.S.C.; Hulatt, C.J.; Morales-Sánchez, D.; Wijffels, R.H.; Kiron, V. Fatty acids and proteins from marine cold adapted microalgae for biotechnology. Algal Res. 2019, 42, 101604. [Google Scholar] [CrossRef]
- Pereira, S.B.; Sousa, A.; Santos, M.; Araújo, M.; Serôdio, F.; Granja, P.; Tamagnini, P. Strategies to Obtain designer polymers based on cyanobacterial extracellular polymeric substances (EPS). Int. J. Mol. Sci. 2019, 20, 5693. [Google Scholar] [CrossRef] [PubMed]
- Deschoenmaeker, F.; Bayon-Vicente, G.; Sachdeva, N.; Depraetere, O.; Pino, J.C.C.; Leroy, B.; Muylaert, K.; Wattiez, R. Impact of different nitrogen sources on the growth of Arthrospira sp. PCC 8005 under batch and continuous cultivation-A biochemical, transcriptomic and proteomic profile. Bioresour. Technol. 2017, 237, 78–88. [Google Scholar] [CrossRef]
- Dorina, S.; Judith, S.; Björn, W.; Julia, S.; Andrea, S.; Muffler, K.; Roland, U. A new strategy for a combined isolation of EPS and pigments from Cyanobacteria. J. Appl. Phycol. 2020, 35, 1–12. [Google Scholar] [CrossRef]
| Strain | EPS Fraction | Compositional Monosaccharides | Non-Carbohydrates Substituents | EPS Characteristic | Reference |
|---|---|---|---|---|---|
| Aphanothece sacrum | CPS | Glc, Gal, Man, Xyl, Rha, Fuc, GalA, GlcA | sulfate and carboxyl groups | anti-inflammatory; anti-allergic; adsorption of metal ions; liquid crystallization | [32] |
| Cyanothece PCC 0010 | RPS | Man, Glc, uronic acids, Gal, Xyl, Rha, Fuc Ara | sulfate and acetate groups, peptides | thermostable, amorphous, pseudoplastic behavior | [31] |
| Cyanothece sp. 113 | CPS | D-Glc | nd† | [33] | |
| Cyanothece sp. ATCC 51142 | RPS | 2-C-Me-Glc, Ido-2-C-Carboxylic acid, 2-deoxy-Ido | calcium; sulfate groups | gel formation; adsorption of metal ions | [34] |
| Nostoc commune DRH-1 | RPS | dXyl, dGlc, dGal, dRib, GlcA, Man | nd† | [35] | |
| Nostoc flagelliforme | CPS | Glu, Gal, Xyl, Man, GlcA | anti-viral activity, negative antithrombin activity | [36] | |
| Nostoc insulare | RPS | Ara, 3-O-Methyl-Ara, Glc, GlcA | Nd† | [37] | |
| Nostoc sphaeroids kütz | CPS | Man, Glc, Xyl, Gal, GlcA | immunological activity | [38] | |
| Spirulina platensis | CPS | Rha, Fru, Rib, Man, Gal, Xyl, Glc, GlcA, GalA | sulfate groups, calcium | anti-viral activity | [39] |
| Origin | Order (No. Strains) | Screening Conditions (Medium, Temperature, Light/Dark Cycle, Light Intensity, Air Supply/Mixing, Inoculum Conditions, Working Volume, Cultivation Days) | EPS Target | Reference |
|---|---|---|---|---|
| Soil, Soil/water, Water, Plant symbiosis | Nostocales (40) | BG110, 30 °C, L/D (24/0 h), 100 μE, 5% (v/v) CO2-air, agitation, axenic, working volume: 0.4 L, 10–15 days | RPS | [40] |
| nd† | Nostocales, Chroococcales, Synechococcales (15) | BG110, 25 °C, L/D (16/10 h), 35 μE, L/D, axenic, working volume: 0.1 L, 44 days | RPS | [44] |
| Freshwater | Nostocales, Synechococcales, Oscillatoriales (25) | Z medium 2x concentrated, 20 °C, L/D (24/0 h) 15 W/m2, 12-36 months | RPS | [45] |
| Marine | Oscillatoriales (4) | modified f/2 plus sea mud extract, 29 °C, L/D (24/0 h), 2700 Lux, aeration, inoculum 8 × 105 –9 × 105 cells/mL, working volume: 0.05 L, 15 days | CPS | [46] |
| Indo-Burma hotspot | Nostocales, Oscillatoriales (40) | BG110/ BG11#, 28 °C, L/D (14/10h), 54–67 μE, mixing 2x day, 50 mg of wet pellet, working volume: 0.1 L, 30 days | EPS | [47] |
| Freshwater (Indo-Burma hotspot) | Nostocales, Oscillatoriales (10) | BG110/ BG11#, 28 °C, L/D (14/10 h), 54–67 μE, mixing 2x day, 50 mg of wet pellet, working volume: 0.1 L, 30 days | CPS, RPS | [48] |
| Marine microbial mat French Polynesia | Oscillatoriales, Chroococcales, Synechococcales (6) | Conway (Fed-batch), 32 °C, L/D (12/12 h), 300 μE, 0.125 (v/v/min) pH 8.35 (CO2 on demand), 250 rpm, 10% inoculum non-axenic, working volume: 2 L, 25–35 days | CPS, RPS | [49] |
| Soil contaminated, Gujarat, India nd† | Nostocales, Oscillatriales (4) | BG11 and ASN III, 27 °C, L/D (12/12 h), 3 kLux. Axenic inoculum (chlorophyll a concentration to ∼2.0 mg/L), working volume 0.6 L, 30 days | RPS | [50] |
| Eroded soils; wastewater treatment plant; sediments; Cabras Lagoon | Nostocales, Oscillatoriales, Synechocchales, (7) | BG110/BG11#, 18 °C, L/D (14:10 h), 18 μE, working volume 0.3 L, 25–30 days (until stationary phase) | RPS | [51] |
| Freshwater lakes, Turkey | Synechocchales (3) | BG11, 25 °C, L/D (12:12 h), 1200 μE, 100rpm, working volume: 0.1 L, 20 days | CPS | [52] |
| Soil, garden | Nostocales (3) | BG110 and BG11, 30 °C, continuous illumination, 70–160 μE, aeration pH control (7–8.5) with CO2-air, working volume: 0.25 L, inoculum: chlorophyll a concentration of 1.5 mg/mL, 8–15 days (until stationary phase) | RPS | [53] |
| Miscellaneous / Culture Collections; hard sands Pantelleria island, Italy; Antarctic lake, Antarctic | Nostocales, Oscillatoriales (16) | BG110/ BG11# or Allen and Arnon or alkaline medium, 25 °C or 11 °C (psychrophilic strains), 1.500 lux, aeration pH 7–8.5 (CO2 on demand), inoculum chlorophyll concentration 1.5 or 3 mg/mL, working volume: 1.5 L, 30 days | CPS, RPS | [54] |
| Baltic Sea, Pacific Ocean, Atlantic Ocean, Mediterranean Sea, Red Sea | Synechocchales, Spirulinales, Pleurocapsales, Nostocales, Chroococcales (16) | PCR-11 medium, 20 °C, L/D (16:8 h), 150–300 μE, 120 rpm, working volume: 0.02 L, 30 days | RPS | [41] |
| Strains | Optimization Factor | EPS Titer/Productivity /Yield | ||
|---|---|---|---|---|
| Culture Media | Process Parameters | Literature | ||
| Nostocales | ||||
| Anabaena augstmalis VRUC163 | nd† | Fed-batch; Film forming PBR | 14.73 mg/g (CPS) | [51] |
| Anabaena cylindrica 10 C | N source (NaNO3) Mixotrophy | nd† | 2.36 mg/L (RPS) | [58] |
| Anabaena WSAF | N source (absence; NaNO3); P source (K2PHO4); | L/D cycle (continuous); Shear stress (aeration); | 1.86 mg/L/day (EPS) | [54] |
| Anabaena sp. ATCC 33047 | N source (N2, KNO3; NH4Cl); Salinity (NaCl/absence) | Temperature; Light intensity (medium); Shear stress (high aeration); Dilution rate (0.03 h−1) | 1100 mg/L/day (RPS + CPS) | [70] |
| Anabaena sp. BTA997 | nd† | Initial pH (8.5) | 1.7 g/L (RPS) | [48] |
| Anabaena turolosa | N source (absence; NaNO3); P source (K2PHO4); | L/D cycle (continuous); Shear stress (aeration); | 0.73 mg/L/day | [54] |
| Cyanospira capsulata | C flux metabolism (glyoxylate; nitrogen inhibitor) | nd† | 7.5 mg/L/day | [99] |
| Nostoc flagelliforme | N source (NaNO3); P source (K2PHO4); | Temperature (low); Light intensity (high); Initial pH (alkaline) | 14.29 mg/L/day (RPS) | [57] |
| Nostoc flagelliforme | nd† | Cultivation mode (Fed-batch); pH (8–9) | 8.86 mg/L/day (CPS) | [72] |
| Nostoc flagelliforme | nd† | light quality (monochromatic red, yellow, green, blue, purple) | 47.39 mg/g (RPS) | [79] |
| Nostoc flagelliforme | nd† | light quality (white fluorescent and monochromatic red, yellow, green, blue, purple); Red light intensity (medium) | 275 mg/g (CPS) | [80] |
| Nostoc flagelliforme | C source (absence; NaHCO3); N source (absence; NaNO3) | light quality (monochromatic red, blue) | nd† | [63] |
| Nostoc flagelliforme | N source (Urea, NaNO3, NH4Cl; Arginine) | Light intensity (low); Light quality (mixed wavelengths, red, blue, green); Wavelength shift | 5.42 mg/L/day (RPS) | [61] |
| Nostoc flagelliforme | C source (glucose); Salinity (NaCl) | nd† | 234.82 mg/g (CPS) | [84] |
| Nostoc sp. | nd† | Light intensity (high) | 134.26 mg/g DW (RPS) | [85] |
| Nostoc sp. BTA97 | N source (NaNO3, absence); | Initial pH (alkaline) | 53.3 mg/L/day (RPS + CPS) | [47] |
| Nostoc sp. PCC 7413 | N source (NaNO3; presence/absence) | Light intensity (low, high) | 150 mg L/day (RPS) | [53] |
| Scytonema tolypothrichoides | nd† | Temperature and light intensity crossed gradients | 310–360 mg/L (CPS) | [77] |
| Tolypothrix bouteillei | nd† | Temperature and light intensity crossed gradients | 186–216 mg/L (CPS) | [77] |
| Oscillatoriles | ||||
| Arthrospira platensis PCC 8005 | nd† | Light intensity (low) | nd† | [86] |
| Arthrospira platensis | C source (NaHCO3) | nd† | nd† | [66] |
| Arthrospira platensis “Compére 1968/3786” | nd† | Temperature; Light intensity | 11.76 mg/L/day (RPS) | [98] |
| Arthrospira platensis “Compére 1968/3786” | Photoautotrophic (light), Mixotrophic (light, glucose), Heterotrophic (glucose) | nd† | 26.4 mg/L/day (RPS) | [68] |
| Cyanothece epiphytica AUS-JR/DB/NT-021 | N source (absence; NaNO3); Salinity (NaCl); Micronutrients (MgSO4); Ozone | nd† | 9.66 mg/L/day (RPS + CPS) | [62] |
| Cyanothece sp. 113 | N source (absence; NaNO3); Salinity (NaCl); Micronutrients (MgSO4; NaH2PO4) | Aeration; Temperature; Light intensity; Time course | 1300 g/L/day (CPS) | [46,65] |
| Cyanothece sp. CCY 0110 | C source (glycerol); N source (absence/combined); Salinity (NaCl); Micronutrients (MgCl2) | Temperature; Light intensity; L/D cycle; shear stress (aeration) | 42.86 mg/L/day (RPS) | [56] |
| Microcoleus vaginatus | nd† | Light intensity; | 139 mg/g (RPS) | [100] |
| Synechococcales | ||||
| Limnothrix redekei PUPCCC 116 | N source (KNO3); Salinity (NaCl) | nd† | 14.48 mg/L/day | [59] |
| Oscillatoria formosa | N source (KNO3); Salinity (NaCl); Micronutrients (CaCl2) | Temperature (high); L/D cycles (14/10) | 9.88 mg/L/day (RPS) | [60] |
| Synechococcus sp. | N source (N2, nitrate, combined/absence) | Light intensity; L/D cycle | 330 mg/L/day | [64] |
| Synechocystis sp. BASO | Salinity (NaCl) | nd† | 500 mg/L (CPS) | [52] |
| Chroococcales | ||||
| Cyanobacterium aponinum | C source (5% CO2; NaHCO3) | Temperature (high); Light intensity (high) | 20 mg/L/day (RPS) | [76] |
| Spirulinales | ||||
| Spirulina sp. | N source (NaNO3); P source (K2PHO4); Salinity (NaCl) | Temperature | 1.83 mg/L/day (EPS) | [54] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cruz, D.; Vasconcelos, V.; Pierre, G.; Michaud, P.; Delattre, C. Exopolysaccharides from Cyanobacteria: Strategies for Bioprocess Development. Appl. Sci. 2020, 10, 3763. https://doi.org/10.3390/app10113763
Cruz D, Vasconcelos V, Pierre G, Michaud P, Delattre C. Exopolysaccharides from Cyanobacteria: Strategies for Bioprocess Development. Applied Sciences. 2020; 10(11):3763. https://doi.org/10.3390/app10113763
Chicago/Turabian StyleCruz, Diogo, Vitor Vasconcelos, Guillaume Pierre, Philippe Michaud, and Cédric Delattre. 2020. "Exopolysaccharides from Cyanobacteria: Strategies for Bioprocess Development" Applied Sciences 10, no. 11: 3763. https://doi.org/10.3390/app10113763
APA StyleCruz, D., Vasconcelos, V., Pierre, G., Michaud, P., & Delattre, C. (2020). Exopolysaccharides from Cyanobacteria: Strategies for Bioprocess Development. Applied Sciences, 10(11), 3763. https://doi.org/10.3390/app10113763









