Marine Microalgae Biomolecules and Their Adhesion Capacity to Salmonella enterica sv. Typhimurium
Abstract
1. Introduction
2. Materials and Methods
2.1. Algae Culture Conditions
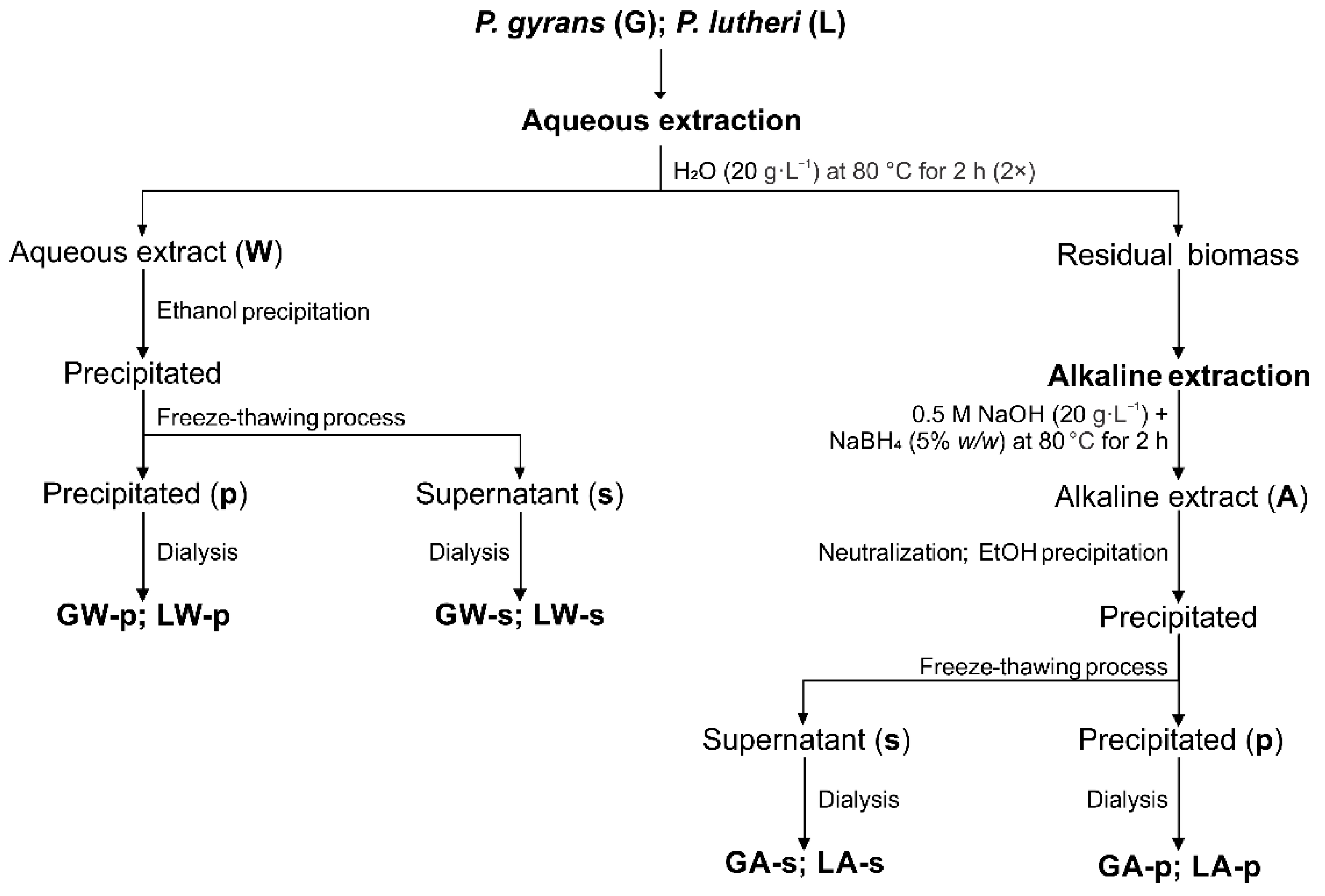
2.2. Microalgae Extraction
2.3. Characterization of Microalgal Extracts and Fractions
2.3.1. General Methods
2.3.2. Methylation Analyses
2.3.3. Nuclear Magnetic Resonance Spectroscopy (NMR)
2.4. Adhesion Assay
2.4.1. Bacteria Strain and Culture Conditions
2.4.2. Calibration Curve
2.4.3. Assay
2.4.4. Data Processing and Statistics
3. Results
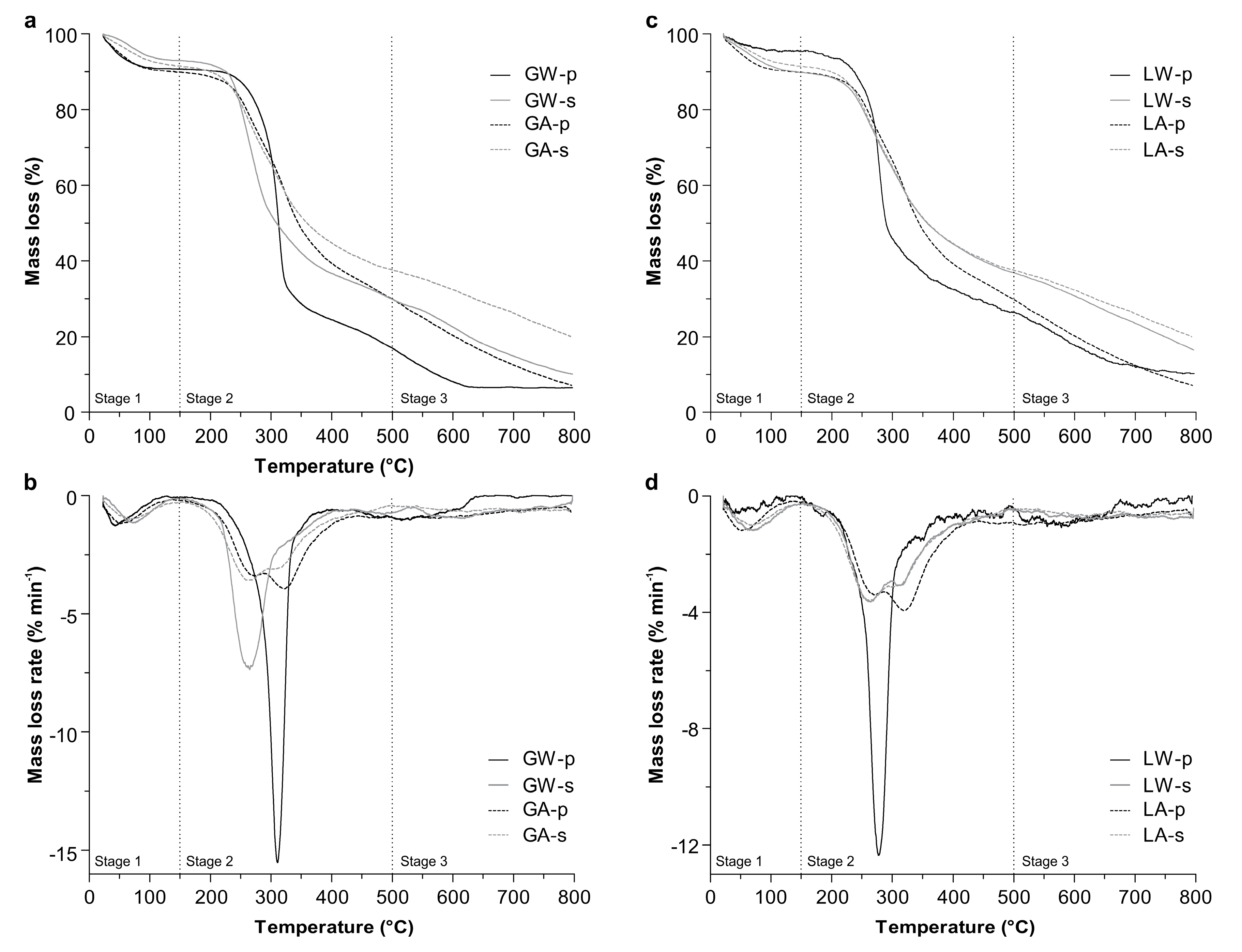
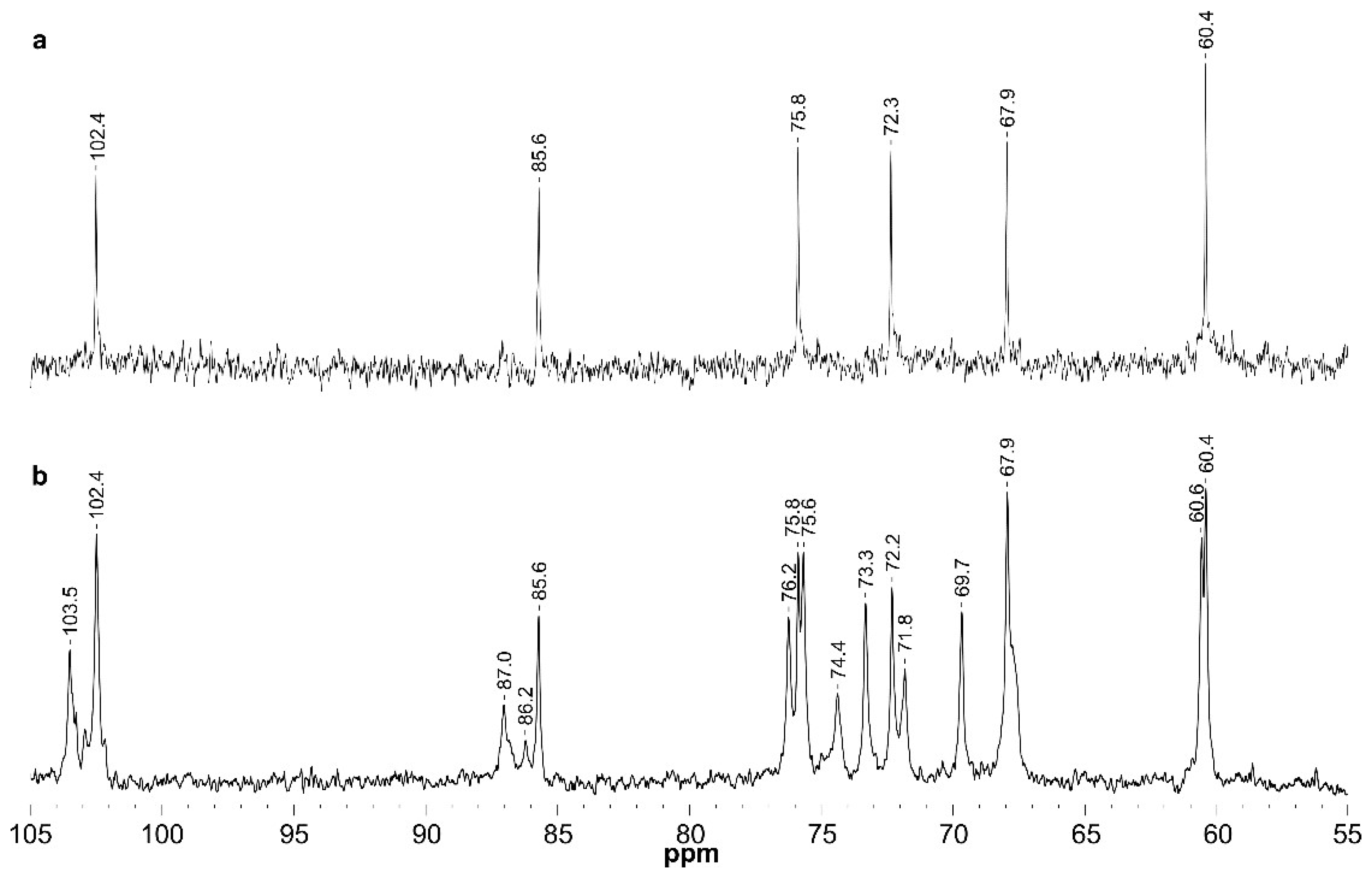
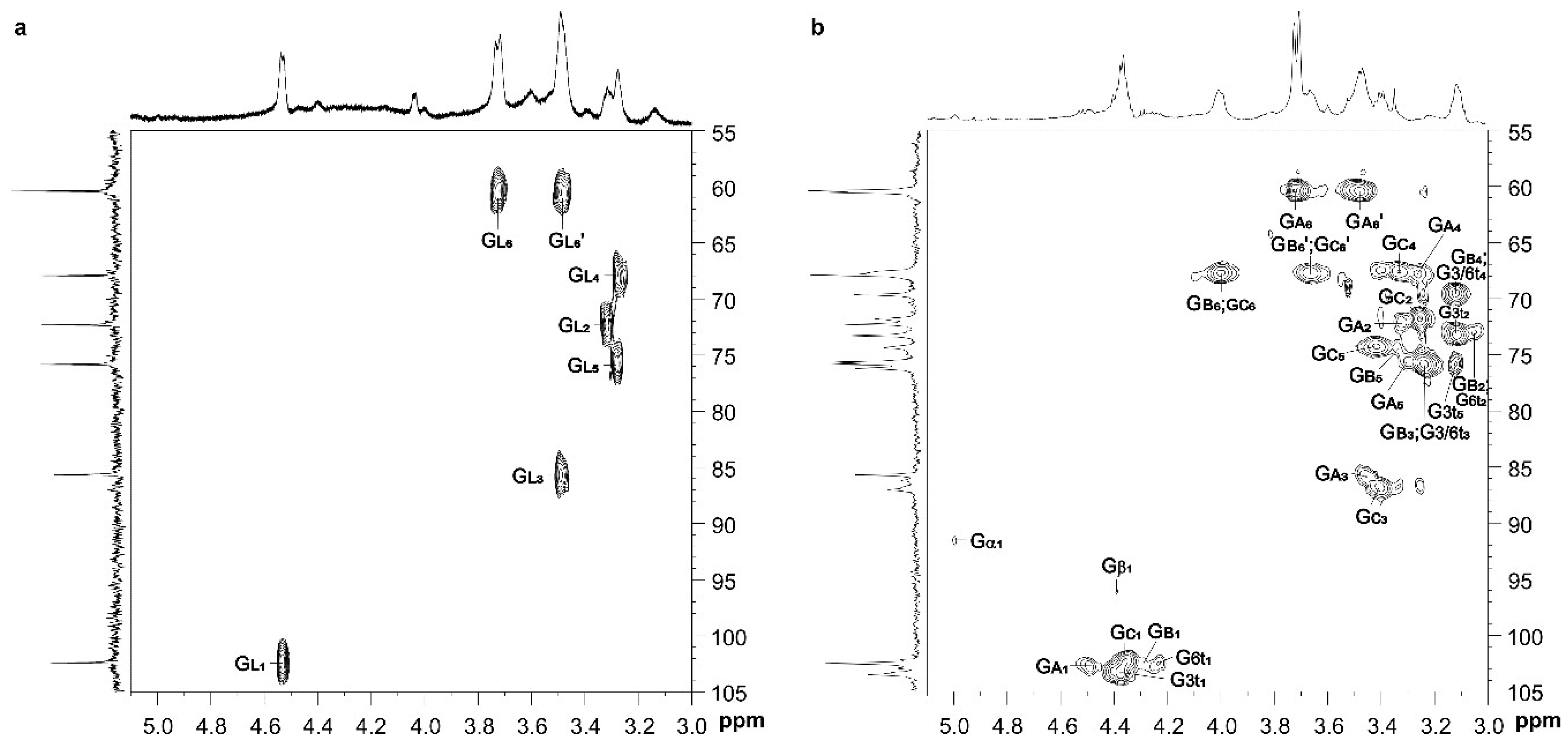
3.1. Extraction and Characterization of Polysaccharides from P. gyrans and P. lutheri
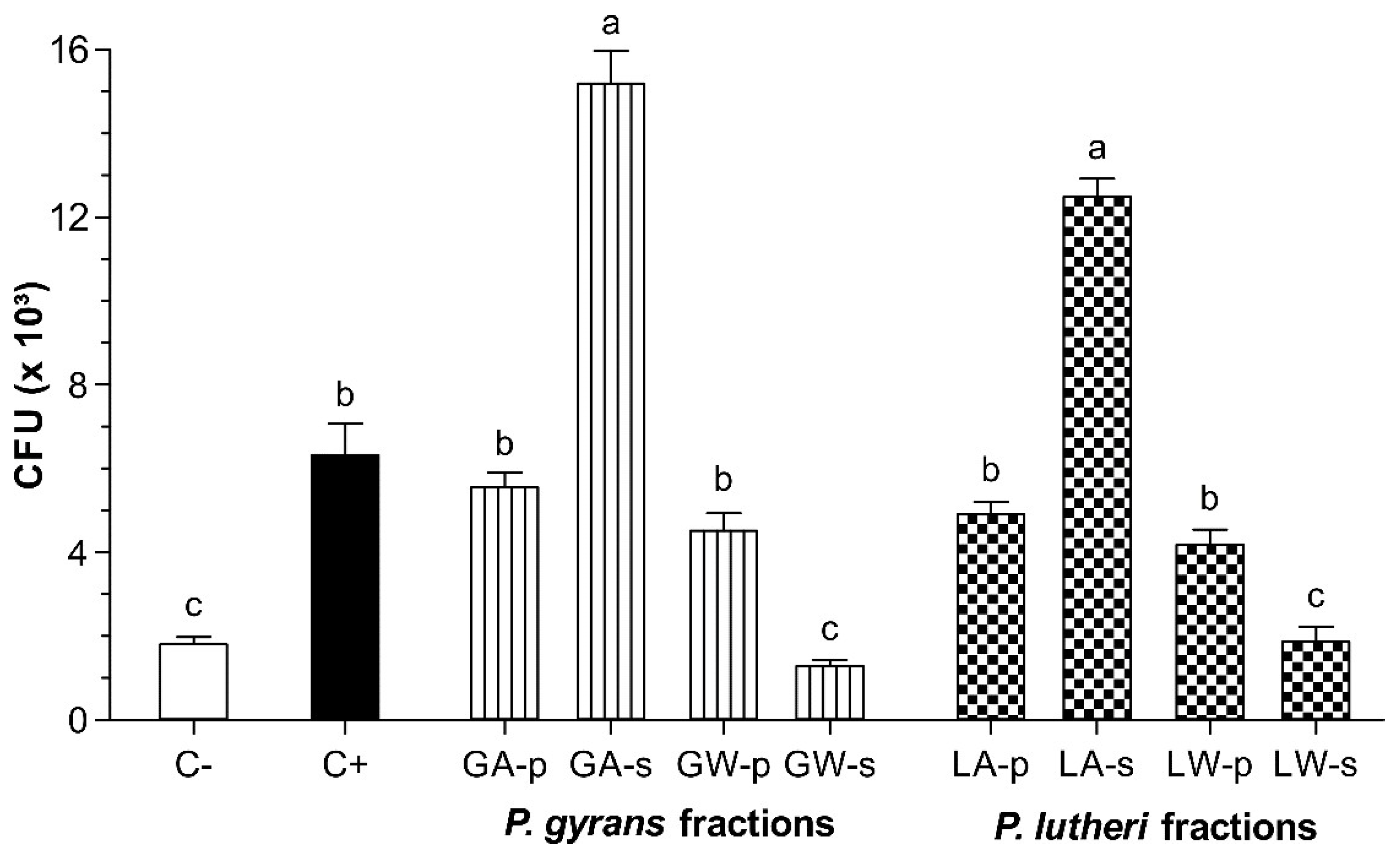
3.2. Adhesion Test
4. Discussion
5. Conclusions
6. Patents
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- López, Y.; Cepas, V.; Soto, S.M. The marine ecosystem as a source of antibiotics. In Grand Challenges in Marine Biotechnology; Rampelotto, P.H., Trincone, A., Eds.; Springer: Cham, Switzerland, 2018; pp. 3–48. ISBN 978-3-319-69074-2. [Google Scholar]
- Chu, W.L. Biotechnological applications of microalgae. IeJSME 2012, 6, S24–S37. [Google Scholar]
- Villarruel-López, A.; Ascencio, F.; Nuño, K. Microalgae, a potential natural functional food source—A review. Polish J. Food Nutr. Sci. 2017, 67, 251–264. [Google Scholar] [CrossRef]
- Bendif, M.E.; Probert, I.; Hervé, A.; Billard, C.; Goux, D.; Lelong, C.; Cadoret, J.P.; Véron, B. Integrative taxonomy of the Pavlovophyceae (Haptophyta): A reassessment. Protist 2011, 162, 738–761. [Google Scholar] [CrossRef] [PubMed]
- Rehberg-Haas, S.; Meyer, S.; Lippemeier, S.; Schulz, C. A comparison among different Pavlova sp. products for cultivation of Brachionus plicatilis. Aquaculture 2015, 435, 424–430. [Google Scholar] [CrossRef]
- Haas, S.; Bauer, J.L.; Adakli, A.; Meyer, S.; Lippemeier, S.; Schwarz, K.; Schulz, C. Marine microalgae Pavlova viridis and Nannochloropsis sp. as n-3 PUFA source in diets for juvenile European sea bass (Dicentrarchus labrax L.). J. Appl. Phycol. 2016, 28, 1011–1021. [Google Scholar] [CrossRef]
- Ponis, E.; Probert, I.; Véron, B.; Le Coz, J.R.; Mathieu, M.; Robert, R.; Coz, J.R.L.; Mathieu, M.; Robert, R. Nutritional value of six Pavlovophyceae for Crassostrea gigas and Pecten maximus larvae. Aquaculture 2006, 254, 544–553. [Google Scholar] [CrossRef]
- Bhalamurugan, G.L.; Valerie, O.; Mark, L. Valuable bioproducts obtained from microalgal biomass and their commercial applications: A review. Environ. Eng. Res. 2018, 23, 229–241. [Google Scholar] [CrossRef]
- de Jesus Raposo, M.F.; de Morais, A.M.B.; de Morais, R.M.S.C. Marine polysaccharides from algae with potential biomedical applications. Mar. Drugs 2015, 13, 2967–3028. [Google Scholar] [CrossRef]
- Xiao, R.; Zheng, Y. Overview of microalgal extracellular polymeric substances (EPS) and their applications. Biotechnol. Adv. 2016, 34, 1225–1244. [Google Scholar] [CrossRef]
- Maldonado-Gomez, M.X.; Lee, H.; Barile, D.; Lu, M.; Hutkins, R.W. Adherence inhibition of enteric pathogens to epithelial cells by bovine colostrum fractions. Int. Dairy J. 2015, 40, 24–32. [Google Scholar] [CrossRef]
- Quintero-Villegas, M.I.; Aam, B.B.; Rupnow, J.; Sorlie, M.; Eijsink, V.G.H.; Hutkins, R.W.; Sørlie, M.; Eijsink, V.G.H.; Hutkins, R.W. Adherence inhibition of enteropathogenic Escherichia coli by chitooligosaccharides with specific degrees of acetylation and polymerization. J. Agric. Food Chem. 2013, 61, 2748–2754. [Google Scholar] [CrossRef] [PubMed]
- Parkar, S.G.; Redgate, E.L.; Wibisono, R.; Luo, X.; Koh, E.T.H.; Schröder, R. Gut health benefits of kiwifruit pectins: Comparison with commercial functional polysaccharides. J. Funct. Foods 2010, 2, 210–218. [Google Scholar] [CrossRef]
- Gonzalez-Ortiz, G.; Perez, J.F.; Hermes, R.G.; Molist, F.; Jimenez-Diaz, R.; Martin-Orue, S.M. Screening the ability of natural feed ingredients to interfere with the adherence of enterotoxigenic Escherichia coli (ETEC) K88 to the porcine intestinal mucus. Br. J. Nutr. 2014, 111, 633–642. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, J.M.; Duarte, M.E.R.; Noseda, M.D. Modified soybean meal polysaccharide with high adhesion capacity to Salmonella. Int. J. Biol. Macromol. 2019, 139, 1074–1084. [Google Scholar] [CrossRef] [PubMed]
- Ofek, I.; Sharon, N.; Abraham, S.N. Bacterial adhesion. In The Prokaryotes; Dworkin, M., Falkow, S., Rosenberg, E., Schleifer, K.H., Stackebrandt, E., Eds.; Springer: Singapore, 2006; Volume 2, pp. 16–31. [Google Scholar]
- Laxminarayan, R.; Duse, A.; Wattal, C.; Zaidi, A.K.; Wertheim, H.F.; Sumpradit, N.; Vlieghe, E.; Hara, G.L.; Gould, I.M.; Goossens, H.; et al. Antibiotic resistance-the need for global solutions. Lancet Infect. Dis. 2013, 13, 1057–1098. [Google Scholar] [CrossRef]
- Kogan, G.; Kocher, A. Role of yeast cell wall polysaccharides in pig nutrition and health protection. Livest. Sci. 2007, 109, 161–165. [Google Scholar] [CrossRef]
- Ganner, A.; Stoiber, C.; Uhlik, J.T.; Dohnal, I.; Schatzmayr, G. Quantitative evaluation of E. coli F4 and Salmonella typhimurium binding capacity of yeast derivatives. AMB Express 2013, 3, 62–69. [Google Scholar] [CrossRef]
- Guzman-Murillo, M.A.; Ascencio, F. Anti-adhesive activity of sulphated exopolysaccharides of microalgae on attachment of red sore disease-associated bacteria and Helicobacter pylori to tissue culture cells. Lett. Appl. Microbiol. 2000, 30, 473–478. [Google Scholar] [CrossRef]
- Loke, M.F.; Lui, S.Y.; Ng, B.L.; Gong, M.; Ho, B. Antiadhesive property of microalgal polysaccharide extract on the binding of Helicobacter pylori to gastric mucin. FEMS Immunol. Med. Microbiol. 2007, 50, 231–238. [Google Scholar] [CrossRef]
- Guillard, R.R.L. Culture of phytoplankton for feeding marine invertebrates. In Culture of Marine Invertebrate Animals; Smith, W.L., Chanley, M.H., Eds.; Plenum Press: New York, NY, USA, 1975; pp. 29–60. ISBN 978-1-4615-8714-9. [Google Scholar]
- Gorin, P.A.J.; Iacomini, M. Structural diversity of D-galacto-D-mannan components isolated from lichens having Ascomycetous mycosymbionts. Carbohydr. Res. 1985, 142, 253–261. [Google Scholar] [CrossRef]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [PubMed]
- DuBois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F. Colorimetric method for determination of sugars and related substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Jansson, P.E.; Kenne, L.; Liedgren, H.; Lindberg, B.; Lönngren, J. A practical guide to the methylation analysis of carbohydrates. Chem. Commun. Univ. Stock. 1976, 8, 1–75. [Google Scholar]
- Ciucanu, I.; Kerek, F. A simple and rapid method for the permethylation of carbohydrates. Carbohydr. Res. 1984, 131, 209–217. [Google Scholar] [CrossRef]
- Bao, X.; Liu, C.; Fang, J.; Li, X. Structural and immunological studies of a major polysaccharide from spores of Ganoderma lucidum (Fr.) Karst. Carbohydr. Res. 2001, 332, 67–74. [Google Scholar] [CrossRef]
- Sassaki, G.L.; Gorin, P.A.J.; Souza, L.M.; Czelusniak, P.A.; Iacomini, M. Rapid synthesis of partially O-methylated alditol acetate standards for GC-MS: Some relative activities of hydroxyl groups of methyl glycopyranosides on Purdie methylation. Carbohydr. Res. 2005, 340, 731–739. [Google Scholar] [CrossRef]
- Ascêncio, S.D.; Orsato, A.; França, R.A.; Duarte, M.E.R.; Noseda, M.D. Complete 1H and 13C NMR assignment of digeneaside, a low-molecular-mass carbohydrate produced by red seaweeds. Carbohydr. Res. 2006, 341, 677–682. [Google Scholar] [CrossRef]
- Becker, P.M.; Galletti, S.; Roubos-van den Hil, P.J.; van Wikselaar, P.G. Validation of growth as measurand for bacterial adhesion to food and feed ingredients. J. Appl. Microbiol. 2007, 103, 2686–2696. [Google Scholar] [CrossRef]
- Ganner, A.; Stoiber, C.; Wieder, D.; Schatzmayr, G. Quantitative in vitro assay to evaluate the capability of yeast cell wall fractions from Trichosporon mycotoxinivorans to selectively bind gram negative pathogens. J. Microbiol. Methods 2010, 83, 168–174. [Google Scholar] [CrossRef]
- Batista, A.P.; Gouveia, L.; Bandarra, N.M.; Franco, J.M.; Raymundo, A. Comparison of microalgal biomass profiles as novel functional ingredient for food products. Algal Res. 2013, 2, 164–173. [Google Scholar] [CrossRef]
- Fernandes, T.; Fernandes, I.; Andrade, C.A.P.; Ferreira, A.; Cordeiro, N. Marine microalgae monosaccharide fluctuations as a stress response to nutrients inputs. Algal Res. 2017, 24, 340–346. [Google Scholar] [CrossRef]
- Marcilla, A.; Gómez-Siurana, A.; Gomis, C.; Chápuli, E.; Catalá, M.C.; Valdés, F.J. Characterization of microalgal species through TGA/FTIR analysis: Application to Nannochloropsis sp. Thermochim. Acta 2009, 484, 41–47. [Google Scholar] [CrossRef]
- Ponder, G.R.; Richards, G.N.; Stevenson, T.T. Influence of linkage position and orientation in pyrolysis of polysaccharides: A study of several glucans. J. Anal. Appl. Pyrolysis 1992, 22, 217–229. [Google Scholar] [CrossRef]
- Leng, E.; Costa, M.; Peng, Y.; Zhang, Y.; Gong, X.; Zheng, A.; Huang, Y.; Xu, M. Role of different chain end types in pyrolysis of glucose-based anhydro-sugars and oligosaccharides. Fuel 2018, 234, 738–745. [Google Scholar] [CrossRef]
- Dobruchowska, J.M.; Jonsson, J.O.; Fridjonsson, O.H.; Aevarsson, A.; Kristjansson, J.K.; Altenbuchner, J.; Watzlawick, H.; Gerwig, G.J.; Dijkhuizen, L.; Kamerling, J.P.; et al. Modification of linear (β 1→3)-linked gluco-oligosaccharides with a novel recombinant beta-glucosyltransferase (trans-beta-glucosidase) enzyme from Bradyrhizobium diazoefficiens. Glycobiology 2016, 26, 1157–1170. [Google Scholar] [PubMed]
- Sadovskaya, I.; Souissi, A.; Souissi, S.; Grard, T.; Lencel, P.; Greene, C.M.; Duin, S.; Dmitrenok, P.S.; Chizhov, A.O.; Shashkov, A.S.; et al. Chemical structure and biological activity of a highly branched (1→3,1→6)-β-D-glucan from Isochrysis galbana. Carbohydr. Polym. 2014, 111, 139–148. [Google Scholar] [CrossRef] [PubMed]
- Kono, H.; Kondo, N.; Hirabayashi, K.; Ogata, M.; Totani, K.; Ikematsu, S.; Osada, M. NMR spectroscopic structural characterization of a water-soluble beta-(1→3, 1→6)-glucan from Aureobasidium pullulans. Carbohydr. Polym. 2017, 174, 876–886. [Google Scholar] [CrossRef]
- Lowman, D.W.; West, L.J.; Bearden, D.W.; Wempe, M.F.; Power, T.D.; Ensley, H.E.; Haynes, K.; Williams, D.L.; Kruppa, M.D. New Insights into the structure of (1→3,1→6)-β-D-Glucan side chains in the Candida glabrata cell wall. PLoS ONE 2011, 6, e27614. [Google Scholar] [CrossRef]
- Sassaki, G.L.; Machado, M.J.; Tischer, C.A.; Gorin, P.A.J.; Iacomini, M. Glycosyldiacylglycerolipids from the lichen Dictyonema glabratum. J. Nat. Prod. 1999, 62, 844–847. [Google Scholar] [CrossRef]
- de Souza, L.M.; Iacomini, M.; Gorin, P.A.J.; Sari, R.S.; Haddad, M.A.; Sassaki, G.L. Glyco- and sphingophosphonolipids from the medusa Phyllorhiza punctata: NMR and ESI-MS/MS fingerprints. Chem. Phys. Lipids 2007, 145, 85–96. [Google Scholar] [CrossRef]
- Wang, H.; Li, Y.L.; Shen, W.Z.; Rui, W.; Ma, X.J.; Cen, Y.Z. Antiviral activity of a sulfoquinovosyldiacylglycerol (SQDG) compound isolated from the green alga Caulerpa racemosa. Bot. Mar. 2007, 50, 185–190. [Google Scholar] [CrossRef]
- Almoselhy, R.I.M.; Allam, M.H.; El-Kalyoubi, M.H.; El-Sharkawy, A.A. 1H NMR spectral analysis as a new aspect to evaluate the stability of some edible oils. Ann. Agric. Sci. 2014, 59, 201–206. [Google Scholar] [CrossRef]
- Cavanagh, J.; Fairbrother, W.J.; Palmer, A.G.; Rance, M.; Skelton, N.J.B. Sequential assignment, structure determination, and other applications. In Protein NMR Spectroscopy: Principles and Practice; Cavanagh, J., Fairbrother, W.J., Palmer, A.G., Rance, M., Skelton, N.J.B., Eds.; Academic Press: Burlington, VT, USA, 2007; pp. 781–817. ISBN 978-0-12-164491-8. [Google Scholar]
- Ma, L.; Yang, Y.; Yao, J.; Shao, Z.; Huang, Y.; Chen, X. Selective chemical modification of soy protein for a tough and applicable plant protein-based material. J. Mater. Chem. B 2015, 3, 5241–5248. [Google Scholar] [CrossRef]
- Myklestad, S.M.; Granum, E. Biology of (1,3)-β-Glucans and related glucans in protozoans and chromistans. In Chemistry, Biochemistry, and Biology of 1-3 β Glucans and Related Polysaccharides; Bacic, A., Fincher, G.B., Stone, B.A., Eds.; Academic Press: San Diego, CA, USA, 2009; pp. 353–385. ISBN 978-0-12-373971-1. [Google Scholar]
- Hirokawa, Y.; Fujiwara, S.; Suzuki, M.; Akiyama, T.; Sakamoto, M.; Kobayashi, S.; Tsuzuki, M. Structural and physiological studies on the storage beta-polyglucan of haptophyte Pleurochrysis haptonemofera. Planta 2008, 227, 589–599. [Google Scholar] [CrossRef] [PubMed]
- Varum, K.M.; Kvam, B.J.; Myklestad, S.; Paulsen, B.S. Structure of a food-reserve β-D-glucan produced by the haptophyte alga Emiliania huxleyi (Lohmann) Hay and Mohler. Carbohydr. Res. 1986, 152, 243–248. [Google Scholar] [CrossRef]
- Janse, I.; van Rijssel, M.; van Hall, P.J.; Gerwig, G.J.; Gottschal, J.C.; Prins, R.A. The storage glucan of Phaeocystis globosa (Prymesiophyceae) cells. J. Phycol. 1996, 32, 382–387. [Google Scholar] [CrossRef]
- Renaud, S.M.; Van Thinh, L.; Parry, D.L. The gross chemical composition and fatty acid composition of 18 species of tropical Australian microalgae for possible use in mariculture. Aquaculture 1999, 170, 147–159. [Google Scholar] [CrossRef]
- Mauer, L. Heat Treatment for Food Proteins. In Encyclopedia of Food Sciences and Nutrition; Caballero, B., Trug, L., Finglas, P.M., Eds.; Academic Press: Oxford, UK, 2003; pp. 4868–4872. ISBN 978-0-12-227055-0. [Google Scholar]
- Tatsuzawa, H.; Takizawa, E. Changes in lipid and fatty acid composition of Pavlova lutheri. Phytochemistry 1995, 40, 397–400. [Google Scholar] [CrossRef]
- Eichenberger, W.; Gribi, C. Lipids of Pavlova lutheri: Cellular site and metabolic role of DGCC. Phytochemistry 1997, 45, 1561–1567. [Google Scholar] [CrossRef]
- Thompson, G.A. Lipids and membrane function in green algae. Biochim. Biophys. Acta Lipids Lipid Metab. 1996, 1302, 17–45. [Google Scholar] [CrossRef]
- Shoaf-Sweeney, K.D.; Hutkins, R.W. Chapter 2 Adherence, anti-adherence, and oligosaccharides: Preventing pathogens from sticking to the host. Adv. Food Nutr. Res. 2008, 55, 101–161. [Google Scholar]
- Romling, U.; Bian, Z.; Hammar, M.; Sierralta, W.D.; Normark, S. Curli fibers are highly conserved between Salmonella typhimurium and Escherichia coli with respect to operon structure and regulation. J. Bacteriol. 1998, 180, 722–731. [Google Scholar] [CrossRef] [PubMed]
- Wagner, C.; Barlag, B.; Gerlach, R.G.; Deiwick, J.; Hensel, M. The Salmonella enterica giant adhesin SiiE binds to polarized epithelial cells in a lectin-like manner. Cell. Microbiol. 2014, 16, 962–975. [Google Scholar] [CrossRef] [PubMed]
- Griessl, M.H.; Schmid, B.; Kassler, K.; Braunsmann, C.; Ritter, R.; Barlag, B.; Stierhof, Y.-D.; Sturm, K.U.; Danzer, C.; Wagner, C.; et al. Structural insight into the giant Ca2+-Binding Adhesin SiiE: Implications for the adhesion of Salmonella enterica to polarized epithelial cells. Structure 2013, 21, 741–752. [Google Scholar] [CrossRef] [PubMed]
- Dorsey, C.W.; Laarakker, M.C.; Humphries, A.D.; Weening, E.H.; Bäumler, A.J. Salmonella enterica serotype Typhimurium MisL is an intestinal colonization factor that binds fibronectin. Mol. Microbiol. 2005, 57, 196–211. [Google Scholar] [CrossRef]
- Weimer, B.C.; Chen, P.; Desai, P.T.; Chen, D.; Shah, J. Whole cell cross-linking to discover host–microbe protein cognate receptor/ligand pairs. Front. Microbiol. 2018, 9, 1585. [Google Scholar] [CrossRef]
- Chowdhury, S.M.; Shi, L.; Yoon, H.; Ansong, C.; Rommereim, L.M.; Norbeck, A.D.; Auberry, K.J.; Moore, R.J.; Adkins, J.N.; Heffron, F.; et al. A method for investigating protein−protein interactions related to Salmonella Typhimurium pathogenesis. J. Proteome Res. 2009, 8, 1504–1514. [Google Scholar] [CrossRef]
- Brück, W.M.; Kelleher, S.L.; Gibson, G.R.; Graverholt, G.; Lonnerdal, B.L. The effects of a-lactalbumin and glycomacropeptide on the association of CaCo-2 cells by enteropathogenic Escherichia coli, Salmonella typhimurium and Shigella flexneri. FEMS Microbiol. Lett. 2006, 259, 158–162. [Google Scholar] [CrossRef]
- Nakajima, K.; Tamura, N.; Kobayashi-Hattori, K.; Yoshida, T.; Hara-Kudo, Y.; Ikedo, M.; Sugita-Konishi, Y.; Hattori, M. Prevention of intestinal infection by glycomacropeptide. Biosci. Biotechnol. Biochem. 2005, 69, 2294–2301. [Google Scholar] [CrossRef]
- Ouwehand, A.C.; Salminen, S.J.; Skurnik, M.; Conway, P.L. Inhibition of pathogen adhesion by β-lactoglobulin. Int. Dairy J. 1997, 7, 685–692. [Google Scholar] [CrossRef]
- Newburg, D.S.; McCormick, B.; Chaturvedi, P.; Siber, A.M.; Warren, C.D. Inhibition of Salmonella typhimurium by human milk sulfatides. Pediatr. Res. 1999, 45, 116. [Google Scholar] [CrossRef][Green Version]
- Bouckaert, J.; Berglund, J.; Schembri, M.; De Genst, E.; Cools, L.; Wuhrer, M.; Hung, C.S.; Pinkner, J.; Slattegard, R.; Zavialov, A.; et al. Receptor binding studies disclose a novel class of high-affinity inhibitors of the Escherichia coli FimH adhesin. Mol. Microbiol. 2005, 55, 441–455. [Google Scholar] [CrossRef] [PubMed]
- Hung, C.S.; Bouckaert, J.; Hung, D.; Pinkner, J.; Widberg, C.; DeFusco, A.; Auguste, C.G.; Strouse, R.; Langermann, S.; Waksman, G.; et al. Structural basis of tropism of Escherichia coli to the bladder during urinary tract infection. Mol. Microbiol. 2002, 44, 903–915. [Google Scholar] [CrossRef] [PubMed]
- Ganner, A.; Fink, L.; Schatzmayr, G. Quantitative in vitro assay to evaluate yeast products concerning their binding activity of enteropathogenic bacteria. J. Anim. Sci. 2008, 86, 54. [Google Scholar]
- Ofek, I.; Doyle, R.J. Principles of Bacterial Adhesion. In Bacterial Adhesion to Cells and Tissues; Ofek, I., Doyle, R.J., Eds.; Springer: Boston, MA, USA, 1994; pp. 1–15. ISBN 978-1-4684-6435-1. [Google Scholar]
- Ofek, I.; Hasty, D.L.; Sharon, N. Anti-adhesion therapy of bacterial diseases: Prospects and problems. FEMS Immunol. Med. Microbiol. 2003, 38, 181–191. [Google Scholar] [CrossRef]
- Sletmoen, M.; Stokke, B.T. Higher order structure of (1,3)-β-D-Glucans and its influence on their biological activities and complexation abilities. Biopolymers 2008, 89, 310–321. [Google Scholar] [CrossRef]

| Fraction | Carbohydrate (%) | Protein (%) | Monosaccharides (mol%) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Rha | Fuc | Ara | Xyl | Man | Gal | Glc | |||
| P. gyrans | |||||||||
| GW-p | 83.5 | 10.7 | tr | tr | tr | tr | tr | tr | 100 |
| GW-s | 68.6 | 18.1 | 1.4 | 1.2 | 1.1 | tr | 2.8 | 2.7 | 90.8 |
| GA-p | 11.6 | 30.0 | 5.2 | 6.5 | 3.7 | 10.6 | 21.6 | 16.0 | 36.4 |
| GA-s | 15.3 | 38.4 | 4.1 | 6.6 | 8.9 | 11.5 | 12.2 | 24.2 | 32.5 |
| P. lutheri | |||||||||
| LW-p | 55.6 | 14.5 | 2.1 | 2.5 | tr | tr | 1.5 | 2.0 | 91.8 |
| LW-s | 59.9 | 17.9 | 1.4 | 3.7 | 1.8 | tr | 3.4 | 4.3 | 85.4 |
| LA-p | 15.2 | 49.1 | 8.3 | 7.8 | 3.0 | 5.1 | 14.2 | 17.6 | 44.0 |
| LA-s | 17.5 | 34.7 | 5.5 | 7.7 | 8.2 | 8.1 | 15.4 | 24.9 | 30.2 |
| Fraction | Total Volatile Matter Content (%) a | Ash and Fixed Carbon (%) b | ||
|---|---|---|---|---|
| Stage 1 | Stage 2 | Stage 3 | ||
| P. gyrans | ||||
| GW-p | 9.2 | 78.7 | 5.5 | 6.5 |
| GW-s | 7.0 | 66.1 | 16.8 | 10.1 |
| GA-p | 8.8 | 63.6 | 17.4 | 10.1 |
| GA-s | 8.6 | 58.0 | 22.5 | 10.8 |
| P. lutheri | ||||
| LW-p | 4.6 | 72.8 | 12.3 | 10.3 |
| LW-s | 10.1 | 55.6 | 17.8 | 16.5 |
| LA-p | 10.0 | 64.7 | 18.1 | 7.1 |
| LA-s | 8.3 | 56.1 | 15.4 | 20.1 |
| Sugar Derivative a | Deduced Linkages b | Molar Ratio c | |
|---|---|---|---|
| GW-p | GW-s | ||
| 2,3,4,6-Me4-Glc d | Glcp-(1→ | 7.3 | 38.0 |
| 2,4,6-Me3-Glc | →3)-Glcp-(1→ | 90.0 | 26.7 |
| 2,3,4-Me3-Glc | →6)-Glcp-(1→ | 2.5 | 11.2 |
| 2,4-Me2-Glc | →3,6)-Glcp-(1→ | 5.3 | 24.1 |
| Fraction | Glycosyl Residues | Chemical Shifts a | ||||||
|---|---|---|---|---|---|---|---|---|
| H-1/C-1 | H-2/C-2 | H-3/C-3 | H-4/C-4 | H-5/C-5 | H-6;H-6′/C-6 | |||
| GW-p | →3)-β-d-Glcp-(1→ | GL | 4.53/102.4 | 3.32/72.3 | 3.49/85.6 | 3.28/67.9 | 3.28/75.8 | 3.72; 3.48/60.4 |
| GW-s | →3)-α-d-Glcp | Gα | 5.00/91.5 | nd | nd | nd | nd | nd |
| →3)-β-d-Glcp | Gβ | 4.39/96.1 | nd | nd | nd | nd | nd | |
| β-d-Glcp-(1→3 | G3t | 4.38/103.5 | 3.11/73.5 | 3.24/75.6 | 3.12/69.7 | 3.12/75.8 | 3.72;3.47/60.7 | |
| β-d-Glcp-(1→6 | G6t | 4.23/102.5 | 3.05/73.3 | 3.23/75.6 | 3.13/69.7 | 3.19/nd | 3.71;3.49/60.7 | |
| →3)-β-d-Glcp-(1→ | GA | 4.49/102.4 | 3.32/72.2 | 3.46/85.6 | 3.26/67.9 | 3.29/75.8 | 3.72; 3.48/60.4 | |
| →6)-β-d-Glcp-(1→ | GB | 4.28/102.6 | 3.05/73.3 | 3.23/75.8 | 3.15/69.7 | 3.34/74.4 | 4.00; 3.61/67.9 | |
| →3,6)-β-d-Glcp-(1→ | GC | 4.36/102.4 | 3.25/72.0 | 3.40/87.0 | 3.33/67.8 | 3.42/74.4 | 4.00; 3.66/67.9 | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Machado, T.W.M.; Rodrigues, J.M.; Moro, T.R.; Duarte, M.E.R.; Noseda, M.D. Marine Microalgae Biomolecules and Their Adhesion Capacity to Salmonella enterica sv. Typhimurium. Appl. Sci. 2020, 10, 2239. https://doi.org/10.3390/app10072239
Machado TWM, Rodrigues JM, Moro TR, Duarte MER, Noseda MD. Marine Microalgae Biomolecules and Their Adhesion Capacity to Salmonella enterica sv. Typhimurium. Applied Sciences. 2020; 10(7):2239. https://doi.org/10.3390/app10072239
Chicago/Turabian StyleMachado, Tatiane Winkler Marques, Jenifer Mota Rodrigues, Tatiana Rojo Moro, Maria Eugênia Rabello Duarte, and Miguel Daniel Noseda. 2020. "Marine Microalgae Biomolecules and Their Adhesion Capacity to Salmonella enterica sv. Typhimurium" Applied Sciences 10, no. 7: 2239. https://doi.org/10.3390/app10072239
APA StyleMachado, T. W. M., Rodrigues, J. M., Moro, T. R., Duarte, M. E. R., & Noseda, M. D. (2020). Marine Microalgae Biomolecules and Their Adhesion Capacity to Salmonella enterica sv. Typhimurium. Applied Sciences, 10(7), 2239. https://doi.org/10.3390/app10072239





