Experimental Investigation of Chloride Uptake Performances of Hydrocalumite-Like Ca-Al LDHs with Different Microstructures
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of Hydrocalumite-Like CaAl-NO3- LDHs
2.3. Characterization
2.4. Adsorption Studies
3. Results and Discussion
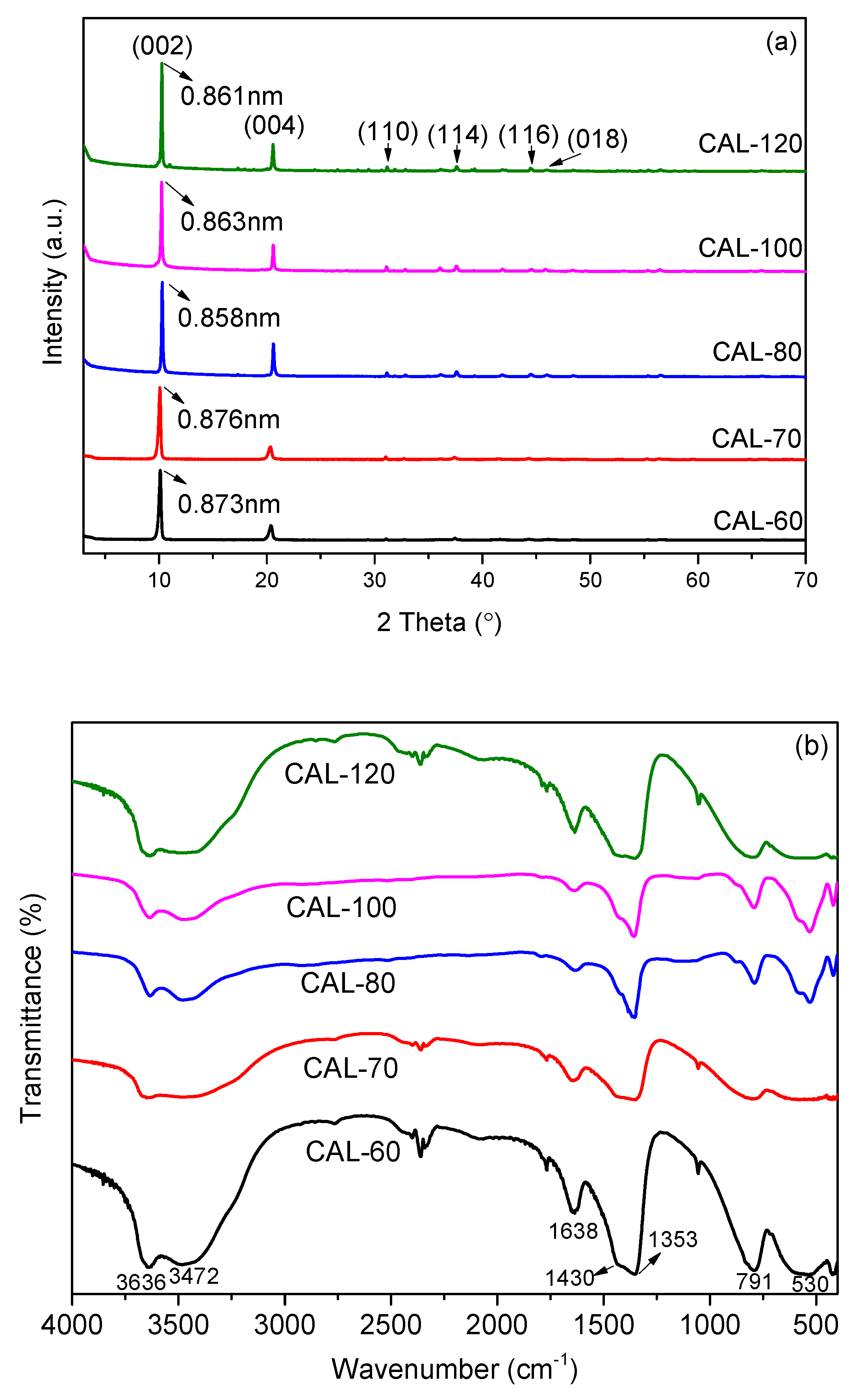
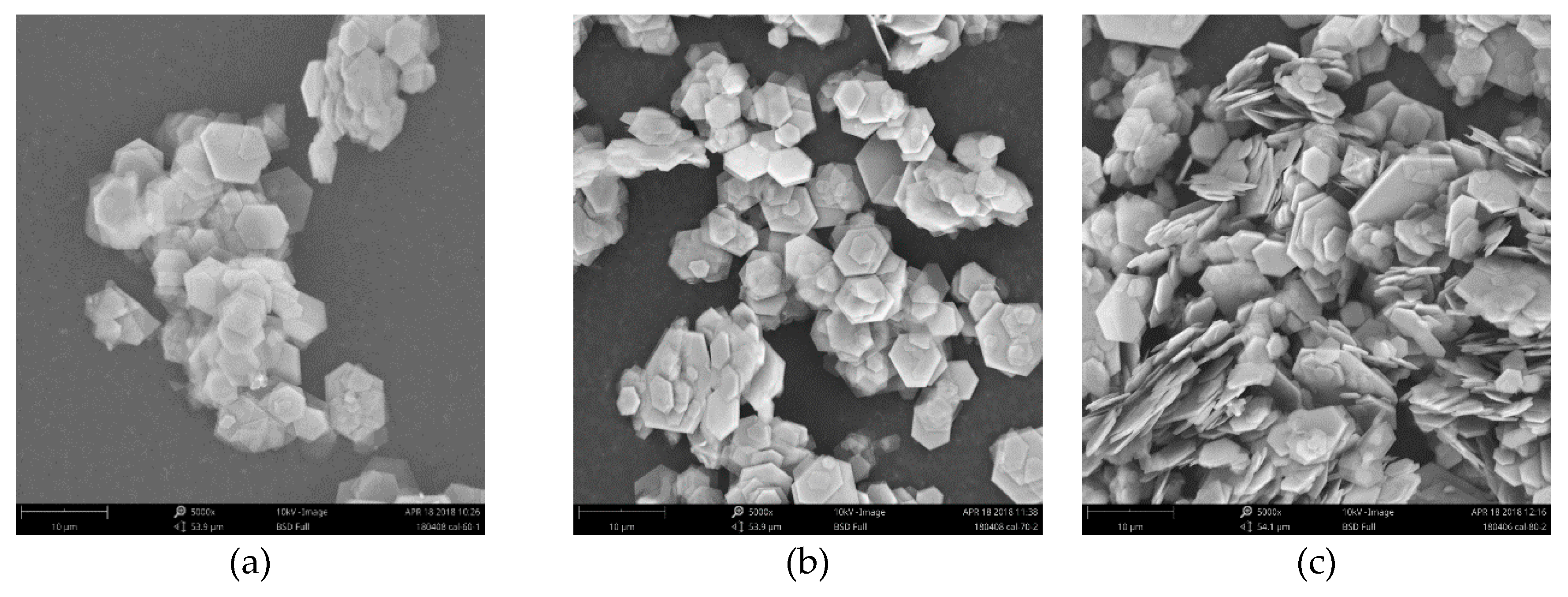
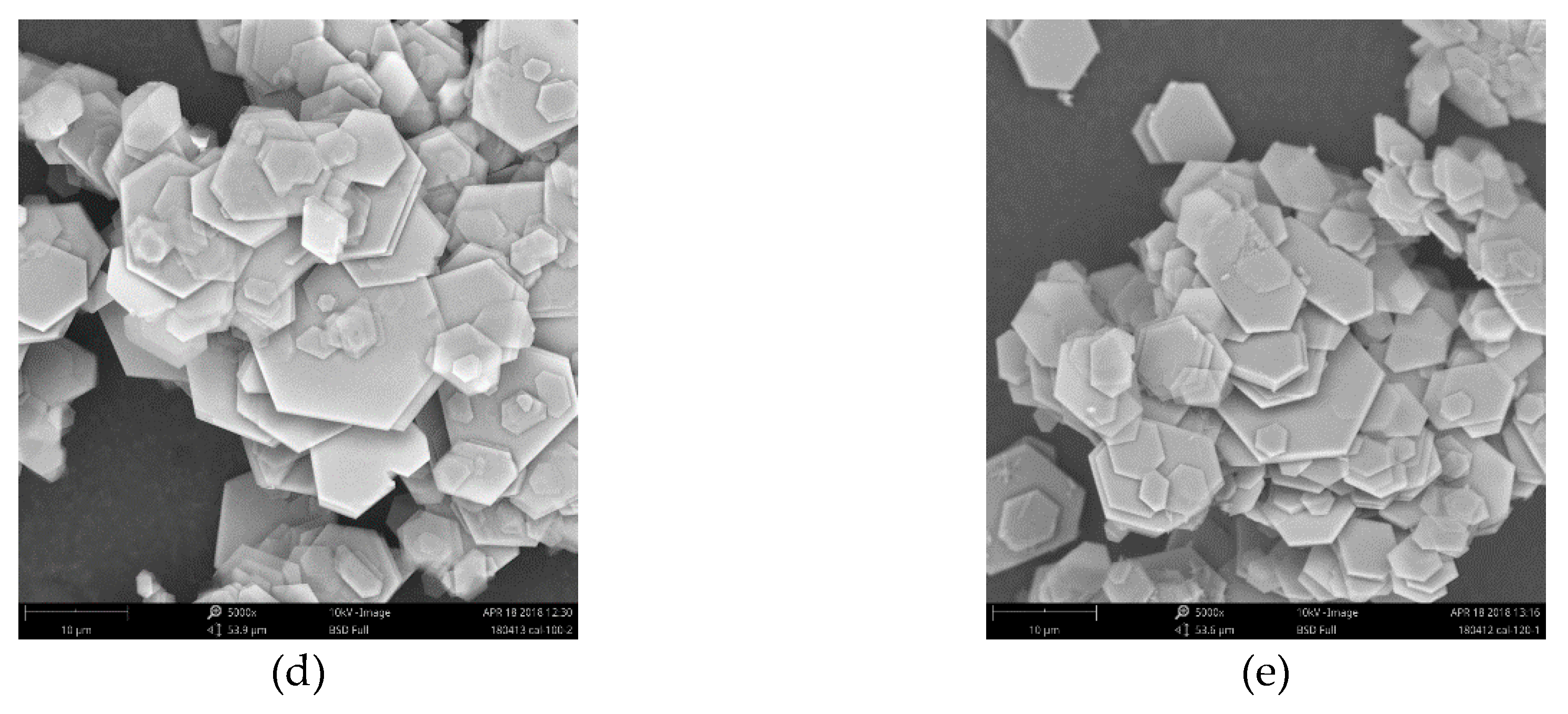
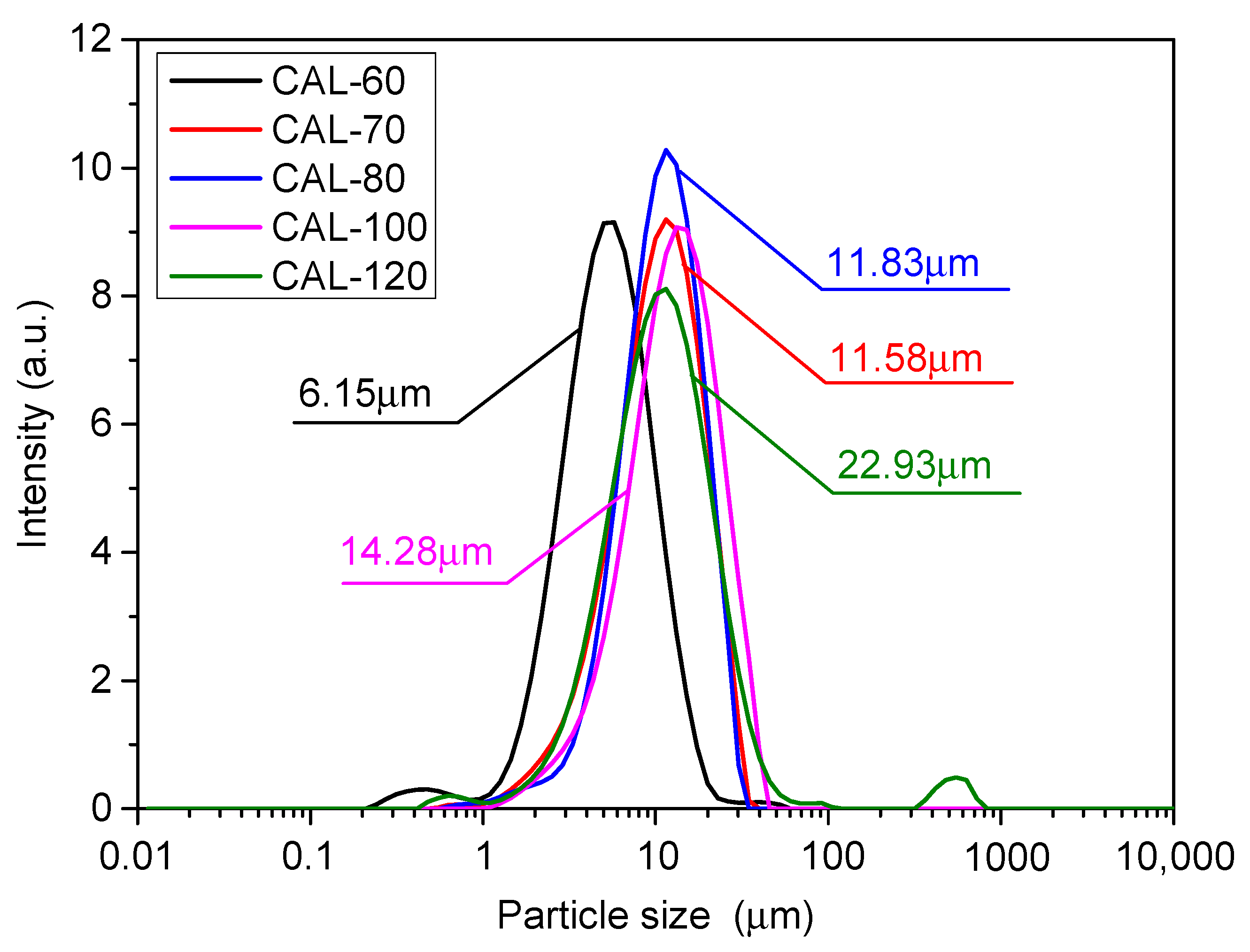
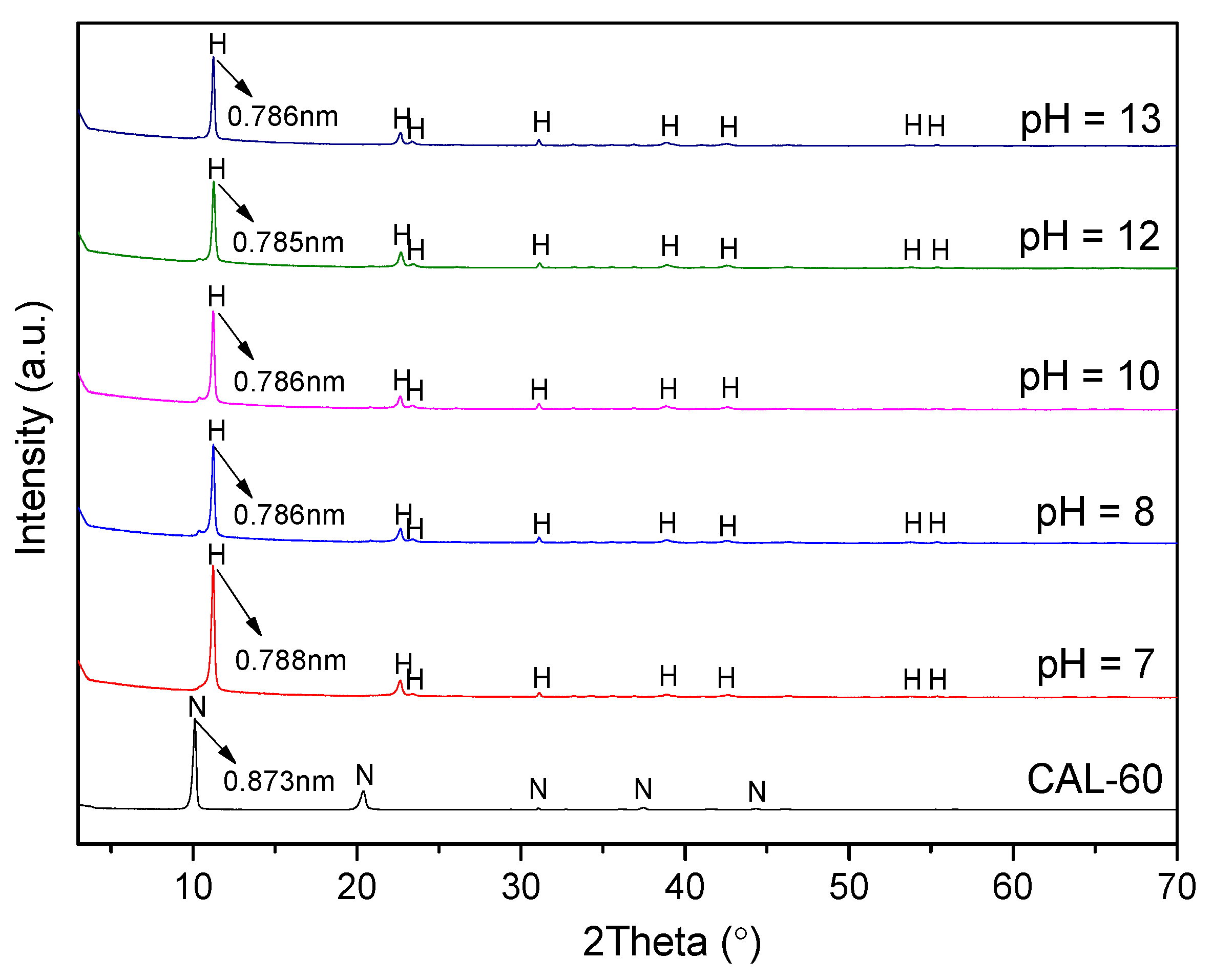
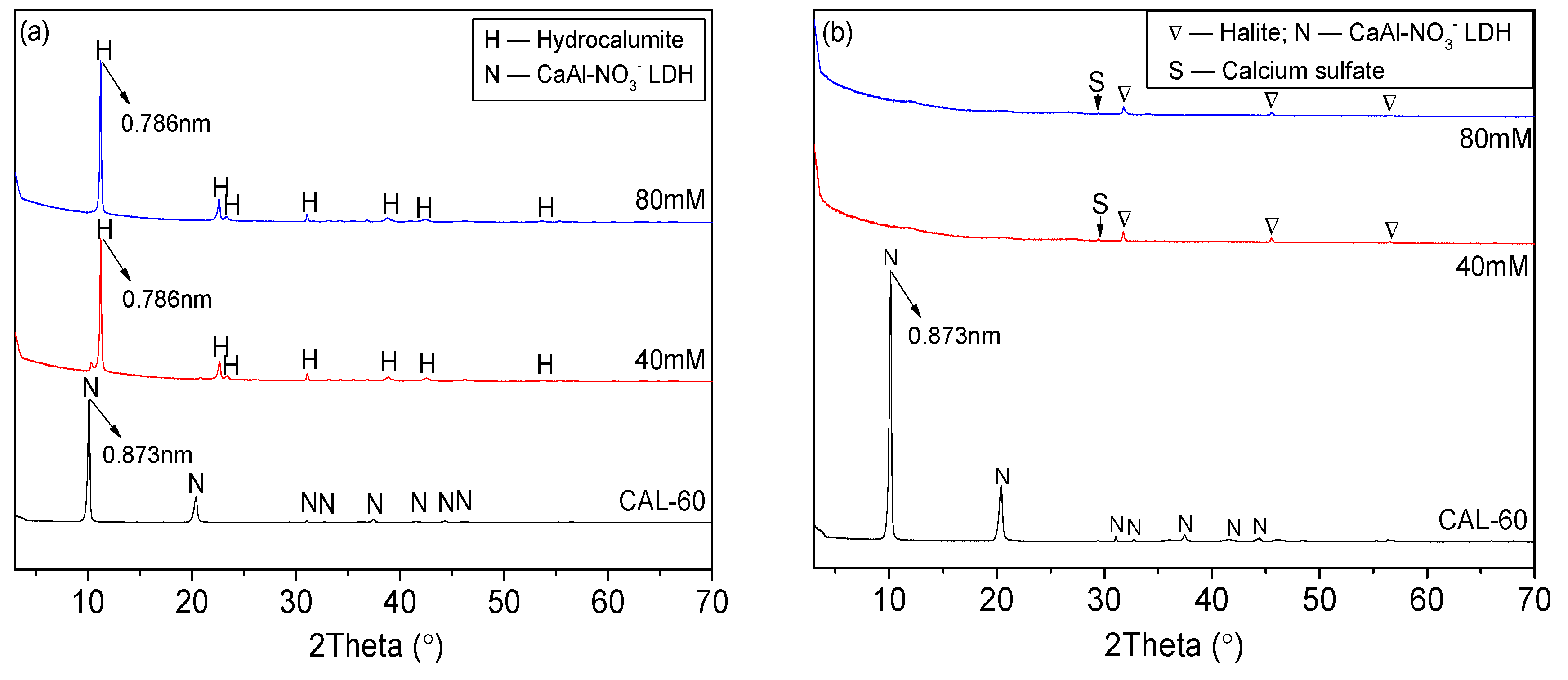
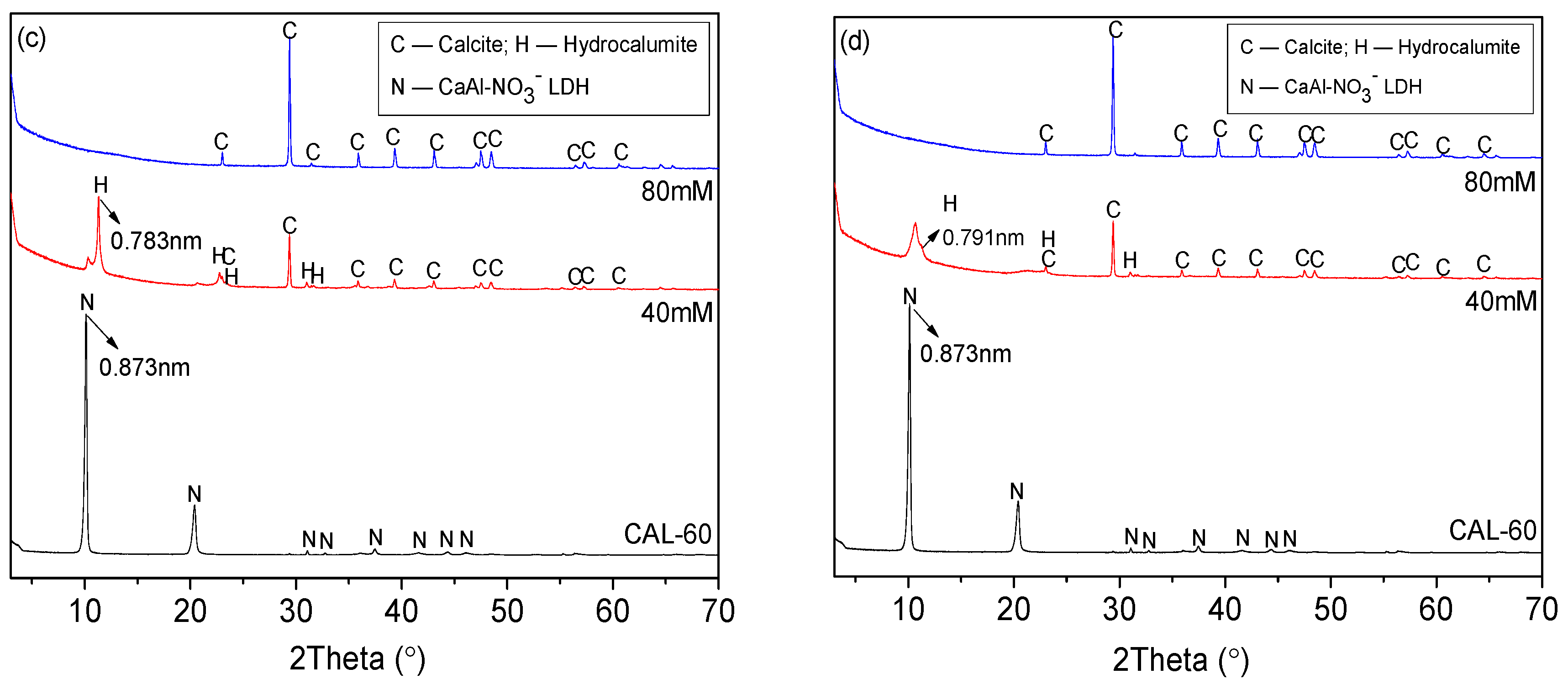
3.1. Characterization of CAL
3.2. Effect of Contact Time
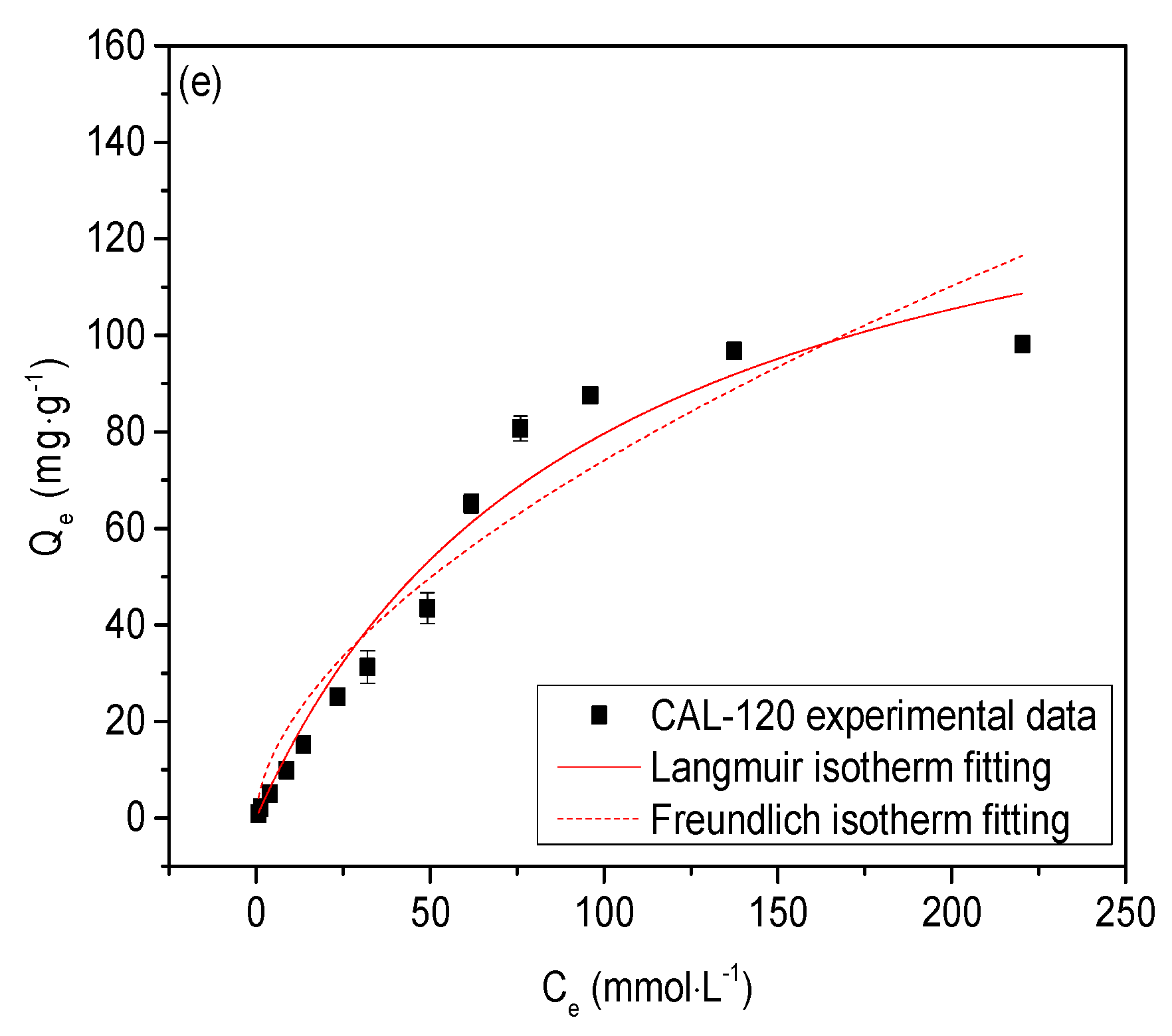
3.3. Effect of Initial Cl− Concentration
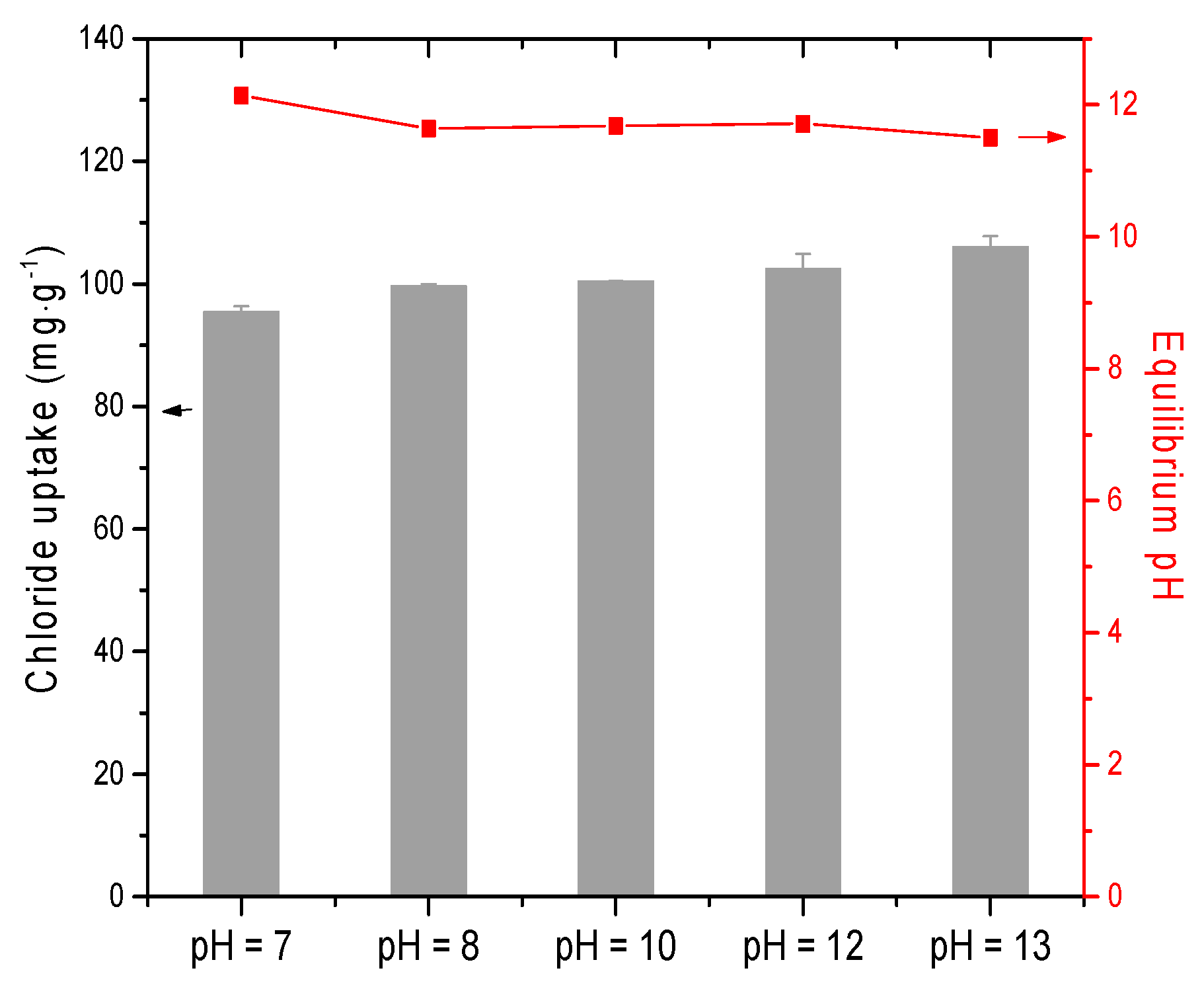
3.4. Effect of Initial pH
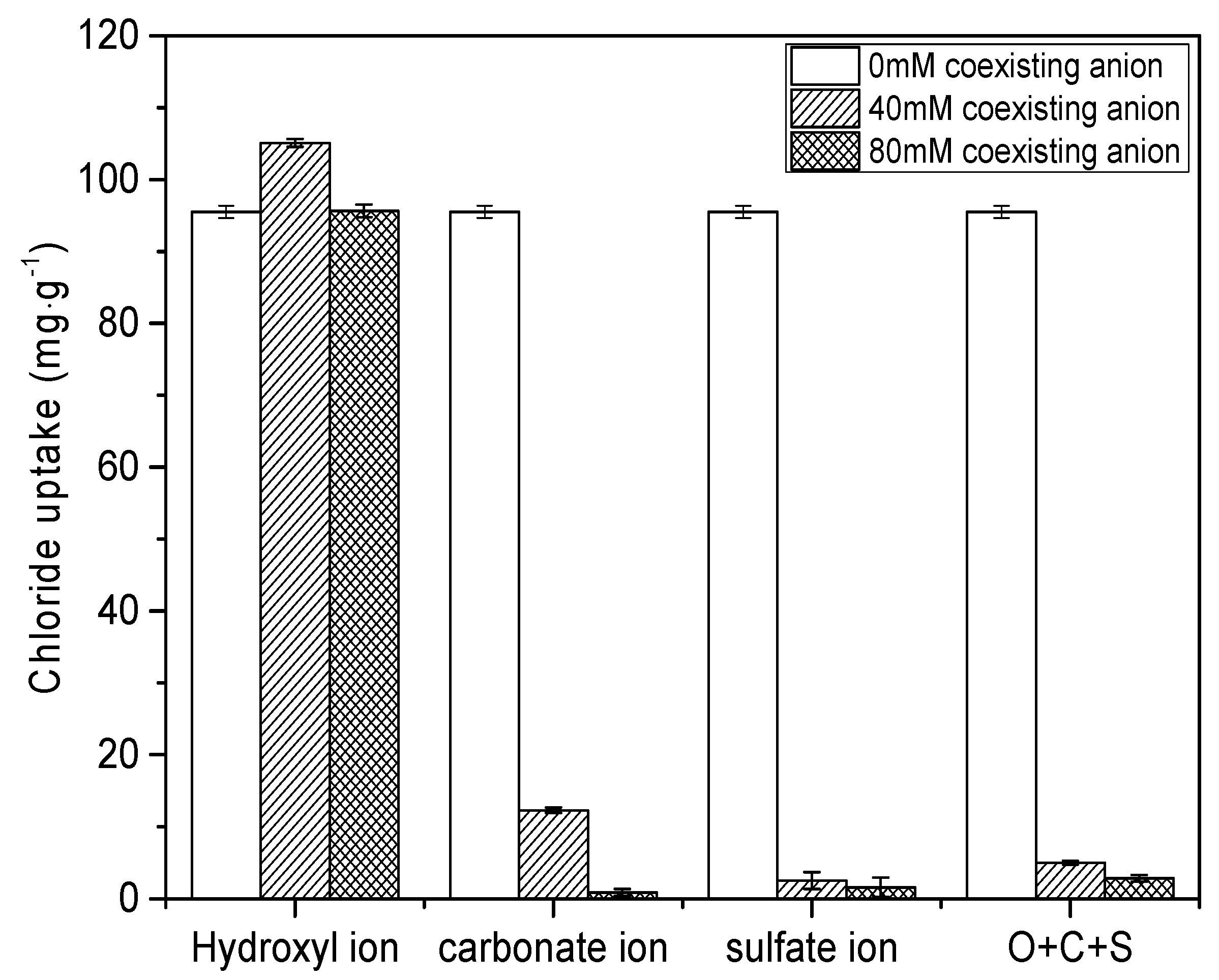
3.5. Effect of Coexistence Anions and Adsorptive Selectivity of CAL
4. Conclusions
- (1)
- CAL samples synthesized at 60–120 °C performed a typical double hydroxide structure and the crystallinity of CAL increased slightly with increasing the aging temperature, which can be observed from XRD patterns. Enhancement of aging temperature also results in a sharper edge and larger particle size of CAL. The average particle size of CAL samples synthesized at 60 °C, 70 °C, 80 °C, 100 °C, and 120 °C was 6.148 μm, 11.580 μm, 11.829 μm, 14.278 μm and 22.932 μm, respectively.
- (2)
- The kinetic process of chloride ion adsorption by CAL was more in line with the description of a Pseudo-second-order kinetic model and the Langmuir isotherm could fit the adsorption results of CAL samples better than the Freundlich isotherm. Theoretical maximum adsorption capacity of Cl− on CAL prepared at 60–120 °C was 211.324 mg/g, 182.117 mg/g, 174.903 mg/g, 160.914 mg/g and 155.960 mg/g, respectively. Chloride uptake of CAL could be improved by increasing aging temperature due to particle size reduction.
- (3)
- The main adsorption mechanism of chloride ion on CAL was an interlayer anion exchange reaction other than the dissolution-precipitate mode. With increasing the pH of solution (7–13), chloride uptake of CAL increased slightly and a high pH level (11–12) was kept after adsorption. The presence of CO32− and SO42− with high concentration reduced the adsorption capacity of CAL significantly as compared with OH− due to the destruction of layered structure and precipitates formed (CaCO3 or CaSO4). Interference sequence of three anions on the chloride uptake of CAL was SO42− > CO32− > OH−, and the order of interlayer anionic affinity was Cl− > OH− > NO3−.
- (4)
- Although the results showed that the presence of CO32− and SO42− could affect the application of LDH in concrete, in actual conditions the concentration of CO32− and SO42− in concrete is low and the carbonation and sulfate attacks happen from the exterior surface of the concrete structure. So, it is believed incorporation of LDHs into concrete would still maintain its adsorption effect on Cl−.
Author Contributions
Funding
Conflicts of Interest
References
- Mehta, P.K.; Paulo, J.; Monteiro, M. Concrete Microstructure, Properties and Materials; McGraw-Hill Education: New York, NY, USA, 2017. [Google Scholar]
- Tam, V.W.; Butera, A.; Le, K.N.; Li, W. Utilising CO2 technologies for recycled aggregate concrete: A critical review. Constr. Build. Mater. 2020, 250, 118903. [Google Scholar] [CrossRef]
- Sagoe-Crentsil, K.; Brown, T.; Taylor, A. Performance of concrete made with commercially produced coarse recycled concrete aggregate. Cem. Concr. Res. 2001, 31, 707–712. [Google Scholar] [CrossRef]
- Pacheco, J.; De Brito, J.; Chastre, C.; Evangelista, L. Experimental investigation on the variability of the main mechanical properties of concrete produced with coarse recycled concrete aggregates. Constr. Build. Mater. 2019, 201, 110–120. [Google Scholar] [CrossRef]
- Bao, J.; Li, S.; Zhang, P.; Ding, X.; Xue, S.; Cui, Y.; Zhao, T. Influence of the incorporation of recycled coarse aggregate on water absorption and chloride penetration into concrete. Constr. Build. Mater. 2020, 239, 117845. [Google Scholar] [CrossRef]
- Quattrone, M.; Angulo, S.C.; John, V.M. Energy and CO2 from high performance recycled aggregate production. Resour. Conserv. Recycl. 2014, 90, 21–33. [Google Scholar] [CrossRef]
- Zhu, Y.-G.; Kou, S.-C.; Poon, C.-S.; Dai, J.-G.; Li, Q.-Y. Influence of silane-based water repellent on the durability properties of recycled aggregate concrete. Cem. Concr. Compos. 2013, 35, 32–38. [Google Scholar] [CrossRef]
- Nandhini, K.; Ponmalar, V. Effect of Blending Micro and Nano Silica on the Mechanical and Durability Properties of Self-Compacting Concrete. Silicon 2020, 1–9. [Google Scholar] [CrossRef]
- García, R.; Reyes, E.; Villanueva, P.; De La Rubia, M.Á.; Fernández, J.; Moragues, A. Service Life and Early Age Durability Enhancement due to Combined Metakaolin and Nanosilica in Mortars for Marine Applications. Materials (Basel) 2020, 13, 1169. [Google Scholar] [CrossRef]
- Prakasam, G.; Murthy, A.R.; Reheman, M.M.S.; Ganesh, P. Mechanical, Durability and Fracture Properties of Nano Modified FA-GGBS Geopolymer Mortar. Mag. Concr. Res. 2020, 72, 207–216. [Google Scholar] [CrossRef]
- Yang, Z.; Sui, S.; Wang, L.; Feng, T.; Gao, Y.; Mu, S.; Tang, L.; Jiang, J. Improving the chloride binding capacity of cement paste by adding nano-Al2O3: The cases of blended cement pastes. Constr. Build. Mater. 2020, 232. [Google Scholar] [CrossRef]
- Lv, S.; Hu, H.; Zhang, J.; Lei, Y.; Sun, L.; Hou, Y. Structure, performances, and formation mechanism of cement composites with large-scale regular microstructure by distributing uniformly few-layered graphene oxide in cement matrix. Struct. Concr. 2018, 20, 471–482. [Google Scholar] [CrossRef]
- Li, G.; Zhou, J.; Yue, J.; Gao, X.; Wang, K. Effects of nano-SiO2 and secondary water curing on the carbonation and chloride resistance of autoclaved concrete. Constr. Build. Mater. 2020, 235, 117465. [Google Scholar] [CrossRef]
- Duan, P.; Chen, W.; Ma, J.; Shui, Z. Influence of layered double hydroxides on microstructure and carbonation resistance of sulphoaluminate cement concrete. Constr. Build. Mater. 2013, 48, 601–609. [Google Scholar] [CrossRef]
- Yuan, Q.; Shi, C.; De Schutter, G.; Audenaert, K.; Deng, D. Chloride Binding of Cement-Based Materials Subjected to External Chloride Environment—A Review. Constr. Build. Mater. 2009, 23, 1–13. [Google Scholar] [CrossRef]
- Chi, L.; Wang, Z.; Zhou, Y.; Lu, S.; Yao, Y. Layered Double Hydroxides Precursor as Chloride Inhibitor: Synthesis, Characterization, Assessment of Chloride Adsorption Performance. Materials (Basel) 2018, 11, 2537. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Shui, Z.; Chen, W.; Chen, G. Chloride binding of synthetic Ca–Al–NO3 LDHs in hardened cement paste. Constr. Build. Mater. 2015, 93, 1051–1058. [Google Scholar] [CrossRef]
- Chung, C.-W.; Jung, H.-Y.; Kwon, J.-H.; Jang, B.-K.; Kim, J.-H. Use of calcium aluminum–layered double hydroxide to control chloride ion penetration of cement-based materials. J. Struct. Integr. Maint. 2019, 4, 37–42. [Google Scholar] [CrossRef]
- Qu, Z.; Yu, Q.; Brouwers, H. Relationship between the particle size and dosage of LDHs and concrete resistance against chloride ingress. Cem. Concr. Res. 2018, 105, 81–90. [Google Scholar] [CrossRef]
- Rojas, R. Copper, lead and cadmium removal by Ca Al layered double hydroxides. Appl. Clay Sci. 2014, 87, 254–259. [Google Scholar] [CrossRef]
- Xu, S.; Zhang, B.; Chen, Z.; Yu, J.; Evans, D.G.; Zhang, F. A General and Scalable Formulation of Pure CaAl-Layered Double Hydroxide via an Organic/Water Solution Route. Ind. Eng. Chem. Res. 2011, 50, 6567–6572. [Google Scholar] [CrossRef]
- Sipos, P.; Pálinkó, I. As-prepared and intercalated layered double hydroxides of the hydrocalumite type as efficient catalysts in various reactions. Catal. Today 2018, 306, 32–41. [Google Scholar] [CrossRef]
- Yang, Z.; Fischer, H.; Polder, R. Synthesis and characterization of modified hydrotalcites and their ion exchange characteristics in chloride-rich simulated concrete pore solution. Cem. Concr. Compos. 2014, 47, 87–93. [Google Scholar] [CrossRef]
- Grover, K.; Komarneni, S.; Katsuki, H. Synthetic hydrotalcite-type and hydrocalumite-type layered double hydroxides for arsenate uptake. Appl. Clay Sci. 2010, 48, 631–637. [Google Scholar] [CrossRef]
- Oladoja, N.; Adelagun, R.; Ololade, I.; Anthony, E.; Alfred, M. Synthesis of nano-sized hydrocalumite from a Gastropod shell for aqua system phosphate removal. Sep. Purif. Technol. 2014, 124, 186–194. [Google Scholar] [CrossRef]
- Zhang, P.; Wang, T.; Zhang, L.; Wu, D.; Frost, R.L. XRD, SEM and infrared study into the intercalation of sodium hexadecyl sulfate (SHS) into hydrocalumite. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2015, 151, 673–678. [Google Scholar] [CrossRef]
- Milagres, J.L.; Bellato, C.R.; Vieira, R.S.; Ferreira, S.O.; Reis, C. Preparation and evaluation of the Ca-Al layered double hydroxide for removal of copper(II), nickel(II), zinc(II), chromium(VI) and phosphate from aqueous solutions. J. Environ. Chem. Eng. 2017, 5, 5469–5480. [Google Scholar] [CrossRef]
- Li, Y.; Wang, J.; Li, Z.; Liu, Q.; Liu, J.; Liu, L.; Zhang, X.; Yu, J. Ultrasound assisted synthesis of Ca–Al hydrotalcite for U (VI) and Cr (VI) adsorption. Chem. Eng. J. 2013, 218, 295–302. [Google Scholar] [CrossRef]
- Tathod, A.P.; Gazit, O.M. Fundamental Insights into the Nucleation and Growth of Mg–Al Layered Double Hydroxides Nanoparticles at Low Temperature. Cryst. Growth Des. 2016, 16, 6709–6713. [Google Scholar] [CrossRef]
- Galvão, T.L.P.; Neves, C.; Caetano, A.P.; Maia, F.; Mata, D.; Malheiro, E.; Ferreira, M.J.; Bastos, A.; Salak, A.N.; Gomes, J.R.B.; et al. Control of crystallite and particle size in the synthesis of layered double hydroxides: Macromolecular insights and a complementary modeling tool. J. Colloid Interface Sci. 2016, 468, 86–94. [Google Scholar] [CrossRef]
- Ji, H.; Wu, W.; Li, F.; Yu, X.; Fu, J.; Jia, L. Enhanced adsorption of bromate from aqueous solutions on ordered mesoporous Mg-Al layered double hydroxides (LDHs). J. Hazard. Mater. 2017, 334, 212–222. [Google Scholar] [CrossRef]
- Wang, T.; Li, C.; Wang, C.; Wang, H. Biochar/Mnal-Ldh Composites for Cu (Ιι) Removal from Aqueous Solution. Colloids Surf. A Physicochem. Eng. Asp. 2018, 538, 443–450. [Google Scholar]
- Zhou, H.; Jiang, Z.; Wei, S. A New Hydrotalcite-Like Absorbent Femnmg-Ldh and Its Adsorption Capacity for Pb 2+ Ions in Water. Appl. Clay Sci. 2018, 153, 29–37. [Google Scholar]
- Hatami, H.; Fotovat, A.; Halajnia, A. Comparison of adsorption and desorption of phosphate on synthesized Zn-Al LDH by two methods in a simulated soil solution. Appl. Clay Sci. 2018, 152, 333–341. [Google Scholar] [CrossRef]
- Tamaki, T.; Nakanishi, N.; Ohashi, H.; Yamaguchi, T. The effect of particle size and surface area on the ion conductivity of layered double hydroxide. Electrochem. Commun. 2012, 25, 50–53. [Google Scholar] [CrossRef]
- Kuroda, Y.; Miyamoto, Y.; Hibino, M.; Yamaguchi, K.; Mizuno, N. Tripodal Ligand-Stabilized Layered Double Hydroxide Nanoparticles with Highly Exchangeable CO32–. Chem. Mater. 2013, 25, 2291–2296. [Google Scholar] [CrossRef]
- Zhang, P.G.; Qian, Z.P.; Xu, H.; Shi, X.; Ruan, J.; Yang, R.L. Effective Adsorption of Sodium Dodecylsulfate (Sds) by Hydrocalumite (Caal-Ldh-Cl) Induced by Self-Dissolution and Re-Precipitation Mechanism. J. Colloid. Interface Sci. 2012, 367, 264–271. [Google Scholar] [CrossRef]
- Qian, G.; Feng, L.; Zhou, J.; Xu, Y.; Liu, J.; Zhang, J.; Xu, Z.P. Solubility product (Ksp)-controlled removal of chromate and phosphate by hydrocalumite. Chem. Eng. J. 2012, 181, 251–258. [Google Scholar] [CrossRef]
- Dadwhal, M.; Sahimi, M.; Tsotsis, T.T. Adsorption Isotherms of Arsenic on Conditioned Layered Double Hydroxides in the Presence of Various Competing Ions. Ind. Eng. Chem. Res. 2011, 50, 2220–2226. [Google Scholar] [CrossRef]


| Samples | Qe,ex (mg·g−1) | Pseudo-First-Order | Psudo-Second-Order | ||||
|---|---|---|---|---|---|---|---|
| Qe,ca (mg·g−1) | k1 (1·min−1) | R2 | Qe,ca (mg·g−1) | k1 (g·mg−1·min−1) | R2 | ||
| CAL-60 | 93.548 | 88.417 | 0.064 | 0.906 | 91.844 | 0.00119 | 0.948 |
| Sample | Qm,ex (mg·g−1) | Langmuir Isotherm | Freundlich Isotherm | ||||
|---|---|---|---|---|---|---|---|
| Qm,ca (mg·g−1) | kL (L·mg−1) | R2 | kF (mg·g−1) (L·mg−1)1/n | 1/n | R2 | ||
| CAL-60 | 137.929 | 211.324 | 0.01185 | 0.972 | 7.89054 | 0.56491 | 0.922 |
| CAL-70 | 124.890 | 182.117 | 0.01279 | 0.968 | 7.40587 | 0.55328 | 0.913 |
| CAL-80 | 117.921 | 174.903 | 0.01217 | 0.970 | 7.01206 | 0.55201 | 0.921 |
| CAL-100 | 110.340 | 160.914 | 0.01381 | 0.977 | 7.68250 | 0.52628 | 0.920 |
| CAL-120 | 98.144 | 155.960 | 0.01043 | 0.967 | 5.27952 | 0.57350 | 0.925 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, S.; Yu, F.; He, W.; Zheng, D.; Cui, H.; Lv, L.; Tang, W.; Han, N. Experimental Investigation of Chloride Uptake Performances of Hydrocalumite-Like Ca-Al LDHs with Different Microstructures. Appl. Sci. 2020, 10, 3760. https://doi.org/10.3390/app10113760
Zhang S, Yu F, He W, Zheng D, Cui H, Lv L, Tang W, Han N. Experimental Investigation of Chloride Uptake Performances of Hydrocalumite-Like Ca-Al LDHs with Different Microstructures. Applied Sciences. 2020; 10(11):3760. https://doi.org/10.3390/app10113760
Chicago/Turabian StyleZhang, Shupeng, Feng Yu, Wenting He, Dapeng Zheng, Hongzhi Cui, Leyang Lv, Waiching Tang, and Ningxu Han. 2020. "Experimental Investigation of Chloride Uptake Performances of Hydrocalumite-Like Ca-Al LDHs with Different Microstructures" Applied Sciences 10, no. 11: 3760. https://doi.org/10.3390/app10113760
APA StyleZhang, S., Yu, F., He, W., Zheng, D., Cui, H., Lv, L., Tang, W., & Han, N. (2020). Experimental Investigation of Chloride Uptake Performances of Hydrocalumite-Like Ca-Al LDHs with Different Microstructures. Applied Sciences, 10(11), 3760. https://doi.org/10.3390/app10113760








